Abstract
Epigenetic alteration of P16INK4a is conventionally thought to induce the initiation of carcinoma. However, the role of P16INK4a methylation in ovarian cancer still remains controversial. Therefore, we performed a meta-analysis to further elucidate the relationship between P16INK4a promoter methylation and ovarian cancer. A total of 24 studies, including 20 on risk, 10 on clinicopathological features, and 3 on prognosis, were included in our meta-analysis. Our results indicated that the frequency of P16INK4a methylation in cancer tissues was significantly higher than normal tissues and low malignant potential tumor tissues (odds ratio [OR] =5.01, 95% CI=1.55–16.14; OR =1.88, 95% CI=1.10–3.19, respectively), but similar to benign tissues (OR =1.18, 95% CI=0.52–2.65). Furthermore, P16INK4a promoter methylation was not strongly correlated with age, clinical stage, tumor differentiation, or histological subtype in patients with ovarian cancer. Additionally, survival analysis showed that patients with P16INK4a promoter methylation had a shorter progression-free survival in univariate and multivariate Cox regression models (hazard ratio =1.68, 95% CI=1.26–2.24; hazard ratio =1.55, 95% CI=1.15–2.08; respectively). In The Cancer Genome Atlas datasets, the methylation levels of seven out of nine CpG sites were significantly increased in the ovarian tumor tissues compared with the normal tissues. In conclusion, the present meta-analysis suggests that P16INK4a promoter methylation may be useful in distinguishing malignant cancer from healthy ovarian tissues, and it may be a potential predictive marker for prognosis in patients with ovarian cancer.
Introduction
Ovarian cancer is the fifth leading cause of cancer-related deaths in women. According to the GLOBOCAN 2012 database, the incidences of ovarian cancer are 9.1 per 1,00,100 in developed countries and 5.0 per 1,00,000 in developing countries.Citation1 There-into, approximately 70% is high-grade serous carcinomas.Citation2 Up to now, despite the effective treatments including radical resection, systemic chemotherapy, and targeted drugs for patients, the average 5-year survival is still only at 46%.Citation3 Ovarian cancer is a multifactorial disease caused by the interaction of genetic and epigenetic factors.Citation4,Citation5 (TSGs). Hypermethylation in the proximal promoter region often contributes to the transcriptional downregulation but methylation in exons is associated with active transcription.Citation6,Citation7 Recently, mounting evidences demonstrated that DNA methylation was involved in ovarian cancer.Citation8–Citation10 Therefore, identifying the role of TSG methylation in patients with ovarian cancer is of value.
P16INK4a (also known as CDKN2A), a classical TSG, is located on chromosome 9p21 and plays an important role in cell cycle regulation by decelerating cells progression from G1 to S phase.Citation11,Citation12 It has become clear that the expression of P16 is reduced by DNA methylation.Citation13–Citation15 Also, P16INK4a inactivation upregulates retinoblastoma (RB) protein by stimulating the cyclin-dependent kinases (CDKs) and RB pathway, which leads to dysfunction of cell proliferation and apoptosis, thereby further facilitating carcinogenesis.Citation16 Indeed, several types of cancer, including ovarian cancer, exhibit a methylation phenotype of P16INK4a.Citation17–Citation19
To date, even though abundant studies have been conducted to explore the role of P16INK4a promoter methylation in ovarian cancer, the results are still inconclusive. Several studies reported that P16INK4a promoter methylation was associated with an increasing trend in ovarian cancer,Citation20–Citation23 while, other studies suggested that P16INK4a promoter methylation was not related to the occurrence of ovarian cancer.Citation24–Citation30 Interestingly, even the conclusions in two published meta-analyses were inconsistent. Xiao et al reported that aberrant methylation of P16INK4a was significantly associated with ovarian carcinogenesis,Citation31 while Jiang et al suggested no association between P16INK4a methylation and epithelial ovarian cancer.Citation32
Considering these conflicting conclusions on the role of methylated P16INK4a in ovarian cancer, we performed an adaptive synthesized analysis to quantitatively evaluate the occurrence frequency, clinicopathological features, and potential prognostic significance of P16INK4a promoter methylation in ovarian cancer. Moreover, we searched The Cancer Genome Atlas (TCGA) database, collecting hundreds of ovarian cancer samples with whole genome DNA methylation datasets to validate our meta-analysis.
Materials and methods
Search strategy and selection criteria
PubMed, Embase, Web of Science, and China National Knowledge Infrastructure were searched up to April 12, 2017, by the following keywords and search items: (P16 OR P16INK4a OR CDKN2A) AND (methylation OR hypermethylation OR demethylation) AND (ovarian OR ovary) AND (cancer OR carcinoma OR neoplasm). The search was limited to human studies, without language restriction. Moreover, a manual search of the relevant references was implemented to identify the potentially additional articles.
The following criteria were used for screening eligible studies: 1) case–control studies evaluating the association between P16INK4a promoter methylation and ovarian cancer risk, or case only studies evaluating the association of P16INK4a promoter methylation with clinicopathological features or prognosis in ovarian cancer; 2) articles providing sufficient information for calculating an odds ratio (OR) and corresponding 95% CI, or study offering hazard ratio (HR) and 95% CI directly; 3) sample types limited to tissues; and 4) studies with full-text articles. It is worth noting that when multiple reports were published from a same study population, only the most recent or complete information was included in this meta-analysis. Meanwhile, studies with Newcastle Ottawa Scale (NOS) scores greater than or equal to five were enrolled.
Data extraction and quality assessment
With a preformed unified form, data were extracted independently by two investigators, and disagreements were resolved by discussion till consensus was achieved. The following information was extracted from studies: the first author’s name, publication year, country, geographical location, sample size, age of patients in the case group, the frequencies of methylation in the case and control groups, methods for detecting methylation, methylation site, disease stage, tumor grade, histological subtype, and effects on survival outcomes.
The quality of eligible case–control studies was assessed according to the NOS criteria.Citation33 The NOS criteria are based on three aspects: 1) subject selection: 0–4; 2) comparability of subject: 0–2; 3) clinical outcome: 0–3.
Statistical analysis
Statistical analysis was conducted with Review Manager 5.2 (Cochrane Collaboration, Oxford, UK) and the Stata 12.0 (Stata Corporation, College Station, TX, USA). ORs with corresponding 95% CIs were calculated to estimate the association between P16INK4a promoter methylation and ovarian cancer risk or clinicopathological features. Meanwhile, HRs and 95% CIs were used to assess the prognosis of P16INK4a promoter methylation on ovarian cancer. Inter-study heterogeneity was estimated with the Cochran’s Q statistic and I2 tests. P<0.05 or I2>50% indicated substantial heterogeneity, and then the random-effects model was applied. Otherwise, the fixed-effects model was selected. We also explored sources of heterogeneity using meta-regression and subgroup analyses by publication year, geographical location, method, and case sample size. Additionally, sensitivity analysis was performed to investigate the influence of individual study. Publication bias was evaluated by funnel plots and Begg’s test, and P<0.05 was considered statistically significant. It is worth mentioning that, for some trials containing no events in both case and control arms, as no information supplied about the likely magnitude of the effect, we excluded such trials when synthesizing data.Citation34
TCGA datasets extraction and analysis
We collected DNA methylation datasets of 582 ovarian cancer cases and 12 ovarian normal tissues from TCGA (“TCGA-ovary [OV]” project) program.Citation35 The methylation measurement was performed using Illumina HumanMethylation27 BeadChip. Beta value of each CpG site was extracted to assess the methylation level of CDKN2A gene. Beta value was calculated based on the intensities of the methylated (M) and unmethylated (U) bead types: beta value = M/(M+U).Citation36 The difference of DNA methylation level of CpG sites between ovarian tumor tissues and normal ovarian tissues in TCGA database was analyzed by Student’s t-test on the means. P16INK4a gene expression value (fragments per kilobase of transcript per million mapped reads) in ovarian tumor tissues (TCGA, “TCGA-OV” project) was also extracted. Pearson’s product-moment correlation between P16INK4a gene expression levels and methylation of its CpG islands was evaluated. Data analysis was performed using R software (R i386 3.4.0). P-values were adjusted via Bonferroni correction.
Results
Identification of relevant studies
The procedure of study selection is outlined in . We identified 233 articles in the initial literature search. A total of 153 references remained after removing duplicates. After reading titles and abstracts, 84 records were identified for further full-text assessment, which further excluded 60 more articles. Finally, 24 studies from 1997 to 2015 were included in this meta-analysis.Citation17,Citation20,Citation22–Citation30,Citation37–Citation49
Baseline characteristics of included studies
Out of the 24 studies, 11 studies were conducted in Asia, 7 in Europe, 4 in America, 1 in Africa, and 1 in Oceania. The detection methods of methylation in 20 studies were methylation-specific PCR (MSP) and real-time quantitative MSP, while methylation-specific multiplex ligation-dependent probe amplification was used in two studies, MethyLight was used in one study, and Southern analysis was used in one study. Among the 24 articles, 20 studiesCitation17,Citation20,Citation22–Citation30,Citation37–Citation40,Citation42,Citation45–Citation47,Citation49 addressed the risk of P16INK4a promoter methylation in ovarian cancer, 10 studiesCitation20,Citation25,Citation28,Citation29,Citation38,Citation41,Citation43,Citation44,Citation47,Citation48 covered clinicopathological features, and 3 studiesCitation20,Citation42,Citation43 discussed prognosis. To explore the relationship between P16INK4a promoter methylation and ovarian cancer risk, three groups, that is, normal tissues, benign tissues, and low malignant potential or borderline tumor tissues (LMP), were compared. The NOS scores of all case–control studies were ≥5. The basic characteristics of all included studies are summarized in and .
Table 1 Characteristics of studies included for the association between P16INK4a methylation and ovarian cancer risk
Table 2 Characteristics of studies included for the association between P16INK4a methylation and clinicopathological features of ovarian cancer
Quantitative data synthesis
Association between P16INK4a promoter methylation and ovarian cancer risk
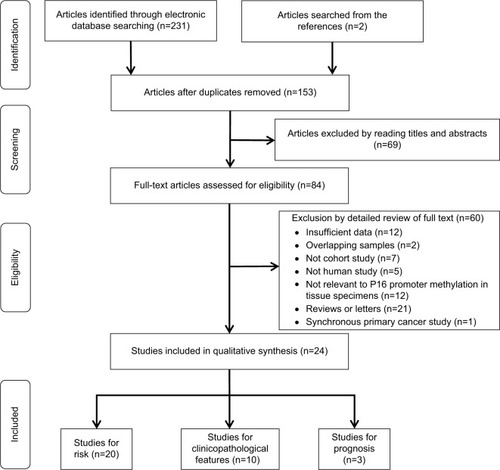
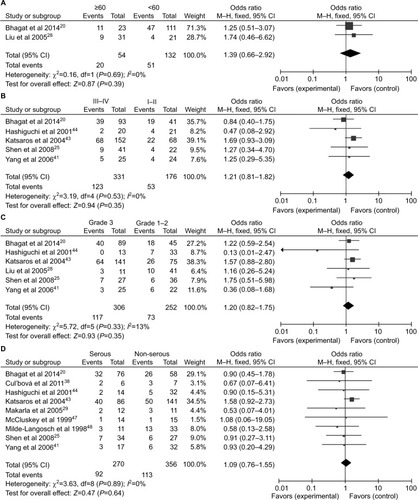
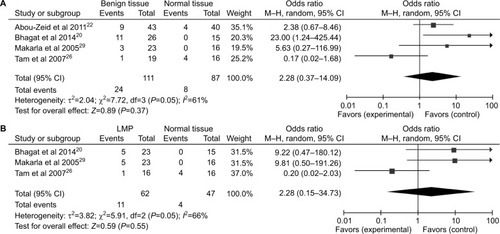
A total of 1,217 ovarian cancers, 116 LMP cancers, 271 benign patients, and 351 normal controls were quantitatively synthesized in this analysis. Results indicated that the frequency of P16INK4a promoter methylation in cancer tissues was significantly elevated than that in normal tissues (OR=5.01, 95% CI=1.55–16.14) and LMP tissues (OR =1.88, 95% CI=1.10–3.19), but similar to benign tissues (OR =1.18, 95% CI=0.52–2.65; ). Further analyses showed that the frequencies of P16INK4a promoter methylation in benign tissues and LMP tissues were not higher than those in normal tissues (OR =2.28, 95% CI=0.37–14.09; OR =2.28, 95%CI=0.15–34.73, respectively; ).
Figure 2 Forest plots for the association between P16INK4a methylation and ovarian cancer risk.
Notes: (A) Cancer tissues vs normal tissues; (B) cancer tissues vs benign tissues; (C) cancer tissues vs LMP tissues.
Abbreviations: LMP, low malignant potential or borderline tumor tissues; M–H, Mantel–Haenszel.
Figure 3 Forest plots for the association between P16INK4a methylation and ovarian diseases.
Notes: (A) Benign tissues vs normal tissues; (B) LMP tissues vs normal tissues.
Abbreviations: LMP, low malignant potential or borderline tumor tissues; M–H, Mantel–Haenszel.
With large heterogeneity, meta-regression and subgroup analyses were conducted by the publication year, geographical location, method, and case sample size in the comparison of cancer tissues vs normal tissues. Meta-regression found that case sample size was significantly correlated with the inter-study heterogeneity (P=0.041) while other covariates were not (). Furthermore, as shown in , subgroup analyses revealed that the OR was 5.69 (95% CI=0.42–76.14) for the publication year ≤2005 and 4.71 (95% CI=1.30–17.07) for >2005 under the random-effects model. For geographical location, the OR was 7.85 (95% CI=1.33–46.32) in Asia, 2.31 (95% CI=0.24–22.01) in America, and 6.10 (95% CI=1.89–19.69) in Africa under random-effects model. For test method, the OR for MSP was 4.49 (95% CI=0.97–20.64) under random-effects model and 8.11 (95% CI=2.93–22.40) for other methods under fixed-effects model. In addition, the OR was 15.75 (95% CI=4.05–61.34) for sample size <50 in fixed-effects model and 2.21 (95% CI=1.33–3.67) for that ≥50 in random-effects model.
Table 3 Meta-regression and subgroup analyses of P16INK4a methylation in comparison of cancer tissues vs normal tissues
Association between P16INK4a promoter methylation and clinicopathological features in patients with ovarian cancer
Ten studies comprising 680 samples were enrolled to assess whether or not the abnormal P16INK4a promoter methylation was associated with ovarian cancer clinicopathological characteristics. As displayed in , no statistically significant correlation was found between P16INK4a promoter methylation and age of patients (≥60 vs<60: OR =1.39, 95% CI=0.66–2.92), clinical stage (III–IV vs I–II: OR =1.21, 95% CI=0.81–1.82), grade (3 vs 1–2: OR=1.20, 95% CI=0.82–1.1.75) as well as histological subtype (serous vs non-serous: OR=1.09, 95% CI=0.76–1.55).
Prognostic value of P16INK4a promoter methylation in patients with ovarian cancer
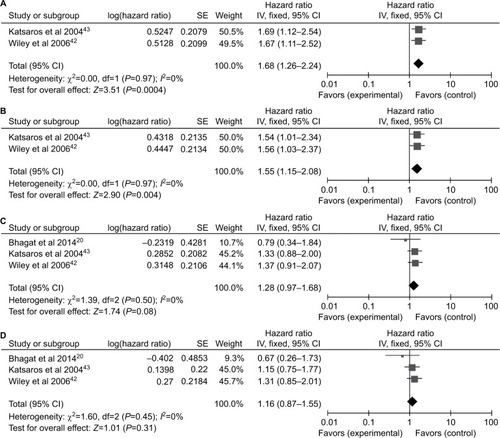
Only two studiesCitation42,Citation43 containing 464 patients evaluated the P16INK4a promoter methylation on progression-free survival (PFS) and three studiesCitation20,Citation42,Citation43 containing 600 patients on overall survival (OS). The combined results revealed P16INK4a promoter methylation was significantly associated with a poor PFS by univariate Cox proportional hazards regression model (HR=1.68, 95% CI=1.26–2.24; ). After considering potential confounders by adjusting for age at diagnosis or surgery, disease stage, histological grade, and residual tumor size, the pooled HR was 1.55 (1.15–2.08; ). Survival analysis also showed that P16INK4a promoter methylation reduced OS in univariate and multivariate Cox regression models (HR =1.28, 95% CI=0.97–1.68; HR =1.16, 95% CI=0.87–1.55, respectively; ), but the differences were not statistically significant.
Figure 5 Forest plots for P16INK4a methylation on survival analysis in univariate and multivariate Cox regression model.
Notes: (A) PFS in univariate Cox regression model; (B) PFS in multivariate Cox regression model; (C) OS in univariate Cox regression model; (D) OS in multivariate Cox regression model.
Abbreviations: OS, overall survival; PFS, progression-free survival; SE, standard error.
Sensitivity analysis and publication bias
As presented in , no single study significantly affected the pooled ORs in the sensitivity analysis, indicating our analysis was relatively stable and credible. Funnel plots and Begg’s test were used to evaluate the publication bias. The funnel plots were largely symmetric suggesting there were no publication biases in the meta-analysis of P16INK4a promoter methylation and ovarian cancer risk, which was confirmed by the Begg’s test ().
Figure 6 Sensitivity analyses and Begg’s test for publication bias of P16INK4a methylation during the carcinogenesis of ovarian cancer.
Notes: (A and D) Cancer tissues vs normal tissues; (B and E) sensitivity analysis for the comparison of cancer tissues vs benign tissues; (C and F) sensitivity analysis for the comparison of cancer tissues vs LMP tissues.
Abbreviations: LMP, low malignant potential or borderline tumor tissues; SE, standard error.
Methylation level of P16INK4a measured by TCGA program
To further explore the methylation level of P16INK4a in ovarian tumor tissues, we extracted DNA methylation data of P16INK4a CpG sites measured with Illumina HumanMethylation27 BeadChip from TCGA program. As shown in , the beta values of 582 ovarian tumor tissues and 12 normal ovarian tissues were extracted for analysis. Obviously, the methylation levels of seven out of nine CpG sites were significantly increased in the ovarian tumor tissues compared with the normal tissues (cg03079681, cg07752420, cg09099744, cg10895543, cg11653709, cg12840719, and cg26673943). Among these regions, methylation level of probe cg26673943 region (located at the promoter region of P16INK4a) was negatively associated with P16INK4a expression in ovarian cancer patients (adjusted P-value <0.000001). However, methylation levels of the rest six probes, which are located at non-promoter region tended to be positively associated with P16INK4a gene expression. Additionally, we found that methylation level of probe cg13479669 region was lower in tumor tissues compared with normal tissues, and negatively associated with P16INK4a gene expression in tumor tissues. These results suggest that hypermethylation of P16INK4a might be correlated with ovarian carcinogenesis and development. Nevertheless, it seems that the methylation at promoter region or non-promoter region has contrary effects on P16INK4a gene expression.
Table 4 Methylation of P16INK4a CpG sites on Illumina HumanMethylation 27 BeadChip from TCGA datasets
Discussion
Ovarian cancer is one of the leading causes of cancer-related deaths in women.Citation50 Identification of early disease indicators for diagnosis and prognosis is of clinical value. P16INK4a, which resembles classic TSGs such as P53, is an important negative regulator of cell growth and proliferation.Citation16 It has been synthetically evaluated for aberrant P16INK4a methylation in numerous cancers,Citation51–Citation54 including ovarian cancer.Citation31,Citation32 Considering the conflicting conclusions in two meta-analyses and the lack of comprehensive assessment on the role of methylated P16INK4a in ovarian cancer, we performed an adaptive synthesized analysis to investigate the relationships between P16INK4a promoter methylation and ovarian cancer risk, as well as clinicopathological features and prognostic value in ovarian cancer. Meanwhile, we searched TCGA data to validate our meta-analysis.
Our meta-analysis demonstrated that P16INK4a promoter methylation in cancer tissues was significantly higher than that in normal tissues (P<0.05), but not much increased than that in benign tissues. Compared with normal tissues, the frequency of P16INK4a promoter methylation was 2.28-fold higher in both benign tissues and LMP tissues (P>0.05), but the differences were not statistically significant. The reason for this phenomenon may be that the transformation of normal cells to cancer cells is a long-term, gradual, and multiphase process.Citation55 Although not establishing a strong correlation between P16INK4a promoter methylation and cancer progression, the above results do suggest a possibility that epigenetic alteration of P16INK4a promoter methylation might play a certain role in ovarian carcinogenesis and might be useful in distinguishing malignant tumor from healthy ovarian tissues. Considering the evident heterogeneity, we conducted subgroup analyses based on probable covariates in the comparison of cancer tissues vs normal tissues. For geographical location, P16INK4a promoter methylation is a risk factor in Asia and Africa, but not in America. The divergence may be underscored in a large part to a combination of differences in allele frequencies and complex epistasis or gene–environment interactions.Citation56 A review also outlined that some factors such as distinct physical appearance, behavior, and response to environ mental agents and drugs between human populations could have contributed to the epigenetic variations.Citation57 Similar findings appeared in the subgroup analyses of different methods and publication year. Kurdyukov and BullockCitation58 suggested that it was essential to choose an appropriate method in a suitable region to answer a particular biological question in studies of DNA methylation. Additionally, the 95% CI was large in the group of small sample size while relatively small in the group of large sample size, implying the conclusion may not be reliable unless studies should be conducted using a sufficient number of samples. Previous studies also demonstrated that the methylation status in blood samples or fluids might be different from that in tissues.Citation59,Citation60 Thus, our results should be interpreted with caution because sample types were limited to tissues in studies included in this meta-analysis.
Previous studies indicated that P16INK4a promoter methylation was associated with poorly differentiated tumors and was different in histological subtype in ovarian cancer.Citation22,Citation43 However, we could not establish any significant correlations between P16INK4a promoter methylation and clinicopathological features, including age, clinical stage, tumor differentiation or histological subtype in this study. Therefore, it might not be essential to predict the invasion and metastasis of ovarian cancer.
Katsaros et alCitation43 and Wiley et alCitation42 reported association of P16INK4a promoter methylation with PFS and OS in ovarian cancer, while Bhagat et alCitation20 found no significant value in predicting prognosis. In the present study, we discovered that P16INK4a promoter methylation represented a risk factor for PFS. For OS, patients with P16INK4a promoter methylation also had a slightly elevated risk, though the differences are not statistically significant. This trend was also observed in other types of cancer.Citation51,Citation54 However, its statistical confirmation requires large studies. The data from TCGA also indicated that methylation level of probe cg26673943 region (located at the promoter region of P16INK4a) in the ovarian tumor tissues was higher than normal ovarian tissues. Increased methylation of CpG island at the promoter region was negatively associated with P16INK4a gene expression, while methylation of CpG islands at non-promoter regions was positively associated with P16INK4a expression.
Compared with previous meta-analyses,Citation31,Citation32 our meta-analysis had several improvements. First, the development of ovarian cancer is a multistep procedure involving normal tissues, benign disease, LMP or borderline tumor, and malignant tumor.Citation20 We compared malignant ovarian cancer with LMP tumors, benign disease, and normal samples to give more rigorously to the analysis. Second, with 1,217 malignant ovarian cancer patients, 116 LMP, 271 benign patients, and 351 normal samples, the sample size in our study is much larger than that of all previous meta-analyses. Finally, we included the clinicopathological features and prognostic significance of P16INK4a promoter methylation in ovarian cancer for more comprehensive understanding of the underlying pathogenesis of ovarian cancer. These strengths make our study a useful effort in seeking better understanding of the P16INK4a promoter methylation in ovarian cancer.
Limitations
Several potential limitations in our current study should be noted. First, the heterogeneity was still large after subgroup analyses in the assessment of the association between P16INK4a promoter methylation and ovarian cancer risk, which may affect the statistical power. Second, as a retrospective study, a potential unidentified confounding information and selection bias may exist in our meta-analysis. We could not eliminate the possibility of publication bias, where positive results are likely published than negative results. Third, the total sample size was still relatively small for reliably assessing the prognostic value of P16INK4a promoter methylation in ovarian cancer. Fourth, none of the studies included in our meta-analysis defined the region considered as promoter or provided specific methylation sites. Therefore, we are unable to establish whether or not they focused on the same sequence of P16INK4a gene. However, the impact of methylation on transcriptional potential depends on the density of the methylated CpG islands and their location relative to the transcription start site. This highlights the importance of a uniform and full-scale reporting of study designs and outcomes. Additionally, previous researches showed that the occurrence of P16INK4a promoter methylation may depend on the histological subtype.Citation41,Citation48,Citation61 However, we are unable to extract sufficient data to analyze the association between P16INK4a promoter methylation and high-grade serous carcinomas because no detailed information of P16INK4a promoter methylation in high-grade serous carcinomas was provided in the eligible articles.
Although with certain limitations, our study is a comprehensive meta-analysis focusing on the correlation of aberrant P16INK4a promoter methylation with the initiation, development, and prognosis of ovarian cancer to provide a new insight into the pathogenesis of ovarian cancer.
Conclusion
In conclusion, our meta-analysis suggests that aberrant methylation of P16INK4a promoter may be essential to the initiation of ovarian cancer and in distinguishing malignant from healthy ovarian tissues. Besides, P16INK4a promoter methylation is a potential predictive factor for poor prognosis in ovarian cancer. This study indicates the need for multicenter large-scale studies to confirm the role of P16INK4a promoter methylation in ovarian cancer.
Acknowledgments
This study was supported by National Natural Science Funds (numbers 81472033 and 30901308), the National Science Foundation of Hubei Province (numbers 2013CFB233 and 2013CFB235), the Scientific and Technological Project of Wuhan City (number 2014060101010045), Hubei Province Health and Family Planning Scientific Research Project (WJ2015Q021), and Training Program of the Science and Technology Innovation from Zhongnan Hospital of Wuhan University (cxpy20160054). We thank the Beijing Circle & Dot Technology Co., Ltd. for assisting in analyzing the TCGA database.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- PratJNew insights into ovarian cancer pathologyAnn Oncol201223Suppl 10x111x11722987944
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- BaiHCaoDYangJLiMZhangZShenKGenetic and epigenetic heterogeneity of epithelial ovarian cancer and the clinical implications for molecular targeted therapyJ Cell Mol Med201620458159326800494
- YouJSJonesPACancer genetics and epigenetics: two sides of the same coin?Cancer Cell201222192022789535
- KazanetsAShorstovaTHilmiKMarquesMWitcherMEpigenetic silencing of tumor suppressor genes: paradigms, puzzles, and potentialBiochim Biophys Acta20161865227528827085853
- MaunakeaAKChepelevICuiKZhaoKIntragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognitionCell Res201323111256126923938295
- DongALuYLuBGenomic/Epigenomic alterations in ovarian carcinoma: translational insight into clinical practiceJ Cancer20167111441145127471560
- KoukouraOSpandidosDADaponteASifakisSDNA methylation profiles in ovarian cancer: implication in diagnosis and therapy (Review)Mol Med Rep20141013924821107
- GlossBSSamimiGEpigenetic biomarkers in epithelial ovarian cancerCancer Lett2014342225726322245949
- SharplessNEDepinhoRAThe INK4A/ARF locus and its two gene productsCurr Opin Genet Dev199991223010072356
- SerranoMHannonGJBeachDA new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4Nature199336664567047078259215
- QinYLiuJYLiBSunZLSunZFAssociation of low p16INK4a and p15INK4b mRNAs expression with their CpG islands methylation with human hepatocellular carcinogenesisWorld J Gastroenterol20041091276128015112341
- KimBNYamamotoHIkedaKMethylation and expression of p16INK4 tumor suppressor gene in primary colorectal cancer tissuesInt J Oncol20052651217122615809712
- GaoSJZhangGFZhangRPHigh CpG island methylation of p16 gene and loss of p16 protein expression associate with the development and progression of tetralogy of FallotJ Genet201695483183727994181
- SharplessNEINK4a/ARF: a multifunctional tumor suppressor locusMutat Res20055761–2223815878778
- MoselhySSKumosaniTAKamalIHJalalJAJabaarHSDalolAHypermethylation of P15, P16, and E-cadherin genes in ovarian cancerToxicol Ind Health2015311092493023572389
- di VinciAPerdelliLBanelliBp16(INK4a) promoter methylation and protein expression in breast fibroadenoma and carcinomaInt J Cancer2005114341442115578730
- ShimaKNoshoKBabaYPrognostic significance of CDKN2A (p16) promoter methylation and loss of expression in 902 colorectal cancers: cohort study and literature reviewInt J Cancer201112851080109420473920
- BhagatRKumarSSVaderhobliSEpigenetic alteration of p16 and retinoic acid receptor beta genes in the development of epithelial ovarian carcinomaTumour Biol20143599069907824913706
- BammidiLSNeerukondaGNMurthySKanapuramRDp16 gene alterations in human ovarian cancers: comparison between tissue and blood samplesInt J Gynecol Cancer201222455356022344043
- Abou-ZeidAAAzzamAZKamelNAMethylation status of the gene promoter of cyclin-dependent kinase inhibitor 2A (CDKN2A) in ovarian cancerScand J Clin Lab Invest201171754254721728901
- DhillonVSAslamMHusainSAThe contribution of genetic and epigenetic changes in granulosa cell tumors of ovarian originClin Cancer Res200410165537554515328194
- OzdemirFAltinisikJKaratekeACoksuerHBuyruNMethylation of tumor suppressor genes in ovarian cancerExp Ther Med2012461092109623226780
- ShenWJDaiDQGuoKJXmLPromoter hypermethylation of RASSF1A, BRCA1 and p16 gene in epithelial ovarian cancer and its clinical significanceChin J Cancer Prev Treat2008157530533
- TamKFLiuVWLiuSSMethylation profile in benign, borderline and malignant ovarian tumorsJ Cancer Res Clin Oncol2007133533134117177027
- LiMHuangZJDongWHDisfigurement of p16INK4A gene expression in development of ovarian cancer and the mechanismZhonghua Fu Chan Ke Za Zhi200641640841216831366
- LiuZWangLEWangLMethylation and messenger RNA expression of p15INK4b but not p16INK4a are independent risk factors for ovarian cancerClin Cancer Res200511134968497616000597
- MakarlaPBSaboorianMHAshfaqRPromoter hypermethylation profile of ovarian epithelial neoplasmsClin Cancer Res200511155365536916061849
- RathiAVirmaniAKSchorgeJOMethylation profiles of sporadic ovarian tumors and nonmalignant ovaries from high-risk womenClin Cancer Res20028113324333112429618
- XiaoXCaiFNiuXShiHZhongYAssociation between P16INK4a promoter methylation and ovarian cancer: a meta-analysis of 12 published studiesPLoS One2016119e016325727648827
- JiangYYanFLiangLWanYLiuJChengWMeta-analysis demonstrates no association between P16 INK4A promoter methylation and epithelial ovarian cancerArch Gynecol Obstet2017295369770428000027
- StangACritical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol201025960360520652370
- BradburnMJDeeksJJBerlinJARussell LocalioAMuch ado about nothing: a comparison of the performance of meta-analytical methods with rare eventsStat Med2007261537716596572
- National Institutes of HealthNational Cancer InstituteNational Human Genome Resarch InstituteThe Cancer Genome Atlas Available from: https://cancergenome.nih.gov/Accessed August 19, 2018
- Cancer Genome Atlas Research NetworkIntegrated genomic analyses of ovarian carcinomaNature2011474735360961521720365
- HoCMHuangCJHuangCYWuYYChangSFChengWFPromoter methylation status of HIN-1 associated with outcomes of ovarian clear cell adenocarcinomaMol Cancer2012115322871047
- Cul’bováMLasabovaZStanclovaAMetylácia vybraných tumor-supresorických génov v benígnych a malígnych ovariálnych nádoroch [Methylation of selected tumor-suppressor genes in benign and malignant ovarian tumors]Ceska/Gynekol2011764274279 Czech
- GuXHLuYMaDLiuXSGuoSWModel of aberrant DNA methylation patterns and its applications in epithelial ovarian cancerZhonghua Fu Chan Ke Za Zhi2009441075475920078962
- WuQLotheRAAhlquistTDNA methylation profiling of ovarian carcinomas and their in vitro models identifies HOXA9, HOXB5, SCGB3A1, and CRABP1 as novel targetsMol Cancer200764517623056
- YangHJLiuVWWangYTsangPCNganHYDifferential DNA methylation profiles in gynecological cancers and correlation with clinicopathological dataBMC Cancer2006621216928264
- WileyAKatsarosDChenHAberrant promoter methylation of multiple genes in malignant ovarian tumors and in ovarian tumors with low malignant potentialCancer2006107229930816773633
- KatsarosDChoWSingalRMethylation of tumor suppressor gene p16 and prognosis of epithelial ovarian cancerGynecol Oncol200494368569215350359
- HashiguchiYTsudaHYamamotoKInoueTIshikoOOgitaSCombined analysis of p53 and RB pathways in epithelial ovarian cancerHum Pathol200132998899611567230
- BrownIMilnerBRooneyPHaitesNInactivation of the p16INK4A gene by methylation is not a frequent event in sporadic ovarian carcinomaOncol Rep2001861359136211605066
- StrathdeeGAppletonKIllandMPrimary ovarian carcinomas display multiple methylator phenotypes involving known tumor suppressor genesAm J Pathol200115831121112711238060
- MccluskeyLLChenCDelgadilloEFelixJCMuderspachLIDubeauLDifferences in p16 gene methylation and expression in benign and malignant ovarian tumorsGynecol Oncol199972187929889036
- Milde-LangoschKOconEBeckerGLöningTp16/MTS1 inactivation in ovarian carcinomas: high frequency of reduced protein expression associated with hypermethylation or mutation in endometrioid and mucinous tumorsInt J Cancer199879161659495360
- ShihYCKerrJLiuJRare mutations and no hypermethylation at the CDKN2A locus in epithelial ovarian tumoursInt J Cancer19977055085119052747
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- XingXCaiWShiHThe prognostic value of CDKN2A hypermethylation in colorectal cancer: a meta-analysisBr J Cancer2013108122542254823703248
- LiJZhouCZhouHThe association between methylated CDKN2A and cervical carcinogenesis, and its diagnostic value in cervical cancer: a meta-analysisTher Clin Risk Manag2016121249126027574435
- WangXZhuYBCuiHPYuTTTtYAberrant promoter methylation of p15 (INK4b) and p16 (INK4a) genes may contribute to the pathogenesis of multiple myeloma: a meta-analysisTumour Biol20143599035904324908414
- TangBLiYQiGClinicopathological significance of CDKN2A promoter hypermethylation frequency with pancreatic cancerSci Rep201551356326338139
- HoganCImpact of interactions between normal and transformed epithelial cells and the relevance to cancerCell Mol Life Sci201269220321321877117
- FraserHBLamLLNeumannSMKoborMSPopulation-specificity of human DNA methylationGenome Biol2012132R822322129
- KaderFGhaiMDNA methylation-based variation between human populationsMol Genet Genomics2017292153527815639
- KurdyukovSBullockMDNA methylation analysis: choosing the right methodBiology2016513
- ChangHYiBLiLMethylation of tumor associated genes in tissue and plasma samples from liver disease patientsExp Mol Pathol20088529610018691570
- ZhuWQinWHewettJESauterERQuantitative evaluation of DNA hypermethylation in malignant and benign breast tissue and fluidsInt J Cancer2010126247448219618401
- NiederacherDYanHYAnHXBenderHGBeckmannMWCDKN2A gene inactivation in epithelial sporadic ovarian cancerBr J Cancer199980121920192610471040