Abstract
Purpose
Our purpose was to evaluate the association between hematologic markers and mortality and adverse events in patients with esophageal squamous cell carcinoma (ESCC) treated with neoadjuvant chemoradiotherapy (nCRT).
Patients and Methods
A total of 311 patients with ESCC treated with nCRT from 2012 to 2014 were enrolled retrospectively. The Kaplan–Meier method with a Log rank test was used to calculate five-year overall survival (OS). Receiver operating characteristic (ROC) curves were plotted to determine the cut-off values for hematologic markers. Multivariate analysis was performed using Cox regression analysis model. Model performance was evaluated by predicted nomogram, concordance index (C-index) and calibration curve.
Results
Median follow-up was 22 months. High pretreatment platelet to lymphocyte ratio (PLR, p = 0.047) and systemic immune-inflammation index (SII, p = 0.027) were significantly associated with pathologic complete response (pCR). In multivariate analysis, smoking history, Eastern Cooperative Oncology Group (ECOG) performance status, invasion depth, lymph node metastasis, PLR, and SII were independent factors to predict five-year OS. Multivariate analysis showed a lower neutrophil to lymphocyte ratio (NLR) at baseline (p = 0.007) was significantly associated with development of grade ≥3 hematologic toxicity, and none of inflammatory biomarkers could predict grade ≥3 non-hematologic toxicity or radiation pneumonitis (RP).
Conclusion
SII and PLR were independent indicators to predict prognosis in patients with ESCC treated with nCRT, and a lower NLR at baseline was an independent indicator to predict grade ≥3 hematologic toxicity.
Introduction
Esophageal cancer is the eighth most frequently diagnosed cancer and the sixth most common cause of cancer death worldwide.Citation1 In China, esophageal squamous cell carcinoma (ESCC) is the predominant histologic type.Citation2 The prognosis of esophageal cancer is rather poor, with five-year survival rates ranging from 15% to 25% in most countries.Citation3 At present, neoadjuvant chemoradiation (nCRT) followed by esophagectomy is the standard treatment for locally advanced esophageal cancer.Citation4,Citation5 However, the curative effects, prognosis, and adverse events vary widely even for patients with the same clinicopathological factors. Therefore, there is a demand for seeking some biomarkers to accurately identify poor prognosis and severe adverse events in patients with ESCC receiving nCRT and further guide the treatment.
Accumulating evidence has shown that systemic inflammation plays an important role in different stages of tumor development, including initiation, promotion, malignant conversion, invasion, and metastasis.Citation6,Citation7 Previous studies have confirmed that inflammatory biomarkers including preoperative neutrophil to lymphocyte ratio (NLR), preoperative derived neutrophil to lymphocyte ratio (dNLR), preoperative monocyte to lymphocyte ratio (MLR), preoperative platelet to lymphocyte ratio (PLR), preoperative systemic immune-inflammation index (SII), postoperative NLR, and difference in NLR before and after chemoradiotherapy (∆NLR) are associated with worse prognosis in various malignancies,Citation8–Citation12 including esophageal cancer.Citation13–Citation18 However, for ESCC undergoing nCRT, data regarding the relationship between pre- and post-treatment levels of inflammatory biomarkers and prognosis or adverse events was very limited. Recently a study demonstrated that ∆NLR was inversely related to pathologic complete response (pCR) and associated with hazard of recurrence in patients with ESCC receiving chemoradiotherapy alone or nCRT.Citation15 However, the number of patients receiving nCRT was limited (n = 84), and most patients (n = 133) received chemoradiotherapy alone.
Fibrinogen, an important molecular players of the coagulation cascade produced by the liver, is a key regulator of inflammation in disease.Citation19 The hypercoagulable state is common among patients with malignant tumors.Citation20 Serum albumin level, as well as prognostic nutritional index (PNI), has been used to assess cancer patients’ nutritional status. Additionally, lactate dehydrogenase (LDH), a key enzyme in the glycolytic pathway, is directly related to tumor growth, tumor proliferative index, metastasis, and tumor survival.Citation21 Previous studies have demonstrated that preoperative albumin /fibrinogen ratio (AFR), PNI and LDH are significantly associated with prognosis in variety of tumors,Citation22–Citation27 including ESCC.Citation28–Citation30
However, studies assessing the relationship between these hematologic biomarkers and prognosis in patients with ESCC are still few, and controversy exists regarding which is the best hematologic biomarker for predicting prognosis.
Therefore, in this study, we included diverse hematologic biomarkers, aiming to seek the best biomarkers to predict prognosis and adverse events in patients with ESCC treated with neoadjuvant nCRT.
Patients and Methods
Patients
Patients with ESCC receiving nCRT at Shandong Cancer Hospital and Institute between 2011 and 2014 were retrospectively analyzed. The inclusion criteria were as follows: 1) 18–80 years old; 2) pathologically confirmed ESCC; 3) esophagectomy with R0 resection and no presence of postoperative adjuvant therapy; 4) Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; 5) without severe dysfunction of important organs; 6) without distant metastasis; 7) without secondary primary tumor. Those who had acute infection or chronic inflammatory disease, received anti-inflammatory treatment, or had hematological or autoimmune disease before nCRT were excluded from our analysis. All patients were staged based on the seventh edition of the American Joint Committee on Cancer (AJCC) staging manual.Citation31 Pretreatment evaluation included esophageal endoscopy with biopsy, endoscopic ultrasound, barium esophagography, contrast-enhanced thoracic and abdominal computed tomography (CT), and whole-body bone scan. Cardiac and pulmonary function examinations were performed to evaluate surgical tolerance. Positron emission tomography/computed tomography (PET-CT) was optional. The clinical and pathologic TNM stages were performed for all patients (). The difference between clinical and pathologic stages were found in 56 patients. PET-CT were performed for 53 patients to evaluate the TNM stage. This study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute, and written informed consents were obtained from all included individuals. This study was conducted in accordance with the Declaration of Helsinki.
Table 1 Patient Characteristics
Treatment
The details of treatment plan were collected from medical records at our hospital. Most patients (67.5%) received two 3-weekly cycles of cisplatin and fluorouracil. Cisplatin (80 mg/m2) was infused intravenously on day 1 and 5-fluorouracil (1 g/m2 per day) was administered as a continuous infusion on days 1–4. Radiotherapy with a median prescribed dose of 45 Gy (range, 40–50.4 Gy) was administered in 1.8–2.0 Gy per fraction and five fractions per week, starting at the first day of the first cycle of chemotherapy. Radiotherapy was delivered by using linear accelerators with an energy of 6-MV or 10-MV X-ray. Treatment-planning CT scans using intravenous contrast were performed for all patients. Gross tumor volume (GTV), defined by any visible primary tumor and metastatic regional nodes, was determined by radiation oncologists using all available resources (barium swallow, CT, PET-CT, endoscopy, endoscopic ultrasound). Clinical target volume (CTV) was generated using a 3 to 5 cm proximal and distal margin and a 0.5 to 1 cm radial margin around the GTV. Supraclavicular lymph nodes were included electively for upper esophageal cancer and celiac lymph nodes were included for distal esophageal cancer based on the decision of radiation oncologists. The planning target volume (PTV) was defined as a 5 mm margin of CTV in all directions. Radiation technique included three-dimensional conformal radiation therapy (3D-CRT) or intensity-modulated radiation therapy (IMRT). Surgery was scheduled for 4 to 8 weeks after completion of nCRT. All patients received a standardized transthoracic Ivor-Lewis esophagectomy with a two-field or three-field lymphadenectomy. In this study, 182 patients (58.5%) received two-field thoracoabdominal lymphadenectomy which including subcarinal, diaphragmatic, paraesophageal, and paracardiac lymph nodes, as well as those located along the pharyngeal nerve, lesser gastric curvature, left gastric artery, celiac axis and hepatic artery trunk. Three-field lymphadenectomy was performed with 129 patients (41.5%) when the cervical or supraclavicular lymph nodes were thought to be abnormal according to preoperative imaging evaluation.
Measures for Hematologic Biomarkers
The following pretreatment hematologic parameters were collected from peripheral blood within 1 week prior to the initial treatment: serum albumin, LDH, fibrinogen, neutrophil count, lymphocyte count, monocyte count, and platelet count. The following posttreatment hematologic parameters were collected at least 21 days after nCRT (to avoid interference from the acute immunosuppressive effect of nCRT) and before surgery: neutrophil count, lymphocyte count, monocyte count, and platelet count. The definitions of NLR, dNLR, MLR, PLR, SII, AFR, PNI, and ∆NLR are calculated as follows: NLR = neutrophil counts/lymphocyte counts; MLR = monocyte counts/lymphocyte counts; dNLR = neutrophil counts/(white blood cell counts – neutrophil counts); PLR = platelet counts/lymphocyte counts; SII = platelet counts × neutrophil counts/lymphocyte counts; AFR = albumin level (g/L)/fibrinogen level (g/L); PNI = albumin level (g/L) + 5 × total lymphocyte counts (109 /L); and ∆NLR = posttreatment NLR – pretreatment NLR. Finally, the hematologic biomarkers were included in this study as follows: pretreatment levels of LDH, NLR, dNLR, MLR, PLR, SII, AFR, and PNI; posttreatment levels of NLR, MLR, and PLR; and ∆NLR.
Assessment of Response and Adverse Events and Follow-Up
We evaluated the clinical and pathologic response to nCRT. Clinical response to nCRT, evaluated by barium esophagography and thoracic and abdominal CT scan 2–4 weeks after completion of nCRT, was graded by the Response Evaluation Criteria In Solid Tumors (RECIST) criteria.Citation32 For target lesion, clinical complete response (cCR) was defined as disappearance of all target lesions by imaging. For non-target lesion, cCR was defined as disappearance of all non-target lesions and normalisation of tumour marker level. All lymph nodes must be non-pathological in size (<10 mm short axis). We defined pCR as the absence of cancer in the entire surgical specimen, including the resected esophagus and lymph nodes. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.3. Hematologic toxicities included neutropenia, thrombocytopenia, and anemia; non-hematologic toxicities included mucositis, nausea, vomiting, diarrhea, constipation, anorexia, dehydration, fatigue, esophagitis, dysphagia, and neurotoxicity. Non-hematologic and hematologic toxicities were monitored continuously during treatment and for 4 weeks after nCRT, and radiation pneumonitis (RP) was evaluated by radiation oncologists fortnightly during radiotherapy and once a month thereafter until 6 months after radiotherapy. Follow-up evaluation was performed every 3 months for the first 2 years after nCRT and every 6 months thereafter by contrast-enhanced thoracic and abdominal CT scan, endoscopy, and barium esophagography. The last follow-up time was June 2019. Overall survival (OS) was calculated from date of receiving nCRT to the date of death or the follow-up endpoint.
Statistical Analysis
The receiver operated characteristics (ROC) curves were performed to determine the optimal cut-off values for hematologic biomarkers. The cut-off values of NLR, PLR, SII, and AFR were 2.77, 142.17, 583.45, and 10.36, respectively. The survival rates and curves were obtained by the Kaplan-Meier method with a Log rank test. Multicollinearity between hematologic variables was assessed using spearman rank correlation analysis, with correlation coefficient >0.8 representing strong inter-variable correlation.Citation33 Multivariate analyses were performed to evaluate the relationship between hematologic biomarkers and survival outcomes by the Cox proportional hazards regression model. Model performance was evaluated by predicted nomogram, concordance index (C-index), and calibration curve. The chi-squared test was used to compare the differences of treatment response with the patients grouped by inflammatory biomarkers. Univariate and multivariate logistic regression analyses were performed to assess the association between inflammatory biomarkers and adverse events. Variables with a p-value less than 0.10 in univariate analysis were incorporated into the multivariate regression analysis. A two-tailed p-value ≤ 0.05 was considered statistically significant. All statistical analyses were performed by SPSS Statistics V23.0 (IBM Corporation, Armonk, NY, USA) and R (version 3.6.0).
Results
Patient Characteristics
A total of 365 patients were initially enrolled in the study. But owing to the lack of data regarding treatment regimens or hematologic biomarkers in 54 patients, finally, 311 patients were enrolled in our study. The median follow-up was 22 months. Patient characteristics are listed in . There were 247 (79.4%) males and 64 (20.6%) females, with the median age of 63.9 (range, 38–80) years. Median tumor length was 5.5 cm (range, 2–13cm). The most commonly neoadjuvant chemotherapy regimen was cisplatin and 5-fluorouracil (67.5%). The majority of patients (66.9%) received ≥45 Gy of radiation, while all patients received at least 40 Gy.
Treatment Response and Inflammatory Biomarkers
The relationship between inflammatory biomarkers and treatment response to nCRT is shown in . After receiving nCRT, cCR and pCR were observed in 117 (37.6%) and 105 patients (33.8%), respectively. High PLR (p = 0.047) and SII (p = 0.027) were significantly associated with pCR, but none of inflammatory biomarkers was associated with cCR (). Pretreatment dNLR and MLR were not associated with cCR or pCR (data not shown).
Table 2 Associations Between Treatment Response and NLR, PLR, and SII in Patients with ESCC
Prognosis and Hematologic Biomarkers
Median progression-free survival (PFS) and OS were 13 and 22 months, respectively. Five-year OS rate was 25.4%. In univariate analysis, clinicopathological factors including gender, tumor length, current and former smoker, alcoholic, ECOG performance status, invasion depth, lymph node metastasis, pCR, and hematologic biomarkers including NLR, PLR, SII and AFR at baseline were associated with both of five-year PFS and OS (Table A1). All posttreatment hematologic biomarkers and ∆NLR were not associated with PFS or OS. Kaplan-Meier survival curves for OS according to pretreatment NLR, PLR, SII, and AFR are shown in , respectively. Patients with high NLR, PLR and SII and low AFR had a significant worse prognosis than those with low NLR, PLR and SII and high AFR, respectively (p < 0.01, ). shows the ROC curves analysis of NLR, PLR, SII, and AFR for OS prediction. The area under the ROC curve (AUC) for NLR, PLR, SII and AFR was 0.758 (95% confidence interval (CI), 0.694–0.822; p < 0.001), 0.757 (95% CI, 0.695–0.819; p < 0.001), 0.773 (95% CI, 0.710–0.837; p < 0.001), and 0.603 (95% CI, 0.529–0.678; p = 0.006), respectively.
Figure 1 Kaplan-Meier survival curves for OS according to NLR, PLR, SII, and AFR in ESCC patients receiving nCRT. (A) Patients with low NLR had a higher five-year OS rate than those with high NLR (40.8% vs 11.7%; p < 0.001). (B) Patients with low PLR had a higher five-year OS rate than those with high PLR (39.1% vs 13.5%; p < 0.001). (C) Patients with low SII had a higher five-year OS rate than those with high SII (40.6% vs 12.2%; p < 0.001). (D) Patients with high AFR had a higher five-year OS rate than those with low AFR (31.6% vs 21.2%; p = 0.003).
Abbreviations: OS, overall survival; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; SII, systemic immune-inflammation index; AFR, albumin to fibrinogen ratio; ESCC, esophageal squamous cell carcinoma; nCRT, neoadjuvant chemoradiotherapy.
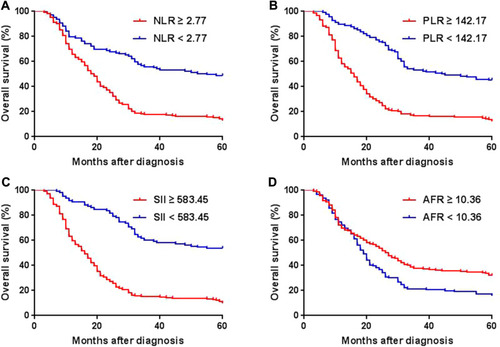
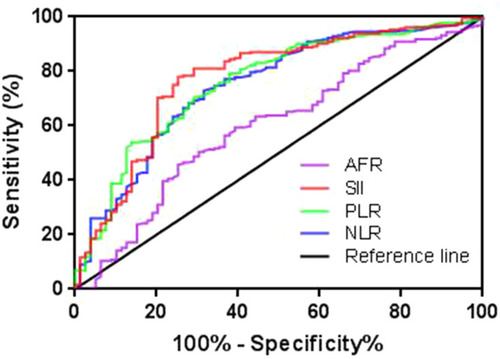
Figure 2 Receiver operating characteristic (ROC) curves of NLR, PLR, SII, and AFR for predicting five-year OS. The area under the curve (AUC) for NLR, PLR, SII, and AFR was 0.758, 0.757, 0.773, and 0.603, respectively.
Abbreviations: NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; SII, systemic immune-inflammation index; AFR, albumin to fibrinogen ratio; OS, overall survival.
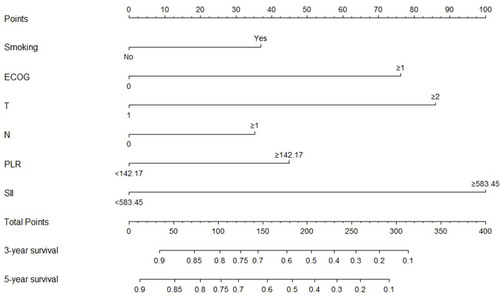
Multicollinearity between NLR, PLR, SII and AFR was assessed using spearman rank correlation analysis, with all correlation coefficients being less than 0.5 (data not shown), demonstrating that high inter-variable correlations were not found between these variables. In multivariate analysis, smoking history (p = 0.015), ECOG performance status (p < 0.001), tumor invasion depth (p = 0.041), lymph node metastasis (p = 0.038), pretreatment PLR (p = 0.002), and pretreatment SII (p < 0.001) were independent prognostic factors for OS in patients with ESCC receiving nCRT (). Correspondingly, tumor length (p = 0.005), smoking history (p = 0.017), ECOG performance status (p < 0.001), lymph node metastasis (p = 0.002), pretreatment PLR (p = 0.005), and pretreatment SII (p < 0.001) as independent prognostic factors associated with PFS ().
Table 3 Multivariate Survival Analysis in Patients with ESCC
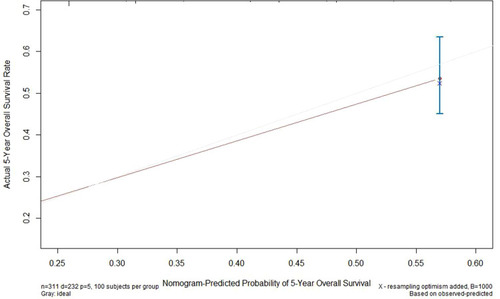
The nomogram and calibration curve for the multivariate Cox regression model were shown in and , respectively. The C-index of the predicted nomogram was 0.772 (95% CI, 0.745–0.800), demonstrating a good predicting accuracy.
Adverse Events and Inflammatory Biomarkers
After nCRT, 73 patients (23.5%) and 84 patients (27.0%) had grade ≥3 non-hematologic and hematologic toxicities, respectively. Seventy-eight patients (25.1%) and twenty-four patients (7.8%) had grade ≥2 and grade ≥3 radiation pneumonitis (RP), respectively. Overall, 143 patients (46.0%) had at least 1 grade ≥3 event and 50 patients (16.1%) with at least 2 grade ≥3 events. There were no grade 5 toxicities in this study. Associations between inflammatory biomarkers and toxicities are shown in . Univariate analysis showed that a lower NLR and a lower SII were significantly associated with grade ≥3 hematologic toxicity (). After adjusting for covariates including gender, age, smoking history, TNM stage, nCRT regimen, performance status, and comorbidities, a lower NLR (hazard ratio (HR), 0.39; 95% CI, 0.19–0.77; p = 0.007) was the only significant factor to predict grade ≥3 hematologic toxicity (). There were no statistically significant differences between inflammatory biomarkers and either grade ≥3 non-hematologic toxicities or RP (). Pretreatment dNLR and MLR were not associated with any kinds of adverse events in univariate analysis (data not shown).
Table 4 Relationships Between NLR, PLR, and SII and Adverse Events
Discussion
In this study, we investigated the association of hematologic indicators, including NLR, dNLR, MLR, PLR, SII, AFR, and PNI, with prognosis and toxicities in patients with ESCC receiving nCRT, demonstrating that pretreatment PLR and SII were the only independent biomarkers to predict prognosis and pretreatment NLR was an independent factor to predict grade ≥3 hematologic toxicity. Previous studies have confirmed that elevated level of pretreatment inflammatory biomarkers were significantly associated with prognosis for esophageal cancer patients who underwent curative esophagectomy or definitive chemoradiotherapy,Citation13–Citation18 but for patients treated with nCRT, the related studies were very limited. Moreover, there has been previously reported that posttreatment NLR was significantly associated with prognosis in malignant tumors treated with chemoradiotherapy,Citation34 but for ESCC patients, there have no related studies. Previous studies also suggested that inflammatory biomarkers were significantly associated with adverse events for cancer patients receiving chemoradiotherapy,Citation35 but for esophageal cancer patients, no research confirmed that inflammatory marker was an independent predictor of adverse events so far. To our best knowledge, this is the first report which evaluates the association between pre- and post-treatment inflammatory markers and prognosis and confirms that pretreatment NLR is an independent factor to predict grade ≥3 hematologic toxicity in ESCC patients treated with nCRT. Moreover, our studies included many kinds of hematologic markers, aiming to seek the best markers to predict prognosis and adverse events in ESCC patients. The hematologic biomarkers, obtained from peripheral blood, were simple and convenient tools to predict the prognosis and adverse events for patients with ESCC. Patients with high SII and low NLR had significant worse prognosis and severe adverse events than those with low SII and high NLR, respectively. Thus these data provide an effective way for clinicians to select high-risk ESCC patients with worse prognosis or severe adverse events before treatment and further timely adjust individualized treatment regimens or perform pretreatment measures.
The median OS for ESCC patients in our study was only 22 months, which was significantly lower than 100.1 months reported in previous Chinese randomized trial.Citation5 The possible reasons were as follows: First, the primary follow-up endpoint in our study was five-year OS. However, in previous Chinese randomized trial,Citation5 the longest follow-up time can reach more than 10 years. Second, in our study, we also included patients with stage N2 or N3. The advanced N stage may lead to an inferior prognosis.
In recent years, growing evidence has demonstrated that inflammatory biomarkers are significantly associated with worse prognosis in ESCC. However, the detailed mechanism is still unclear. The followings are possible explanations for the relationship between inflammatory biomarkers and poor prognosis in patients with solid cancer. Firstly, neutrophils have been shown to promote tumor cell proliferation by producing proteolytic enzymes including matrix metalloproteinases (MMPs) and serine proteases, to stimulate tumor angiogenesis by releasing proangiogenic factors including MMP 9 and vascular endothelial growth factor (VEGF), and to induce local immunosuppression by impairing T-cell responses and inducing T-cell death.Citation36−Citation39 Secondly, there has a growing evidence that T-lymphocytes play a critical anti-tumoral role by inhibiting tumor cell proliferation and metastasization, inducing cytotoxic cell death, and promoting antitumor immune responses.Citation40,Citation41 Thirdly, platelets interact with tumor cells directly and release factors that promote tumor growth, invasion and angiogenesis.Citation42 Platelets could contribute to metastasis by stabilizing tumor cell arrest in the vasculature, stimulating tumor cell proliferation, and promoting tumor cells extravasation.Citation43
Until now, a consensus has not been achieved regarding which hematologic biomarker is the best indicator to predict prognosis in ESCC. Previous studies have demonstrated that NLR, dNLR, MLR, PLR, SII, AFR, and PNI were significantly associated with poor prognosis.Citation13–Citation18,Citation43 In this study, we included all of these biomarkers, demonstrating that elevated SII and PLR were the only independent prognostic indicators. Furthermore, the AUC of these biomarkers was calculated, suggesting that SII had the maximum AUC value for OS, indicating that SII was the best hematologic biomarker to predict OS in patients with ESCC receiving nCRT.
Studies evaluating the predictive value of inflammatory biomarkers for adverse events in patients with esophageal cancer are very limited. Jain et alCitation44 found that in univariate analysis, a high pretreatment PLR was associated with development of hematologic toxicities in patients with esophageal cancer treated with nCRT. But in multivariate analysis, there was no significant association between NLR or PLR and toxicity. However, this study suggests that a low NLR is an independent predictor for grade ≥3 hematologic toxicity, which is inconsistent with previous findings, possibly due to the discrepancies in treatment regimens and histologic types. The mechanism by which a low level of inflammatory biomarker is associated with development of hematologic toxicity is unclear. Given these controversial outcomes, corresponding further studies with large population size and uniform regimen of nCRT are needed to clearly determine this relationship.
There are several limitations in this study. First, the retrospective nature may lead to a bias during patients’ selection and inaccuracies of data. Second, some other reported inflammatory biomarkers such as C-reactive protein and Glasgow prognostic score (GPS) were not included in this study.Citation45 Third, previous study has demonstrated that PET-CT is helpful for detecting regional and distant metastases.Citation46 In our study, PET-CT was optional for ESCC patients, which may reduce the specificity in detecting of lymph nodes and distant metastases, especially for the distant metastases. Last, the chemotherapy regimens and prescribed doses vary among ESCC patients in this study, which may influence the results. Therefore, larger prospective studies with uniform nCRT regimens and more prognostic indicators are needed to confirm and broadly interpret our findings.
Conclusion
Pretreatment SII and PLR were independent predictive markers of prognosis in patients with ESCC treated with nCRT, and a lower NLR at baseline as an independent indicator to predict grade ≥3 hematologic toxicity.
Abbreviations
ESCC, esophageal squamous cell carcinoma; nCRT, neoadjuvant chemoradiotherapy; NLR, neutrophil to lymphocyte ratio; dNLR, derived neutrophil to lymphocyte ratio; MLR, monocyte to lymphocyte ratio; PLR, platelet to lymphocyte ratio; SII, systemic immune-inflammation index; ∆NLR, post-treatment NLR-pretreatment NLR; AFR, albumin to fibrinogen ratio; PNI, prognostic nutritional index; LDH, lactate dehydrogenase; RP, radiation pneumonitis; ROC, receiver operating characteristic; AUC, area under the ROC curve; C-index, concordance index; PFS, progression-free survival; OS, overall survival; pCR, pathologic complete response; cCR, clinical complete response; ECOG, Eastern Cooperative Oncology Group; AJCC, American Joint Committee of Cancer; CT, computed tomography; PET-CT, Positron emission tomography/computed tomography; 3D-CRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; RECIST, Response Evaluation Criteria In Solid Tumors; MMPs, matrix metalloproteinases; VEGF, vascular endothelial growth factor.
Acknowledgments
This article was supported by the National Natural Science Foundation of China (81972864), Academic Promotion Program of Shandong First Medical University (2019RC002), the National Key Research and Development Programme of China (2018YFC1313201), Science and Technology Support Plan for Youth Innovation Teams of Universities in Shandong Province (2019KJL001), and Science and Technology Plan of Jinan (201907113).
Disclosure
The authors report no conflicts of interest in this work.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.2126225651787
- Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233–242. doi:10.2188/jea.JE2012016223629646
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–2509. doi:10.1056/NEJMra131453025539106
- Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet. 2017;390:2383–2396. doi:10.1016/S0140-6736(17)31462-928648400
- Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a Phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36:2796–2803. doi:10.1200/JCO.2018.79.148330089078
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature0132212490959
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation and cancer. Cell. 2010;140:883–899. doi:10.1016/j.cell.2010.01.02520303878
- Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi:10.1016/j.critrevonc.2013.03.01023602134
- Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1204–1212. doi:10.1158/1055-9965.EPI-14-014624793958
- Proctor MJ, McMillan DC, Morrison DS, et al. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012;107:695–699. doi:10.1038/bjc.2012.29222828611
- Miyatani K, Saito H, Kono Y, et al. Combined analysis of the pre- and postoperative neutrophil-lymphocyte ratio predicts the outcomes of patients with gastric cancer. Surg Today. 2018;48(3):300–307. doi:10.1007/s00595-017-1587-628916967
- Muhaxheri G, Vucicevic Boras V, Fucic A, et al. Multivariate analysis of preoperative and postoperative neutrophil-to-lymphocyte ratio as an indicator of head and neck squamous cell carcinoma outcome. Int J Oral Maxillofac Surg. 2018;47(8):965–970. doi:10.1016/j.ijom.2018.02.01129559186
- Xie X, Luo KJ, Hu Y, et al. Prognostic value of preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancer. Dis Esophagus. 2016;29:79–85. doi:10.1111/dote.1229625410116
- Liu X, Li M, Zhao F, et al. The lymphocyte-monocyte ratio predicts tumor response and survival in patients with locally advanced esophageal cancer who received definitive chemoradiotherapy. Onco Targets Ther. 2017;10:871–877. doi:10.2147/OTT.S12491528243122
- Barbetta A, Nobel TB, Sihag S, et al. Neutrophil to lymphocyte ratio as predictor of treatment response in esophageal squamous cell cancer. Ann Thorac Surg. 2018;106:864–871. doi:10.1016/j.athoracsur.2018.04.00729738752
- Sun Y, Zhang L. The clinical use of pretreatment NLR, PLR, and LMR in patients with esophageal squamous cell carcinoma: evidence from a meta-analysis. Cancer Manag Res. 2018;10:6167–6179. doi:10.2147/CMAR.S17103530538564
- Gao Y, Guo W, Cai S, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected esophageal squamous cell carcinoma. J Cancer. 2019;10:3188–3196. doi:10.7150/jca.3028131289589
- Zhang H, Shang X, Ren P, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. 2019;234:1794–1802.30070689
- Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34:43–62. doi:10.1007/s00281-011-0290-822037947
- Schafer AI. The hypercoagulable states. Ann Intern Med. 1985;102(6):814–828. doi:10.7326/0003-4819-102-6-8143158262
- Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017;19(4):353–363. doi:10.3233/CBM-16033628582845
- Zhang Y, Xiao G. Prognostic significance of the ratio of fibrinogen and albumin in human malignancies: a meta-analysis. Cancer Manag Res. 2019;11:3381–3393. doi:10.2147/CMAR.S19841931114374
- Sun F, Tan YA, Gao QF, et al. Circulating fibrinogen to pre-albumin ratio is a promising biomarker for diagnosis of colorectal cancer. J Clin Lab Anal. 2019;33:e22635. doi:10.1002/jcla.2263530047185
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi:10.1186/1475-2891-9-6921176210
- Song S, Li C, Li S, et al. Derived neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio may be better biomarkers for predicting overall survival of patients with advanced gastric cancer. Onco Targets Ther. 2017;10:3145–3154. doi:10.2147/OTT.S13803928706446
- Jin Y, Ye X, Shao L, et al. Serum lactic dehydrogenase strongly predicts survival in metastatic nasopharyngeal carcinoma treated with palliative chemotherapy. Eur J Cancer. 2013;49:1619–1626.23266049
- Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi:10.1200/JCO.2003.06.10012663709
- Tan Z, Zhang M, Han Q, et al. A novel tool of cancer prognosis in esophageal squamous cell carcinoma: the Fibrinogen/Albumin Ratio. J Cancer. 2017;8:1025–1029. doi:10.7150/jca.1649128529615
- Matsuda S, Takeuchi H, Kawakubo H, et al. Prognostic impact of change in the fibrinogen and albumin score during preoperative treatment in esophageal cancer patients. World J Surg. 2017;41:2788–2795. doi:10.1007/s00268-017-4074-828608015
- asWei XL, Zhang DS, He MM, et al. The predictive value of alkaline phosphatase and lactate dehydrogenase for overall survival in patients with esophageal squamous cell carcinoma. Tumour Biol. 2016;37:1879–1887.26323257
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724.20369299
- Eisenhauer EA, Therasse P, Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi:10.1016/j.ejca.2008.10.02619097774
- Wijsman R, Dankers FJWM, Troost EGC, et al. Inclusion of incidental radiation dose to the cardiac atria and ventricles does not improve the prediction of radiation pneumonitis in advanced-stage non-small cell lung cancer patients treated with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:434–441. doi:10.1016/j.ijrobp.2017.04.01128871994
- Kim DY, Kim IS, Park SG. Prognostic value of posttreatment neutrophil-lymphocyte ratio in head and neck squamous cell carcinoma treated by chemoradiotherapy. Auris Nasus Larynx. 2017;44:199–204. doi:10.1016/j.anl.2016.05.01327269133
- Lin YH, Lin YS. Platelet-lymphocyte and neutrophil-lymphocyte ratios: predictive factors of response and toxicity for docetaxel-combined induction chemotherapy in advanced head and neck cancers. J Chin Med Assoc. 2019;82:849–855. doi:10.1097/JCMA.000000000000017831453862
- Bekes EM, Schweighofer B, Kupriyanova TA, et al. Tumorrecruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179:1455–1470. doi:10.1016/j.ajpath.2011.05.03121741942
- Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res. 2016;4:83–91. doi:10.1158/2326-6066.CIR-15-031326839309
- Moses K, Brandau S. Human neutrophils: there role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 2016;28:187–196. doi:10.1016/j.smim.2016.03.01827067179
- Rabinowich H, Cohen R, Bruderman I, et al. Functional analysis of mononuclear cells infiltrating into tumors: lysis of autologous human tumor cells by cultured infiltrating lymphocytes. Cancer Res. 1987;47(1):173–177.3491673
- Lanitis E, Dangaj D, Irving M, et al. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann Oncol. 2017;28:xii18–xii32. doi:10.1093/annonc/mdx23829045511
- Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 2010;30:2362–2367. doi:10.1161/ATVBAHA.110.20751421071699
- Honn KV, Tang DG, Crissman JD. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev. 1992;11:325–351. doi:10.1007/BF013071861423821
- Gao QF, Qiu JC, Huang XH, et al. The predictive and prognostic role of a novel ADS score in esophageal squamous cell carcinoma patients undergoing esophagectomy. Cancer Cell Int. 2018;18:153. doi:10.1186/s12935-018-0648-230305803
- Jain R, Yee JL, Shaikh T, et al. Treatment-related toxicity and outcomes in older versus younger patients with esophageal cancer treated with neoadjuvant chemoradiation. J Geriatr Oncol. 2019;S1879–4068(19):30088–30098.
- Yu X, Wen Y, Lin Y, et al. The value of preoperative glasgow prognostic score and the C-reactive protein to albumin ratio as prognostic factors for long-term survival in pathological T1N0 esophageal squamous cell carcinoma. J Cancer. 2018;9:807–815. doi:10.7150/jca.2275529581759
- Hudson J, Semenkovich T, Puri V. Oncologic quality indicators in thoracic surgery. Thorac Surg Clin. 2017;27(3):227–244. doi:10.1016/j.thorsurg.2017.04.00128647069