Abstract
Androgen deprivation therapy remains the single most effective treatment for the initial therapy of advanced prostate cancer, but is uniformly marked by progression to castration-resistant prostate cancer (CRPC). Residual tumor androgens and androgen axis activation are now recognized to play a prominent role in mediating CRPC progression. Despite suppression of circulating testosterone to castrate levels, castration does not eliminate androgens from the prostate tumor microenvironment and residual androgen levels are well within the range capable of activating the androgen receptor (AR) and AR-mediated gene expression. Accordingly, therapeutic strategies that more effectively target production of intratumoral androgens are necessary. The introduction of abiraterone, a potent suppressor of cytochrome P450 17 α-hydroxysteroid dehydrogenase-mediated androgen production, has heralded a new era in the hormonal treatment of men with metastatic CRPC. Herein, the androgen and AR-mediated mechanisms that contribute to CRPC progression and establish cytochrome P450 17 α-hydroxysteroid dehydrogenase as a critical therapeutic target are briefly reviewed. The mechanism of action and pharmacokinetics of abiraterone are reviewed and its recently described activity against AR and 3-β-hydroxysteroid dehydrogenase is discussed. The Phase I and II data initially demonstrating the efficacy of abiraterone and Phase III data supporting its approval for patients with metastatic CRPC are reviewed. The safety and tolerability of abiraterone, including the incidence and management of side effects and potential drug interactions, are discussed. The current place of abiraterone in CRPC therapy is reviewed and early evidence regarding cross-resistance of abiraterone with taxane therapy, mechanisms of resistance to abiraterone, and observations of an abiraterone withdrawal response are presented. Future directions in the use of abiraterone, including optimal dosing strategies, the role of abiraterone in earlier disease settings, including castration sensitive, biochemically recurrent, or localized disease, and the rationale for combinatorial treatment strategies of abiraterone with enzalutamide and other targeted agents are also discussed.
Introduction to castration-resistant prostate cancer (CRPC)
The primary treatment modality for patients with metastatic prostate cancer is androgen deprivation therapy (ADT). However, treatment is uniformly marked by progression to CRPC over a period of about 18 months, with an ensuing median survival of 1–2 years. Importantly, it is now clear that “androgen independent” or “hormone refractory” tumors remain sensitive to hormonal activation, and that despite suppression of circulating testosterone (T), residual tumor androgens and androgen axis activation play a prominent role in mediating CRPC progression.Citation1 Numerous molecular features have been shown to contribute to AR signaling in CRPC and demonstrate that ongoing AR activation may occur via both ligand-dependent and ligand-independent mechanisms. As a consequence, the efficiency of AR activation at low or absent ligand levels can be enhanced and AR ligand specificity can be broadened, potentiating the persistent activation of AR signaling in CRPC tumors.
Residual tumor androgens in CRPC
Castration does not eliminate androgens from the prostate tumor microenvironment and residual androgen levels are well within the range capable of activating the AR and AR-mediated gene expression,Citation2–Citation5 strongly suggesting that intratumoral androgens are clinically relevant in driving castration-resistant tumors. While the efficacy of ADT is based on achieving castrate levels of serum T (defined as <20 ng/dL), measurement of prostatic tissue androgen levels in locally recurrent and metastatic CRPC has consistently demonstrated the presence of residual tumor androgens. In advanced prostate cancer, Mohler et al found that prostatic T levels in castrate patients with locally recurrent tumors were equivalent to those of benign prostatic hyperplasia patients and that intratumoral dihydrotestosterone (DHT) levels were only reduced 80% (to ~0.4 ng/g).Citation3 In another study, T levels in metastatic tumors obtained via rapid autopsy from men with CRPC were found to be approximately three-fold higher than T levels within primary prostate tumors from untreated (eugonadal) patients (T 0.74 ng/g; DHT 0.25 ng/g).Citation6
Data derived from in vitro and in vivo studies have determined that tissue DHT levels of 0.5–1.0 ng/g (the range observed in prostatic tissue of castrated patients) are sufficient to activate the AR, stimulate expression of AR-regulated genes, and promote androgen-mediated tumor growth.Citation3,Citation7–Citation10 Moreover, residual tissue androgens participate in nearly every mechanism by which AR-mediated signaling leads to the development of castration-resistant disease, including AR overexpression, AR mutations that alter ligand binding, and alterations in AR coregulators, all of which result in hypersensitization of AR to activation by low levels of residual androgens.Citation11 The maintenance of intratumoral androgens can be accounted for, in part, by intratumoral or intracrine biosynthesis of steroid hormones, either via the uptake and conversion of adrenal androgens (as initially put forward by Labrie et al),Citation12 or potentially via de novo steroidogenesis.Citation6,Citation13–Citation18
AR alterations in CRPC
AR overexpression is a well-recognized feature of CRPC and believed to be a critical driver of CRPC progression.Citation3,Citation17,Citation19–Citation27 Potential mechanisms responsible for increased AR expression include amplification of the AR locus itself, increased transcription rates, or stabilization of the messenger RNA or protein. AR overexpression not only mediates sensitivity to low ligand concentrations, but has been demonstrated to convert anti-androgens such as bicalutamide and flutamide from antagonists to agonists via changes in composition of coactivators recruited to the AR promoter.Citation28,Citation29
Several well-characterized AR mutations can result in promiscuous binding and activation of the AR by weak adrenal androgens and other steroid hormones, including dehydroepiandrosterone (DHEA), progesterone, estrogens, and cortisol.Citation30–Citation35 Other mutations convert AR antagonists (flutamide and bicalutamide) into potent agonists.Citation36 Notably, in vitro selection with enzalutamide has revealed a new mutation (F876L) which mediates conversion of enzalutamide to an AR agonist while maintaining sensitivity to bicalutamide.Citation37,Citation38 This mutation also confers resistance to the second-generation AR antagonist ARN509, and has been detected in circulating tumor DNA from ARN509-treated patients.Citation39 While the frequency of AR mutation in CRPC tumors treated with luteinizing hormone (LH)-releasing hormone agonist and first-generation AR antagonists is low (8%–25%),Citation33 the frequency of these mutations may become more frequent with the advent of potent antagonists of AR signaling.
Constitutively active truncated AR splice variants have recently been recognized as a potential mechanism of CRPC progression. The expression of certain variants (eg, AR-V7, which can be detected in hormone-naïve prostate cancer) has been associated with a shorter time to disease recurrence following radical prostatectomy.Citation40,Citation41 High levels of ARV7 and ARV567 were associated with shorter survival in patients with CRPC and bone metastases, consistent with a role in tumor progression.Citation42,Citation43 Although each variant displays a slightly different structure, they each lack portions of the carboxy-terminal ligand binding domain, resulting in a constitutively active AR.Citation44
Clinical evidence of AR axis signaling in CRPC
The clinical importance of ongoing AR pathway activity in CRPC progression is reflected in the rising serum prostate-specific antigen (PSA) levels in patients with CRPC, and is confirmed by clinical responses to treatment strategies that target residual AR axis activity. These include historical responses to adrenalectomy and/or hypophysectomy;Citation45,Citation46 the limited but consistent ~5% overall survival (OS) benefit seen in meta-analyses of combined androgen blockade trials;Citation47–Citation49 the observation that nearly 30% of recurrent prostate tumors demonstrate at least transient clinical responses to secondary or tertiary hormonal manipulation;Citation50 and most recently, the striking clinical response observed with the novel AR axis inhibitors abiraterone and MDV3100.Citation51,Citation52
Mechanism of action and pharmacology of abiraterone
Emerging data suggest residual intratumoral androgens are produced via the uptake and conversion of adrenal androgens, and potentially via de novo synthesis from cholesterol or progesterone precursors within the tumor.Citation6,Citation13–Citation18 Accordingly, therapeutic strategies that more effectively target production of intratumoral androgens are necessary. Abiraterone is the first to enter clinical practice in a series of novel agents designed to potently target adrenal and tumor androgen production.
Cytochrome P450 17 α-hydroxysteroid dehydrogenase (CYP17A) as a therapeutic target in CRPC
The critical enzyme required for androgen synthesis from cholesterol is CYP17A. Adrenal expression of this enzyme accounts for production of circulating adrenal androgens, including DHEA (which primarily circulates in its sulfated form, DHEA-S) and androstenedione (AED), and a number of studies have demonstrated expression of CYP17A in castration-resistant prostate tumors.Citation6,Citation53 Given its central role in the production of either adrenal or tumor-derived extragonadal androgen synthesis, CYP17A has emerged as a primary target of novel therapeutics.
CYP17A is a single enzyme that catalyzes the sequential hydroxylase (required for cortisol synthesis) and lyase (required for adrenal androgen synthesis) steps that are required for conversion of C21 pregnenolone and progesterone precursors to the C19 adrenal androgens, DHEA and AED. While ketoconazole (a weak inhibitor of CYP11α-hydroxylase and CYP17A) has been utilized for suppression of residual adrenal androgens in men with CRPC, its limited efficacy and treatment-related side effects prompted the development of more potent CYP17A inhibitors.Citation54
Abiraterone acetate is an orally administered, rationally designed small molecule derived from the structure of pregnenolone. It irreversibly inhibits both the hydroxylase and lyase activity of CYP17A with approximately 10–30-fold greater potency than ketoconazole.Citation55 Because adrenal inhibition of CYP17A results in blockade of glucocorticoid as well as adrenal androgen synthesis, abiraterone is coadministered with prednisone to ameliorate the secondary rise in adrenocorticotropic hormone (ACTH) that can lead to excess mineralocorticoid synthesis (, discussed further below).Citation56
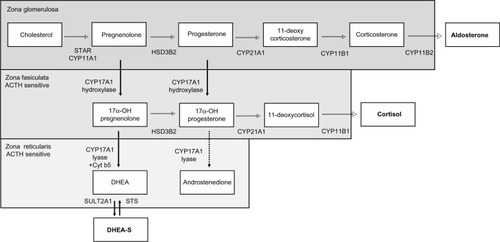
Figure 1 Steroid hormone pathways in zones of the adrenal gland. Steroid synthesis in the adrenal gland occurs in three zones, each with a specific complement of enzymes that determine the steroids produced. The zona glomerulosa contains the enzymes necessary to produce aldosterone. The zona fasciculata and reticularis additionally express CYP17A. The hydroxylase activity of CYP17A is active in the zona fasciculata resulting in the production of cortisol. However, the lyase activity of CYP17A requires the cytochrome b5 coregulator which is only present in the zona reticularis. This drives efficient production of DHEA which is then sulfated to DHEA-S. 17α-OH progesterone is a relatively poor substrate for CYP17A lyase (dotted arrow), and thus androstenedione is formed at lower levels. The zona fasciculata and zona reticularis are sensitive to the ACTH feedback stimulation that occurs when cortisol production is suppressed by inhibition of CYP17A.
Abbreviations: 17α-OH, 17α-hydroxy; ACTH, adrenocorticotropic hormone; CYP11A, cytochrome P450 11α-hydroxylase; CYP11B, cytochrome P450 11β-hydroxylase; CYP17A, 17α-hydroxylase/17,20 lyase/17,20 desmolase; CYP21A, cytochrome P450 21α-hydroxylase; Cyt b5, cytochrome b5; DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone-sulfate; HSD3B, 3-β-hydroxysteroid dehydrogenase; STAR, steroidogenic acute regulatory protein.
Unintended activity of abiraterone against other AR pathway targets
Abiraterone was designed as a selective, irreversible inhibitor of CYP17A. However, most likely due to its steroidal structure, abiraterone has been found to inhibit other AR pathway targets including AR itself, as well as 3β-hydroxysteroid dehydrogenase, another key enzyme required for androgen synthesis.
While not as potent as the first-generation nonsteroidal AR inhibitors (eg, bicalutamide), abiraterone demonstrated measurable AR antagonism at 1–10 μmol/L, a clinically achievable concentration.Citation57 Pharmacokinetic studies (discussed below) have found plasma abiraterone levels to be 1.2–5 μmol/L following a 1,000 mg dose in fasting patients. Galeterone, another novel CYP17A antagonist, has been demonstrated to have anti-AR activity as well, and actually has increased potency compared to abiraterone in this regard.Citation58 Interestingly, an early study demonstrated that ketoconazole can also inhibit the AR although not at clinically achievable doses.Citation59
With regard to its activity against 3β-hydroxysteroid dehydrogenase type I, abiraterone was shown to inhibit two key reactions mediated by this enzyme. Abiraterone, again at clinically achievable concentrations of 2.1–8.8 μmol/L, inhibited the conversion of DHEA to AED, and of 5α-androstanediol to T, with concomitant suppression of AR-regulated gene expression.Citation60 These data suggest that even though maximal inhibition of CYP17A is achieved at the currently approved 1,000 mg dose, dose escalation may increase the efficacy of abiraterone by taking advantage of its ability to inhibit multiple AR pathway targets. Clinical trials evaluating this hypothesis are ongoing.
Pharmacokinetics of abiraterone
Abiraterone is administered as the pro-drug abiraterone acetate which has improved oral bioavailability and shows near complete conversion to abiraterone in the blood. In preclinical toxicology studies, abiraterone reduced the weights of androgen dependent organs such as the prostate and had minimal side effects in other organs.Citation61 First-in-man studies demonstrated the ability of abiraterone to reduce serum T levels in both castrate and noncastrate men. In castrate men dose escalation to 500 mg was required to achieve the target effect, a 75% decrease in T levels. The duration of suppression following a single dose was variable, ranging from 2–5 days. In noncastrate men, repeat dosing at ≥800 mg daily was required to maintain T suppression, and was accompanied by a marked rise in LH that may have prevented sustained T suppression.Citation62
Two Phase I trials in chemotherapy-naïve men with metastatic CRPC were performed to determine the recommended dose for Phase II testing. No dose-limiting toxicities were observed at doses up to 2,000 mg/daily, but 1,000 mg was chosen for expansion as a plateau in pharmacodynamic effect was observed at doses >750 mg. Substantial variability in drug absorption was observed, with up to nine-fold differences in serum pharmacokinetic parameters within the 1,000 mg cohort. Maximal drug concentrations were achieved within 2–4 hours, with a terminal half-life of approximately 12 hours.Citation63,Citation64
Impact of food on abiraterone exposure
Notably, drug exposure was five to seven-fold higher when abiraterone was administered with a low-fat meal (7% fat, 300 calories) and over ten-fold higher with a high-fat meal (57% fat, 825 calories) as compared to the fasted state. To minimize the variability in absorption, abiraterone is labeled for administration as 1,000 mg (four 250 mg tablets) daily on an empty stomach, defined as 1 hour before or 2 hours after a meal.Citation65 Clinical trials evaluating abiraterone in the fed and fasted state are ongoing (NCT01424930),Citation66 as are trials evaluating lower doses of abiraterone taken with food (NCT01543776).Citation67 If borne out, these approaches could decrease drug costs, as well as decrease the potential risk of drug interactions (see below) if a patient accidentally takes the agent with food.Citation65,Citation68
In a dedicated renal impairment trial, renal dysfunction did not impact pharmacokinetic profiles and no dose adjustment is necessary for renal impairment.Citation69 Abiraterone is bound to plasma proteins including albumin and is primarily excreted in the feces.Citation70
Efficacy studies
Phase I and II data
Phase I and II studies demonstrated that abiraterone suppresses serum androgen levels, and achieves PSA and clinical responses in chemotherapy-naïve and docetaxel-treated CRPC patients. No dose-limiting toxicities were associated with abiraterone, and anti-tumor activity and PSA responses were observed at all dose levels. While the highest tolerated dose was 2,000 mg/day, all Phase II and III studies have used the 1,000 mg/day dose, as the impact of therapy on steroids upstream of CYP17A plateaued at doses >750 mg/day.Citation63
In eugonadal men, abiraterone transiently suppresses T levels by >50% (but a corresponding rise in LH levels overcomes inhibition of gonadal androgen synthesis), while in castrate men, abiraterone further suppresses castrate serum T levels by ≥75%.Citation62 In general, abiraterone suppresses serum DHEA levels by approximately 75% and DHEA-S, AED, and T to essentially undetectable.Citation63,Citation64 As observed in studies of ketoconazole, higher baseline levels of DHEA-S, DHEA, and AED were present in patients achieving >50% PSA declines. In contrast to progression on ketoconazole, increases in T, AED, or DHEA levels were not observed on progression with abiraterone.Citation71,Citation72
In men with chemotherapy- and ketoconazole-naïve metastatic CRPC, a Phase I/II single-agent study of abiraterone demonstrated durable PSA declines >50% in 67% of patients, with partial radiographic responses in 37.5% and a median time to progression (TTP) of 32 weeks.Citation72 Based on preclinical data that progesterone precursors upstream of CYP17A can activate certain AR mutations, this study also evaluated the addition of corticosteroids at progression and showed that 33% of patients (10/30) had secondary PSA responses following the addition of dexamethasone 0.5 mg/day.Citation72 A Phase II study of abiraterone combined with prednisone in this population demonstrated PSA declines >50% in 79% of patients and a median TTP of 65 weeks. This study also found that over 48% (11/23) evaluable patients had a transient bone scan flare at 3 months that subsequently showed with improvement or stability.Citation73 In a second Phase I single-agent study of pre-chemotherapy patients in which 58% were ketoconazole pretreated, PSA declines >50% were noted in 55% of patients at 12 weeks.Citation64
In post-docetaxel-treated CRPC patients, a Phase II singleagent study of abiraterone demonstrated PSA declines >50% in 51% of patients (only 17% had received prior ketoconazole), with partial radiographic responses in 27% and a median TTP of 24 weeks.Citation74 In a Phase II study of abiraterone combined with prednisone, in which 47% of patients were ketoconazole pretreated, PSA declines >50% were observed in 36% of patients with a median TTP of 24 weeks.Citation75
Efficacy in ketoconazole-treated patients
Importantly, while ketoconazole-treated patients were specifically excluded in the subsequent Phase III studies, the Phase I/II studies demonstrated that abiraterone has activity in these patients as well. In the pre-chemotherapy Phase I study in which 58% of men (19 of 33) were ketoconazole pretreated, PSA responses >50% were observed in 64% of ketoconazole-naïve patients and 47% of ketoconazole-pretreated patients.Citation64 In the post-docetaxel study in which 47% of patients (27 of 58) had received prior ketoconazole, PSA declines >50% occurred in 45% of ketoconazole-naïve patients and 26% of ketoconazole-treated patients with a median TTP of 28 and 14 weeks, respectively.Citation75 These findings demonstrate that abiraterone maintains a reasonable degree of clinical efficacy in CRPC patients previously treated with docetaxel and/or ketoconazole.
Phase III data
Findings from the Phase I and II studies have been subsequently confirmed in Phase III studies in chemotherapy-naïve (COU-AA-302) and post-docetaxel-treated men (COU-AA-301), resulting in US Food and Drug Administration approval of abiraterone for men with metastatic CRPC either before or after treatment with chemotherapy.
COU-AA-301
In the post-chemotherapy setting, 1,195 men with metastatic CRPC progressing after docetaxel were randomized in a 2:1 ratio to abiraterone/prednisone (n=797) or placebo/prednisone (n=398) with a primary endpoint of OS. The median PSA was ~130 ng/dL, 90% of patients had an Eastern Cooperative Oncology Group performance status (PS) of zero to one, the median age was 70 years, and 28% were ≥75 years. Bone, lymph node, and visceral metastases were present in approximately 90%, 40%, and 10% of patients, respectively, and 30% of patients had received more than one prior chemotherapy regimen. Treatment was continued until clinical or radiographic evidence of progression.
At a median follow-up of 12.8 months, the first interim analysis demonstrated an OS benefit for men receiving abiraterone (14.8 months versus 10.9 months for placebo; hazard ratio [HR] 0.646; P<0.0001), representing a 35% reduction in risk of death and prompting the independent data monitoring committee to recommend that the study be unblinded and men on the placebo arm be offered abiraterone.Citation51 An updated analysis at a median survival of 20.2 months demonstrated a median OS of 15.8 months for abiraterone versus 11.2 months for prednisone (HR 0.74; P<0.0001), extending the OS benefit to 4.6 months.
All secondary endpoints were statistically significant in favor of abiraterone, including median time to PSA progression (8.5 months versus 6.6 months), median radiologic progression-free survival (rPFS; 5.6 months versus 3.6 months), and proportion of patients with >50% PSA response (29.5% versus 5.5%). The impact of abiraterone on OS was observed across all subgroups, including patients who had received one (15.4 versus 11.5 months) or two prior chemotherapy regimens (14.0 versus 10.3 months). Notably, patients with a PS of two had worse outcomes, with a median survival of 7.3 months on abiraterone compared to 15.3 months for those with PS of zero to one receiving abiraterone.Citation76
While visceral disease was associated with a poorer prognosis, an exploratory analysis reported in abstract form found the absolute benefit in OS from abiraterone to be similar in those with and without visceral disease (8.3–12.9 months in those with visceral disease and 12.3–17.3 months in those without). Analysis of patients by site of disease showed worse outcomes in those with hepatic versus pulmonary visceral metastases, but still a benefit favoring abiraterone over placebo (median OS 4.0–7.3 months for liver metastases and 7.9–13.9 months for pulmonary disease).Citation77
Exploratory analyses of COU-AA-301 evaluating the impact of abiraterone on fatigue, pain control, and skeletal-related events suggest abiraterone has efficacy in all these settings. In patients with clinically significant fatigue at baseline, abiraterone significantly increased the number of patients reporting an improvement in fatigue intensity (58.1% versus 40.3%; P=0.0001) as well as the time to fatigue palliation (median 59 days versus 194 days; P=0.0155).Citation78 In patients with clinically significant pain at baseline, abiraterone significantly increased the number of patients reporting palliation of pain (45% versus 28.8%; P=0.0005), as well as faster palliation (median time to palliation 5.6 months versus 13.7 months; P=0.0018). Median time to occurrence of first skeletal-related event (defined as pathologic fracture, spinal cord compression, or palliative surgery or radiation to bone) was also significantly longer in abiraterone treated patients (25 months versus 20.3 months; P=0.0001).Citation79
COU-AA-302
In the pre-chemotherapy setting, 1,088 men with asymptomatic or minimally symptomatic bone and lymph node (but not visceral) metastatic CRPC were randomized 1:1 to abiraterone/prednisone (n=546) or placebo/prednisone (n=542), with co-primary endpoints of rPFS and OS. The median PSA was ~40 ng/dL, about 30% of men were ≥75 years, and approximately 50% had bone-only metastatic disease.
At a median follow-up of 22.2 months, a statistically significant doubling in rPFS from 8.3 months in the placebo arm to 16.5 months was observed in men receiving abiraterone (HR 0.53; P<0.001), accompanied by a trend for increased OS from 27.3 months in the placebo arm to not reached in the abiraterone group (HR 0.75; P=0.01 which did not meet the prespecified P-value of 0.001), again prompting the independent data monitoring committee to recommend that the study be unblinded and men on the placebo arm be offered abiraterone.Citation80 An updated analysis of OS at a median survival of 27.1 months again trended toward favoring abiraterone at 30.1 months in the placebo arm versus 35.3 months in the abiraterone arm (HR 0.79; P=0.015).Citation81
All secondary endpoints were statistically significant in favor of abiraterone, including median time to opiate use (not reached versus 23.7 months), time to initiation of chemotherapy (25.2 months versus 16.8 months), time to PS decline (12.3 months versus 10.9 months), time to PSA progression (11.1 months versus 5.6 months), and proportion of patients with >50% PSA response (62% versus 24%). The impact of abiraterone on rPFS was observed across all subgroups.Citation80 This study did not include patients with visceral disease or moderate to severe pain; however, the exploratory analyses of these subpopulations in the post-chemotherapy setting discussed above suggest these patients are likely to benefit as well.
Safety and tolerability
Abiraterone is generally well tolerated, with 13% and 19% of abiraterone-treated patients in COU-AA-301 and COU-AA-302, respectively, discontinuing therapy for adverse effects versus 18% and 23% of placebo-treated patients. The most common adverse events in both groups were fatigue, back pain, nausea, constipation, bone pain, and arthralgia, all in the range of 25%–30%. The incidence of urinary tract infection was statistically higher in abiraterone-treated patients (12% versus 7% in placebo; P=0.02).
Mineralocorticoid and electrolyte effects
Inhibition of CYP17A by abiraterone increases mineralocorticoid precursors upstream of CYP17A and decreases glucocorticoids downstream of CYP17A (). The latter results in a concomitant elevation of ACTH, which further drives mineralocorticoid production. Phase I and II trials demonstrated that symptoms of mineralocorticoid excess occur in 50%–80% of patients treated with single-agent abiraterone.Citation56 Mineralocorticoid-related symptoms in the Phase III studies were markedly attenuated by inclusion of prednisone 5 mg twice daily, and were generally of Grade I or II in magnitude, including fluid retention (~33% versus 22%–24% in placebo), hypertension (~10% versus 8% in placebo), and hypokalemia (~18% versus 9% in placebo).
Dexamethasone, which lacks mineralocorticoid effects and has a longer duration of action, may theoretically be preferable to prednisone and can be used at a dose of 0.5 mg daily.Citation82 However, a rare incidence (2/100) of orthostatic hypotension was reported following addition of dexamethasone to single-agent abiraterone, possibly due its lack of mineralocorticoid activity in the setting of a rapid decline in circulating mineralocorticoids.Citation63,Citation64
Of note, other CYP17A inhibitors such as orteronel and galeterone demonstrate more selective targeting of the lyase but not hydroxylase activity of CYP17A. These agents may be associated with less inhibition of cortisol synthesis and less ACTH/feedback-driven symptoms of mineralocorticoid excess and are being evaluated for use both with and without corticosteroids.
Careful attention should be paid to hypertension and hypokalemia, which should be corrected before and during therapy with abiraterone. Patients should be monitored for hypertension, hypokalemia, and fluid retention at least once a month. Spironolactone is avoided in patients who develop mineralocorticoid-related side effects due to its mixed AR agonist/antagonist activity. Instead, eplerenone, a second-generation mineralocorticoid receptor antagonist in doses of 50–200 mg/day (in divided doses twice daily) can be used in combination with a salt-restricted diet.Citation83
While eplerenone does not bind the wild-type AR, it can activate certain AR mutations; however, further data is needed regarding the incidence and clinical significance of these mutations. Alternatively, potassium-sparing epithelial sodium channel antagonists such as amiloride and triamterene (in combination with hydrochlorothiazide if hypertension is significant) can be used in place of or added to eplerenone if necessary.Citation82,Citation83 In rare instances, additional anti-hypertensive agents may be necessary in patients already receiving prednisone, eplerenone, and diuretics.
Hepatotoxicity
Grade III or IV hepatic transaminase abnormalities (five times the upper limit of normal [ULN]) occurred in approximately 4% of patients in the Phase III studies, usually within the first 3 months of starting treatment, and more commonly in men whose baseline alanine transaminase or aspartate transaminase were elevated. Serum transaminases should be measured at baseline. Transaminases in patients with normal levels should be checked every 2 weeks for the first 3 months of therapy, and then monthly. No dose adjustment is necessary for mild hepatic impairment. For moderate hepatic impairment (Child–Pugh Class B) abiraterone should be started at 250 mg daily, and transaminases should be checked weekly for the first month, then every 2 weeks for the following 2 months, and then monthly.
If aspartate transaminase or alanine transaminase rise above five times the ULN or bilirubin rises above three times the ULN, abiraterone should be held. It should be discontinued if the patient had moderate hepatic impairment at baseline, but in patients with normal hepatic function at baseline it can be restarted at 750 mg daily when liver function tests decline to less than 2.5 times the ULN and total bilirubin is less than 1.5 times the ULN. If hepatotoxicity recurs, a further dose reduction to 500 mg can be attempted (once levels have fallen below the thresholds given above), but recurrence of hepatotoxicity at the 500 mg dose requires discontinuation of the drug.
Cardiotoxicity
The overall incidence of adverse cardiac effects was not statistically increased by abiraterone in COU-001 (13% versus 11% in placebo), although the frequency of cardiac failure was higher in the abiraterone group (2.1% versus 0.7% in placebo). The most frequently reported cardiac events were Grade I and II tachycardia and Grade III or lower atrial fibrillation. A retrospective study of 51 metastatic CRPC patients with at least one cardiac comorbidity and/or controlled risk factor including hypertension (41%), hyperglycemia (30%), dyslipidemia (18%), cardiac ischemia (12%), stroke (9%), or arrhythmias (6%) reported no cardiac events or variation in left ventricular ejection fraction over 6–12 months of follow-up.Citation84 However, as patients with left ventricular ejection fraction <50% were excluded from the Phase III studies, pretreatment assessment and optimization of cardiac status with electrocardiogram and echocardiography may warrant consideration in elderly patients with reduced cardiac function. A significant effect of abiraterone on the QT/QTc interval in patients with CRPC was not observed.Citation85
Potential drug interactions
Abiraterone is a strong inhibitor of several microsomal drug metabolizing enzymes, including CYP1A2 and CYP2D6.Citation86 Abiraterone increased systemic exposure of dextromethorphan (metabolized by CYP2D6) approximately two to three-fold, while the pharmacokinetics of theophylline (metabolized by CYP1A2) were unaffected. This suggests caution may be warranted when abiraterone is coadministered with known CYP2D6 substrates (including β-blockers, serotonin reuptake inhibitors, anti-arrhythmics, and neuroleptics, as well as codeine, tramadol, and – of relevance to urologic patients – tolterodine).Citation87
Conversely, abiraterone is a substrate of CYP3A4. Interestingly, enzalutamide is a strong inducer of CYP3A4, while ketoconazole and bicalutamide are inhibitors of CYP3A4. Clinical trials evaluating the impact of rifampin (another strong inducer of CYP3A4) on abiraterone exposure (NCT01655147),Citation88 as well as the impact of ketoconazole on prolonging abiraterone exposure (NCT01588782)Citation89 have been completed but not yet reported. This suggests that the combination of bicalutamide and abiraterone merits clinical evaluation.
Current place of abiraterone in CRPC therapy
A number of clinical trials evaluating the sequence and combination of abiraterone with immunotherapy, chemotherapy, enzalutamide, and other novel agents are underway in men with metastatic CRPC. While the optimal place of abiraterone in prostate cancer therapy remains to be determined, current treatment decisions can be guided in part by Phase III data already available.
In men with asymptomatic or minimally symptomatic metastatic CRPC, abiraterone is an attractive first-line option given its ease of administration and relatively low toxicity profile. Similarly, the combination of abiraterone and sipuleucel T would likely be a well-tolerated regimen in this setting and is currently under clinical investigation (NCT01487863).Citation90
The efficacy of abiraterone in men with symptomatic disease prior to chemotherapy has not been specifically demonstrated due to exclusion of these patients from the Phase III trial. The pace of disease may be the best guide to therapy in this setting. Patients with high Gleason scores, poor response to initial ADT, rapidly progressive disease, or poorly controlled symptoms may derive greater benefit from immediate chemotherapy, while a trial of abiraterone may be reasonable in patients with less extensive or more slowly progressing disease.Citation91
In the post-chemotherapy setting, both abiraterone and enzalutamide are supported by Phase III data demonstrating an OS benefit, but the optimal approach to sequencing them is unknown. Retrospective evaluations of patients receiving abiraterone after enzalutamide or vice versa have shown modest response rates with a median TTP of 3–4 months.Citation92–Citation94 Until biomarkers to stratify patients or clinical trial data to support combination or sequencing strategies are available, the sequencing of abiraterone and enzalutamide is likely to be dictated by insurance and regulatory approvals. From a practical perspective, enzalutamide avoids the need for prednisone, although this may become less important if studies show abiraterone can be given with lower doses of prednisone (NCT01867710).Citation95
Cross-resistance with taxanes
An emerging consideration is whether therapy with abiraterone (or enzalutamide) may influence the efficacy of subsequent chemotherapy. Taxanes have been demonstrated to mediate some of their anti-tumor activity in prostate cancer via inhibition of AR transcriptional activity. This has been proposed to occur by various mechanisms including taxane-mediated induction of AR transcriptional corepressors and prevention of microtubule-mediated transit of AR to the nucleus.Citation92,Citation96,Citation97
These data suggest the prostate-specific component of taxane efficacy may be lost in tumors that have developed resistance to AR pathway inhibition. Consistent with this hypothesis, a study reported in abstract form found that metastatic CRPC patients who had early development of castration resistance (<12 months) had a shorter TTP and shorter OS compared to patients with more prolonged sensitivity to androgen axis suppression.Citation98
A published analysis of the efficacy of docetaxel in patients who had progressed on the Phase I/II studies of abiraterone showed >50% PSA declines in only 26% of patients compared to 45% in the TAX 327 study.Citation99 Moreover, all patients who had failed to achieve a PSA response on abiraterone were also refractory to docetaxel. A second study reported in abstract form found median OS from the first dose of docetaxel to be 65 months in patients treated with abiraterone or enzalutamide after salvage cabazitaxel therapy, but only 39 months in those receiving these agents after docetaxel but before cabazitaxel.Citation100 At present these observations remain hypothesis generating.
Mechanisms of resistance to abiraterone
Although clinical responses to abiraterone have been impressive, not all men respond, the duration of response is variable, and a majority eventually progress with a rising PSA. While the mechanisms determining resistance to abiraterone have not been fully elucidated, emerging data from clinical and preclinical studies suggest several possibilities.
Clinical data demonstrate that abiraterone effectively suppresses serum androgens.Citation63,Citation64 In addition, higher levels of AR and CYP17A staining in pretreatment tumor-infiltrated bone marrow biopsies from men with CRPC were associated with longer responses to abiraterone treatment, supporting CYP17A mediated androgen production as the target of abiraterone activity.Citation41 However, the efficacy of abiraterone in suppressing tumor androgens in men with CRPC remains to be demonstrated. In this regard, preclinical studies have provided the first in vivo evidence that the primary mechanism of action of abiraterone is via suppression of tumor androgen levels. Treatment of CRPC xenograft models with abiraterone significantly inhibited tumor growth, serum PSA, and intratumoral androgen levels.Citation101,Citation102 While androgen levels remained suppressed in some tumors recurring after therapy, other tumors demonstrated increasing levels of T and DHT.
Importantly, multiple mechanisms directed at maintaining AR signaling were observed in the abiraterone-treated tumors. These included upregulated expression of full-length AR and ligand-independent AR variants, as well as induction of steroidogenic genes (including the target gene, CYP17A), several of which showed strong correlations with DHT levels in recurrent tumors.Citation101,Citation102 Clinically, development of resistance to abiraterone has not been associated with an increase in serum androgen levels or in bone marrow aspirate T levels (which contrasts with the increase in circulating adrenal androgens that is seen in men progressing on ketoconazole).Citation71 However, numerous studies have shown that serum is not a good indicator of tumor androgen levels.Citation6,Citation103
Abiraterone withdrawal syndrome
Other potential mechanisms of resistance include activation of mutant AR by noncanonical ligands. Interestingly, recent case reports describe instances of an “abiraterone withdrawal syndrome”, in which (generally transient) PSA declines occur following discontinuation of abiraterone.Citation104–Citation106 To date, abiraterone has not been found to elicit AR agonist activity (as is seen following treatment with AR antagonists such as bicalutamide, flutamide, and now enzalutamide),Citation37,Citation107 perhaps because it is a relatively weak AR antagonist.Citation57 An alternative explanation is the development of AR mutations which allow AR activation by exogenous corticosteroids or steroid precursors upstream of CYP17A.
In particular, inhibition of CYP17A is associated with a rise in circulating levels of upstream progesterone precursors,Citation63,Citation64 which have been shown to activate AR bearing certain mutations.Citation31,Citation57 Notably, a Phase II study of single-agent abiraterone found that initiation of dexamethasone at progression (to decrease ACTH-driven stimulation of steroid precursors) reversed resistance in 33% of evaluable patients (although this could also reflect an intrinsic anti-tumor activity of dexamethasone).Citation72 In contrast, however, the exogenous glucocorticoids or mineralocorticoid antagonists used to ameliorate the side effects of abiraterone may themselves be able to activate mutant AR.Citation35 Alternatively, signaling via the glucocorticoid receptor has been shown to activate AR-regulated genes in the absence of ligand,Citation108 suggesting another route by which discontinuation of therapy could lead to a withdrawal response.
While further study is clearly needed, these findings are collectively consistent with the clinical observation that patients progress on abiraterone with a rising PSA, strongly suggesting that the AR axis remains a critical target in abiraterone-resistant tumors.
Conclusion and future directions
Many important questions regarding the use of abiraterone remain to be answered including optimal dosing strategies, its role in different disease settings (eg, castration sensitive, biochemically recurrent, or localized disease), and – in all disease settings – whether abiraterone in sequential or combinatorial treatment strategies will yield the most durable responses.
Both clinical and preclinical data suggest abiraterone-resistance is associated with reactivation of AR signaling. That the AR and a second enzyme involved in androgen synthesis (3β-hydroxysteroid dehydrogenase) are inhibited by higher concentrations of abiraterone suggests dose escalation may be a viable strategy to target AR-related mechanisms of abiraterone resistance. This concept is currently under evaluation in two studies of men with metastatic CRPC (NCT01503229, NCT01637402).Citation109,Citation110
The persistent AR activation in abiraterone-resistant tumors also provides a compelling rationale for combining abiraterone with potent AR inhibitors such as enzalutamide or ARN-509 rather than sequential treatment strategies which may allow alternative pathways of AR activation to emerge. Although historic studies of combined androgen blockade have yielded small gains in OS versus ADT alone, the markedly more potent drug combinations now available hold significant promise for increasing the efficacy of multi-targeted AR pathway blockade. Studies evaluating the combination of abiraterone and enzalutamide or ARN-509 in men with metastatic CRPC (NCT01650194, NCT01949337, NCT01792687)Citation111–Citation113 as well as in men with localized disease prior to prostatectomy (NCT01946165)Citation114 are planned or ongoing.
Early use of abiraterone or potent combined AR blockade may be particularly effective in hormone-naïve tumors which have not yet had the opportunity to develop resistance. The Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE) study is an ongoing trial comparing ADT with and without abiraterone in high-risk patients with biochemical recurrence or newly diagnosed metastatic patients (NCT00268476),Citation115 as are several others (NCT01715285).Citation116 Other studies are evaluating the efficacy of abiraterone and ADT in combination with salvage radiotherapy for biochemical recurrence following prostatectomy (NCT01780220).Citation117
Similarly, the efficacy of abiraterone is being explored in combination with prostatectomy or radiotherapy in men with localized disease (NCT01023061, NCT01717053, NCT01751451).Citation118–Citation120 In this regard, neoadjuvant studies of multi-targeted AR blockade using LH-releasing hormone agonists combined with bicalutamide, dutasteride, and ketoconazole or LH-releasing hormone agonists combined with abiraterone have demonstrated higher pathologic response rates than previously observed in historic studies of ADT prior to prostatectomy.Citation121,Citation122
While the limited number of studies reported to date have identified AR-related mechanisms of resistance to abiraterone, it is likely that other signaling and survival pathways will also be found to play important roles in determining response and resistance. As such, numerous studies evaluating the combination of abiraterone with cytotoxic chemotherapy and targeted agents such as dasatinib (Src inhibitor), veliparib (PARP inhibitor), cabozantinib (c-Met and VEGFR2 inhibitor), alisertib (aurora kinase inhibitor), OGX-427 (HSP27 inhibitor), AT13387 (HSP90 inhibitor), BKM120 (PI3K inhibitor), BEZ235 (dual PI3K/mTOR inhibitor), and ABT-264 (Bcl-2 inhibitor) are planned or underway.
In conclusion, numerous studies evaluating the sequencing and combination of abiraterone in multiple disease settings are underway. Rapid accrual and completion of these studies will be imperative for determining rational treatment strategies with the highest likelihood of durable efficacy. Furthermore, the molecular heterogeneity that characterizes CRPC tumors combined with the growing number of oncogenic drivers for which targeted agents are being developed highlights the critical need for embedding correlative studies within these studies and pursuing the development of predictive biomarkers.
Acknowledgments
Damon Runyon Cancer Research Foundation (Damon Runyon-Genentech Clinical Investigator Award CI-40-08); National Institutes of Health (Pacific Northwest Prostate Cancer SPORE P50 CA97186); Department of Defense Congressionally Directed Medical Research Program; and Prostate Cancer Foundation.
Disclosure
Elahe A Mostaghel has received honoraria from Janssen.
References
- ScherHISawyersCLBiology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axisJ Clin Oncol200523328253826116278481
- GellerJLiuJAlbertJFayWBerryCCWeisPRelationship between human prostatic epithelial cell protein synthesis and tissue dihydrotestosterone levelClin Endocrinol (Oxf)19872621551613665114
- MohlerJLGregoryCWFordOH3rdThe androgen axis in recurrent prostate cancerClin Cancer Res200410244044814760063
- NishiyamaTHashimotoYTakahashiKThe influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancerClin Cancer Res200410217121712615534082
- PageSTLinDWMostaghelEAPersistent intraprostatic androgen concentrations after medical castration in healthy menJ Clin Endocrinol Metab200691103850385616882745
- MontgomeryRBMostaghelEAVessellaRMaintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growthCancer Res200868114447445418519708
- CuligZHoffmannJErdelMSwitch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model systemBr J Cancer199981224225110496349
- GregoryCWJohnsonRTJrMohlerJLFrenchFSWilsonEMAndrogen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgenCancer Res20016172892289811306464
- GregoryCWHamilKGKimDAndrogen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genesCancer Res19985824571857249865729
- MohlerJLMorrisTLFordOH3rdAlveyRFSakamotoCGregoryCWIdentification of differentially expressed genes associated with androgen-independent growth of prostate cancerProstate200251424725511987153
- ScherHIBuchananGGeraldWButlerLMTilleyWDTargeting the androgen receptor: improving outcomes for castration-resistant prostate cancerEndocr Relat Cancer200411345947615369448
- LabrieFBelangerASimardJLuu-TheVLabrieCDHEA and peripheral androgen and estrogen formation: intracinologyAnn N Y Acad Sci199577416288597456
- LockeJAWasanKMNelsonCCGunsESLeonCGAndrogen-mediated cholesterol metabolism in LNCaP and PC-3 cell lines is regulated through two different isoforms of acyl-coenzyme A: cholesterol acyltransferase (ACAT)Prostate2008681203318000807
- LockeJAGunsESLubikAAAndrogen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancerCancer Res200868156407641518676866
- LeonCGLockeJAAdomatHHAlterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft modelProstate201070439040019866465
- MostaghelEASolomonKRPeltonKFreemanMRMontgomeryRBImpact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumorsPLoS One201271e3006222279565
- StanbroughMBubleyGJRossKIncreased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancerCancer Res20066652815282516510604
- HoflandJvan WeerdenWMDitsNFEvidence of limited contributions for intratumoral steroidogenesis in prostate cancerCancer Res20107031256126420086173
- BubendorfLKononenJKoivistoPSurvey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarraysCancer Res199959480380610029066
- FordOH3rdGregoryCWKimDSmithermanABMohlerJLAndrogen receptor gene amplification and protein expression in recurrent prostate cancerJ Urol200317051817182114532783
- LinjaMJSavinainenKJSaramakiORTammelaTLJVessellaRLVisakorpiTAmplification and overexpression of androgen receptor gene in hormone-refractory prostate cancerCancer Res20016193550355511325816
- VisakorpiTHyytinenEKoivistoPIn vivo amplification of the androgen receptor gene and progression of human prostate cancerNat Genet1995944014067795646
- van der KwastTHSchalkenJRuizeveld de WinterJAAndrogen receptors in endocrine-therapy-resistant human prostate cancerInt J Cancer19914821891931708363
- Ruizeveld de WinterJAJanssenPJSleddensHMAndrogen receptor status in localized and locally progressive hormone refractory human prostate cancerAm J Pathol199414447357467512791
- TaplinMEBubleyGJShusterTDMutation of the androgen-receptor gene in metastatic androgen-independent prostate cancerN Engl J Med199533221139313987723794
- HolzbeierleinJLalPLaTulippeEGene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistanceAm J Pathol2004164121722714695335
- LatilABiecheIVidaudDEvaluation of androgen, estrogen (ER alpha and ER beta), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assaysCancer Res20016151919192611280747
- MiyamotoHRahmanMMChangCMolecular basis for the antiandrogen withdrawal syndromeJ Cell Biochem200491131214689576
- ChenCDWelsbieDSTranCMolecular determinants of resistance to antiandrogen therapyNat Med2004101333914702632
- BrookeGNBevanCLThe role of androgen receptor mutations in prostate cancer progressionCurr Genomics2009101182519721807
- CuligZHobischACronauerMVMutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesteroneMol Endocrinol1993712154115508145761
- TanJShariefYHamilKGDehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cellsMol Endocrinol19971144504599092797
- TaplinMEDrug insight: role of the androgen receptor in the development and progression of prostate cancerNat Clin Pract Oncol20074423624417392714
- YuanXBalkSPMechanisms mediating androgen receptor reactivation after castrationUrol Oncol2009271364119111796
- ZhaoXYMalloyPJKrishnanAVGlucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptorNat Med20006670370610835690
- SteinkampMPO’MahonyOABrogleyMTreatment-dependent androgen receptor mutations in prostate cancer exploit multiple mechanisms to evade therapyCancer Res200969104434444219366804
- KorpalMKornJMGaoXAn F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide)Cancer Discov2013391030104323842682
- BalbasMDEvansMJHosfieldDJOvercoming mutation-based resistance to antiandrogens with rational drug designElife20132e0049923580326
- JosephJDLuNQianJA clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509Cancer Discov2013391020102923779130
- GuoZYangXSunFA novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growthCancer Res20096962305231319244107
- HuRDunnTAWeiSLigand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancerCancer Res2009691162219117982
- ZhaoJJLinJLwinTmicroRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphomaBlood2010115132630263920086245
- HornbergEYlitaloEBCrnalicSExpression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survivalPLoS One201164e1905921552559
- HaileSSadarMDAndrogen receptor and its splice variants in prostate cancerCell Mol Life Sci201168243971398121748469
- GreenbergEEndocrine therapy in the management of prostatic cancerClin Endocrinol Metab1980923693816994946
- RobinsonMRShearerRJFergussonJDAdrenal suppression in the treatment of carcinoma of the prostateBr J Urol19744655555594422260
- SamsonDJSeidenfeldJSchmittBSystematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinomaCancer200295236137612124837
- SchmittBBennettCSeidenfeldJSamsonDWiltTMaximal androgen blockade for advanced prostate cancer [review]Cochrane Database Syst Rev20002CD00152610796804
- CaubetJFTostesonTDDongEWMaximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomized controlled trials using nonsteroidal antiandrogensUrology199749171789000189
- SmallEJRyanCJThe case for secondary hormonal therapies in the chemotherapy ageJ Urol20061766 Pt 2S66S7117084172
- de BonoJSLogothetisCJFizaziKAbiraterone acetate (AA) plus low dose prednisone (P) improves overall survival (OS) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) who have progressed after docetaxel-based chemotherapy (chemo): results of COU-AA-301, a randomized double-blind placebo-controlled Phase III study [abstract]Ann Oncol201021Suppl 8LBA5
- ScherHIBeerTMHiganoCSAntitumour activity of MDV3100 in castration-resistant prostate cancer: a Phase I–II studyLancet201037597241437144620398925
- EfstathiouETitusMTsavachidouDEffects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in boneJ Clin Oncol201230663764322184395
- HandrattaVDVasaitisTSNjarVCNovel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft modelJ Med Chem20054882972298415828836
- RowlandsMGBarrieSEChanFEsters of 3-pyridylacetic acid that combine potent inhibition of 17 alpha-hydroxylase/C17,20-lyase (cytochrome P45017 alpha) with resistance to esterase hydrolysisJ Med Chem19953821419141977473546
- AttardGReidAHAuchusRJClinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancerJ Clin Endocrinol Metab201297250751622170708
- RichardsJLimACHayCWInteractions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100Cancer Res20127292176218222411952
- SoiferHSSouleimanianNWuSDirect regulation of androgen receptor activity by potent CYP17 inhibitors in prostate cancer cellsJ Biol Chem201228763777378722174412
- EilCKetoconazole binds to the human androgen receptorHorm Metab Res19922483673701526623
- LiREvaulKSharmaKKAbiraterone inhibits 3β-hydroxysteroid dehydrogenase: a rationale for increasing drug exposure in castration-resistant prostate cancerClin Cancer Res201218133571357922753664
- BarrieSEPotterGAGoddardPMHaynesBPDowsettMJarmanMPharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17–20 lyase)J Steroid Biochem Mol Biol1994505–62672737918112
- O’DonnellAJudsonIDowsettMHormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancerBr J Cancer200490122317232515150570
- AttardGReidAHYapTAPhase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone drivenJ Clin Oncol200826284563457118645193
- RyanCJSmithMRFongLPhase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapyJ Clin Oncol20102891481148820159824
- ToddMMeyersMLCharnasRAcharyaMMolinaAFast and flawed or scientifically sound: the argument for administering oral oncology drugs during fastingJ Clin Oncol201230888888922331950
- Janssen-Ortho Inc., CanadaA Study to Determine the Short-Term Safety of Continuous Dosing of Abiraterone Acetate and Prednisone in Modified Fasting and Fed States to Patients With Metastatic Castration-Resistant Prostate Cancer Available from: http://clinicaltrials.gov/show/NCT01424930. NLM identifier: NCT01424930Accessed December 10, 2013
- University of ChicagoFood Effect Study of Abiraterone Acetate for Treatment of Patients With Castration-Resistant Prostate Cancer Available from: http://clinicaltrials.gov/show/NCT01543776. NLM identifier: NCT01543776Accessed December 10, 2013
- RatainMJFlushing oral oncology drugs down the toiletJ Clin Oncol201129303958395921931020
- MarburyTStonerockRTranNGonzalezMA Phase I single dose open-label reduced/staged pharmacokinetic (PK) and safety study of abiraterone acetate (AA) in men with imapired renal functionEur J Cancer201147Suppl 1S502
- AcharyaMGonzalezMMannensGA Phase I, open-label, single-dose, mass balance study of 14C-labeled abiraterone acetate in healthy male subjectsXenobiotica201343437938923020788
- RyanCJHalabiSOuSSVogelzangNJKantoffPSmallEJAdrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: results from a cancer and leukemia group B studyClin Cancer Res20071372030203717404083
- AttardGReidAHA’HernRSelective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancerJ Clin Oncol200927233742374819470933
- RyanCJShahSEfstathiouEPhase II study of abiraterone acetate in chemotherapy-naïve metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic responseClin Cancer Res201117144854486121632851
- ReidAHAttardGDanilaDCSignificant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetateJ Clin Oncol20102891489149520159823
- DanilaDCMorrisMJde BonoJSPhase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancerJ Clin Oncol20102891496150120159814
- FizaziKScherHIMolinaAAbiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled Phase III studyLancet Oncol2012131098399222995653
- GoodmanOBFlaigTWMolinaAExploratory analysis of the visceral disease (VD) patient subset in COU-AA-301, a Phase III study of abiraterone acetate (AA) in metastatic castration-resistant prostate cancer (mCRPC) [abstract]J Clin Oncol201331Suppl 61423045578
- SternbergCNMolinaANorthSEffect of abiraterone acetate on fatigue in patients with metastatic castration-resistant prostate cancer after docetaxel chemotherapyAnn Oncol20132441017102523152362
- LogothetisCJBaschEMolinaAEffect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trialLancet Oncol201213121210121723142059
- RyanCJSmithMRde BonoJSAbiraterone in metastatic prostate cancer without previous chemotherapyN Engl J Med2013368213814823228172
- RathkopfDESmithMRDe BonoJSUpdated interim analysis (IA) of COU-AA-302, a randomized Phase III study of abiraterone acetate (AA) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) without prior chemotherapy [abstract]J Clin Oncol201331Suppl 6523169505
- FerraldeschiRSharifiNAuchusRJAttardGMolecular pathways: inhibiting steroid biosynthesis in prostate cancerClin Cancer Res201319133353335923470964
- PiaAVignaniFAttardGStrategies for managing ACTH dependent mineralocorticoid excess induced by abirateroneCancer Treat Rev201339896697323582279
- ProcopioGGrassiPTestaISafety of abiraterone acetate in castration-resistant prostate cancer patients with concomitant cardiovascular risk factorsAm J Clin OncolEpub 9212013
- TolcherAWChiKNShoreNDEffect of abiraterone acetate plus prednisone on the QT interval in patients with metastatic castration-resistant prostate cancerCancer Chemother Pharmacol201270230531322752297
- ChiKNTolcherALeePEffect of abiraterone acetate plus prednisone on the pharmacokinetics of dextromethorphan and theophylline in patients with metastatic castration-resistant prostate cancerCancer Chemother Pharmacol201371123724423064959
- BertilssonLDahlMLDalenPAl-ShurbajiAMolecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugsBr J Clin Pharmacol200253211112211851634
- JanssenResearchDevelopmentLLCA Study to Evaluate the Effect of Rifampicin on the Pharmacokinetics of Abiraterone in Healthy Male Participants Available from: http://clinicaltrials.gov/show/NCT01655147. NLM identifier: NCT01655147Accessed December 10, 2013
- JanssenResearchDevelopmentLLCA Study to Assess the Effect of Ketoconazole on the Pharmacokinetics of Abiraterone Following Administration of Abiraterone Acetate Tablets in Healthy Adult Men Available from: http://clinicaltrials.gov/show/NCT01588782. NLM identifier: NCT01588782Accessed December 10, 2013
- DendreonConcurrent Versus Sequential Treatment With Sipuleucel-T and Abiraterone in Men With Metastatic Castrate Resistant Prostate Cancer (mCRPC) Available from: http://clinicaltrials.gov/show/NCT01487863. NLM identifier: NCT01487863Accessed December 10, 2013
- FitzpatrickJMde WitRTaxane mechanisms of action: potential implications for treatment sequencing in metastatic castration-resistant prostate cancerEur UrolEpub 7252013
- GanLChenSWangYInhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancerCancer Res200969218386839419826044
- LoriotYBianchiniDIleanaEAntitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100)Ann Oncol20132471807181223576708
- SchraderAJBoegemannMOhlmannCHEnzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abirateroneEur Urol2014651303623849416
- Janssen Pharmaceutica N.V., BelgiumAbiraterone With Different Steroid Regimens for Side Effect Related to Mineralcorticoid Excess Prevention in Prostate Cancer Prior to Chemotherapy Available from: http://clinicaltrials.gov/show/NCT01867710. NLM identifier:NCT01867710Accessed December 10, 2013
- DarshanMSLoftusMSThadani-MuleroMTaxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancerCancer Res201171186019602921799031
- ZhuMLHorbinskiCMGarzottoMQianDZBeerTMKyprianouNTubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancerCancer Res201070207992800220807808
- HuillardOAlbigesLEymardJCEfficacy of docetaxel chemotherapy in metastatic prostate cancer (mPC) patients (pts) experiencing early castration resistance (CR) [abstract]J Clin Oncol201331Suppl5075
- MezynskiJPezaroCBianchiniDAntitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance?Ann Oncol201223112943294722771826
- AngelerguesAMailletDFlechonAPrognostic factors of survival in patients with metastatic castration resistant prostate cancer (mCRPC) treated with cabazitaxel: sequencing might matter [abstract]J Clin Oncol201331Suppl5063
- MostaghelEAMarckBTPlymateSRResistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variantsClin Cancer Res201117185913592521807635
- CaiCChenSNgPIntratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitorsCancer Res201171206503651321868758
- MostaghelEAPageSTLinDWIntraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancerCancer Res200767105033504117510436
- CaffoOPalermoAVecciaABiochemical and objective response to abiraterone acetate withdrawal: incidence and clinical relevance of a new scenario for castration-resistant prostate cancerUrology20138251090109324001702
- GauthierHBousquetGPouesselDCulineSAbiraterone acetate withdrawal syndrome: does it exist?Case Rep Oncol20125238538723525261
- WitjesJAA case of abiraterone acetate withdrawalEur Urol201364351751823806520
- LabrieFDupontABelangerANew approach in the treatment of prostate cancer: complete instead of partial withdrawal of androgensProstate1983465795946415630
- SahuBLaaksoMPihlajamaaPFoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cellsCancer Res20137351570158023269278
- University of WashingtonAbiraterone Acetate in Treating Patients With Metastatic Hormone-Resistant Prostate Cancer Available from: http://clinicaltrials.gov/show/NCT01508234. NLM identifier: NCT01503229Accessed December 10, 2013
- Terence FriedlanderMDA Phase II Study of Increased-Dose Abiraterone Acetate in Patients With Castration Resistant Prostate Cancer Available from: http://clinicaltrials.gov/show/NCT01637402. NLM identifier: NCT01637402Accessed December 10, 2013
- Astellas Pharma Global Development, IncA Study to Determine Safety and Tolerability of Enzalutamide (MDV3100) in Combination With Abiraterone Acetate in Bone Metastatic Castration-Resistant Prostate Cancer Patients Available from: http://clinicaltrials.gov/show/NCT01650194. NLM identifier: NCT01650194Accessed December 10, 2013
- Alliance for Clinical Trials in OncologyEnzalutamide With or Without Abiraterone Acetate and Prednisone in Treating Patients With Castration-Resistant Metastatic Prostate Cancer Available from: http://clinicaltrials.gov/show/NCT01949337. NLM identifier: CT01949337Accessed December 10, 2013
- Aragon Pharmaceuticals IncSafety, Tolerability, Pharmacokinetics, and Preliminary Anti-tumor Activity of Ascending Doses of ARN 509 in Combination With Abiraterone Acetate Available from: http://clinicaltrials.gov/show/NCT01792687. NLM identifier: NCT01792687Accessed December 10, 2013
- M.D. Anderson Cancer CenterAbiraterone Acetate Plus LHRH Agonist and Abiraterone Acetate Plus LHRH Agonist and Enzalutamide Available from: http://clinicaltrials.gov/show/NCT01946165. NLM identifier: NCT01946165Accessed December 10, 2013
- Medical Research CouncilSTAMPEDE: Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy: A Multi-Stage Multi-Arm Randomised Controlled Trial Available from: http://clinicaltrials.gov/show/NCT00268476. NLM identifier: NCT00268476Accessed December 10, 2013
- Janssen Research & Development LLCA Study of Abiraterone Acetate Plus Low-Dose Prednisone Plus Androgen Deprivation Therapy (ADT) Versus ADT Alone in Newly Diagnosed Patients With High-Risk, Metastatic Hormone-Naive Prostate Cancer (mHNPC) Available from: http://clinicaltrials.gov/show/NCT01715285. NLM identifier: NCT01715285Accessed December 10, 2013
- UNICANCERSafety and Efficacy of Radiotherapy Combined With a 6-month LH-RH Agonist and Abiraterone Hormone Therapy Treatment in Biochemically-relapsing Prostate Cancer Following Surgery (CARLHA) Available from: http://clinicaltrials.gov/show/NCT01780220. NLM identifier: NCT01780220Accessed December 10, 2013
- University of WashingtonAbiraterone Acetate, Prednisone, and Leuprolide Acetate or Goserelin Before and During Radiation Therapy in Treating Patients With Localized or Locally Advanced Prostate Cancer Available from: http://clinicaltrials.gov/show/NCT01023061. NLM identifier: NCT01023061Accessed December 10, 2013
- Duke UniversityAbiraterone, Radiotherapy and Short-Term Androgen Deprivation in Unfavorable Localized Prostate Cancer Available from: http://clinicaltrials.gov/show/NCT01717053. NLM identifier: NCT01717053Accessed December 10, 2013
- Memorial Sloan-Kettering Cancer Center3-arm Study of Abiraterone Acetate Alone, Abiraterone Acetate Plus Degarelix, a GnRH Antagonist, and Degarelix Alone for Patients With Prostate Cancer With a Rising PSA or a Rising PSA and Nodal Disease Following Definitive Radical Prostatectomy Available from: http://clinicaltrials.gov/show/NCT01751451. NLM identifier: NCT01751451Accessed December 10, 2013
- MostaghelEANelsonPSLangePTargeted androgen pathway suppression in localized prostate cancer: a randomized clinical trialJ Clin OncolEpub 2013129
- TaplinMEMontgomeryRBLogothetisCEffect of neoadjuvant abiraterone acetate (AA) plus leuprolide acetate (LHRHa) on PSA, pathological complete response (pCR), and near pCR in localized high-risk prostate cancer (LHRPC): results of a randomized Phase II study [abstract]J Clin Oncol201230Suppl4521
