Abstract
For more than half a century, the different properties of dexrazoxane have captured the attention of scientists and clinicians. Presently, dexrazoxane is licensed in many parts of the world for two different indications: prevention of cardiotoxicity from anthracycline-based chemotherapy, and prevention of tissue injuries after extravasation of anthracyclines. This article reviews the historical, preclinical, and clinical background for the use of dexrazoxane for these indications.
Chemistry and mechanisms of action of dexrazoxane and anthracyclines
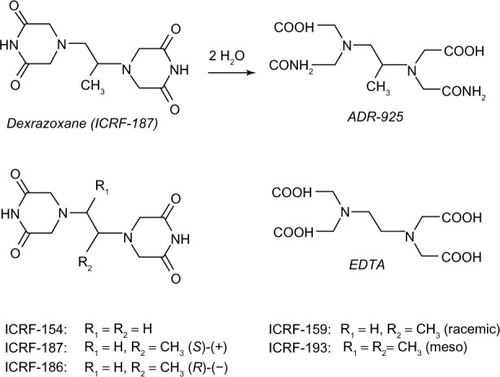
Dexrazoxane belongs to the bisdioxopiperazine compounds and is a water-soluble ring-closed analog of the iron chelator ethylenediaminetetraacetic acid (EDTA) (). Unlike EDTA, dexrazoxane easily passes into cells. Upon hydrolysis, dexrazoxane opens into its EDTA-like form, ADR-925, which is a strong iron chelator that has the ability to displace iron from the anthracycline.Citation1
Figure 1 Chemical structures of dexrazoxane, EDTA, and related bisdioxopiperazines.
Abbreviation: EDTA, ethylenediaminetetraacetic acid.
Dexrazoxane is also a catalytic inhibitor of DNA topoisomerase II that is the same target as the DNA topoisomerase II poisonous anticancer agents: the anthracyclines (eg, doxorubicin, epirubicin, daunorubicin), the anthracenediones (eg, mitoxantrone), and the podophyllotoxins (eg, etoposide, teniposide). However, dexrazoxane does not induce lethal DNA double-strand breaks as do the topoisomerase poisons.Citation2
Historical background of dexrazoxane
In the 1950s, the bisdioxopiperazine compounds were investigated independently by scientists from The Eastman Kodak Company (Rochester, NY, USA) and The Ciba-Geigy Corporation (Basel, Switzerland), for their nonbiological properties and use as jet fuel additives and textile leveling agents.Citation3,Citation4 Based on the hypothesis that intercellular metal ion chelation leads to retardation of neoplastic cell growth, it was later suggested that these chelating compounds might be antineoplastics.Citation5
In due course, this led to the generation of a number of potential antineoplastic bisdioxopiperazine compounds, all bearing the name of the fostering British institution, the Imperial Cancer Research Fund (ICRF). ICRF-159 (razoxane), ICRF-193, and ICRF-154 were initially found to exert in vitro antineoplastic effects in various tumors. In vivo activity was shown in patients with leukemia and lymphosarcoma.Citation6 However, the compounds were soon discarded as antineoplastic agents due to unfavorable toxicity profiles.
The interest in the bisdioxopiperazines as potential protectors against anthracycline-mediated myocardial damage emerged from a large series of preclinical studies, in particular those performed by Herman et al.Citation7 For example, it was observed in 1974 that anthracycline-induced cardiotoxicity was prevented in isolated dog hearts that were pretreated with perfusions of razoxane.Citation7 Pretreatment with dexrazoxane (ICRF-187) reduced cardiotoxicity and lethality in non-cancer-bearing Syrian golden hamsters receiving daunorubicin.Citation8 Furthermore, pretreatment with dexrazoxane was shown to be cardioprotective in doxorubicin- and daunorubicin-treated beagle dogs, rabbits, and miniature swine.Citation9–Citation11 Later, clinical studies in humans confirmed the cardioprotection exerted by dexrazoxane. Hence, a new indication for the use of dexrazoxane was established.
The first report on the experimental amelioration of subcutaneous injuries caused by anthracyclines with dexrazoxane was published in 2000.Citation12 In several series of experiments, ulcers were introduced in mice with subcutaneously injected doxorubicin, daunorubicin, and idarubicin.Citation13–Citation15 Systemic treatment was highly efficacious in protecting against ulcers, and the protection obtained by triple-dose dexrazoxane was superior to the protection obtained by a single dose. The effect was highly significant regarding both the frequency and sizes of wounds, and early treatment was more protective than late treatment. Hence, it was demonstrated that the protection depended on the dose of dexrazoxane as well as on the time and frequency of administration. Additional experiments were carried out to further explore the possible mechanism of action.Citation13–Citation15 outlines the indications and schedules for treatment with dexrazoxane.
Table 1 Schedules and indications for treatment with dexrazoxane for cardiotoxicity and accidental extravasation
Background: anthracycline cardiotoxicity
Presently, the antineoplastic activity of anthracyclines is believed to be almost exclusively due to the ability to bind to DNA and act as a poison to topoisomerase II by inducing lethal double-strand DNA breaks.Citation17,Citation18 However, the anthracyclines also have a number of other potential cytotoxic effects mediated by several mechanisms, including intercalation into nuclear DNA, production of reactive oxygen species, and induction of apoptosis.
The cardiac side effects are believed to result from induction of oxidative stress and apoptosis. The anthracycline quinone can redox cycle after reductive activation by various reductase enzymes to its free semiquinone.Citation19 The semiquinone reacts with oxygen to produce highly reactive oxygen species such as superoxide anion and hydrogen peroxide. The anthracycline also binds iron through its quinone/semiquinone functional groups, which are able to catalyze formation of the toxic and extremely reactive hydroxyl radical in a redox cycling reaction. This iron-dependent anthracycline-based oxidative stress and induction of the proapoptotic pathway is believed to be the main reasons for the myocardial toxicity.Citation20,Citation21 Cardiac mitochondria are easily injured by anthracyclines and the anthracycline–iron complexes, which have a high affinity for the dianionic phospholipid cardiolipin that is present in high concentration in the inner mitochondrial membrane.Citation22 Cardiac progenitor cells may also be damaged by anthracyclines, which clinically translates into the delayed left ventricular dysfunction that may occur decades of years after anthracycline treatment in children.Citation23
Clinically, anthracycline cardiotoxicity roughly exists in two forms: acute and chronic.Citation24 Acute toxicity is associated with intravenous administration of the anthracycline and is characterized by vasodilation, hypotension, and transient rhythm disturbances.
The most serious side effect of repeated anthracycline therapy is late cardiomyopathy. The clinical presentation is that of congestive heart failure, and the major risk factor is the cumulative dose of the anthracycline. Thus, Von Hoff et al demonstrated an incidence of 2.2% in a retrospective analysis of 3,941 patients treated with doxorubicin at various schedules with a median dose of 390 mg/m2. The overall incidence of daunorubicin-induced cardiomyopathy in 5,613 patients was 0.7% in adults and 1.6% in children. Dose dependency was evident by the fact that a cumulative dose of 1,000 mg/m2 was associated with cardiomyopathy in 11%, whereas the incidence was 1.5% at a total dose of 600 mg/m2.Citation25 Aggravating risk factors for developing cardiomyopathy after anthracycline therapy include older age, radiation therapy to the mediastinal region, and combination chemotherapy with anticancer agents such as cyclophosphamide, trastuzumab, and taxanes. The clinical problem is of major importance in the treatment of pediatric patients. Hence, at follow-up 1–15 years after doxorubicin treatment in 115 children with leukemia, changes in ventricular wall thickness and/or contractility were found in 57%.Citation26 The effect of cardiotoxicity increases in long-term survivors, from 2% after 2 years to 5% after 15 years.Citation27 Epirubicin differs from doxorubicin only in the steric position of the 4′-hydroxy group. The resulting slightly lower potency may account for the lower cardiotoxicity. However, fatal late cardiotoxicity also occurs after equi-myelosuppressive doses.Citation28,Citation29 As a consequence of the dose-related risk of cardiomyopathy, epirubicin is usually administered to a maximal total dose of 900–1,000 mg/m2, and doxorubicin and daunorubicin up to a maximum of approximately 550 mg/m2.
Clinical efficacy in cardioprotection
The cardioprotective effect of dexrazoxane has been studied clinically for more than 20 years, and the results have been referenced, study-wise, in meta-analyses, reviews, and clinical guidelines.Citation30–Citation39 There is evidence that the incidence of heart failure is reduced in patients with advanced breast cancer, sarcoma, and lung cancer treated with dexrazoxane added to doxorubicin and epirubicin in these studies. Moreover, in these studies, the cardioprotection does not significantly reduce the overall survival or progression-free survival. In one of the trials, the objective response rate was lower in dexrazoxane-treated patients, but it did not translate into impaired progression-free survival time.Citation39 The most recent multicenter study of women previously exposed to anthracyclines and thereafter receiving doxorubicin or epirubicin confirmed that those treated with dexrazoxane experienced fewer cardiac events (significant reduction in left ventricular ejection fraction or the appearance of clinical signs of cardiac insufficiency) compared with those treated with anthracycline only (13% in the dexrazoxane arm versus 39% in the no-dexrazoxane arm; P<0.001).Citation40 The incidence of congestive heart failure was 1% in the dexrazoxane-treated group compared to 11% in the no-dexrazoxane arm (P=0.015, ie, a reduction in risk of approximately 90%). These findings support the 76% overall risk reduction in congestive heart failure that was estimated in a meta-analysis of several large randomized controlled studies of patients treated with doxorubicin or epirubicin where dexrazoxane was given from the first dose of anthracycline.Citation41
Recently, Kalam and Marwick summarized the clinical efficacy of dexrazoxane in a systematic review of randomized trials and observational studies of cardioprotection in chemotherapeutic treatment of patients with no prior history of heart failure.Citation42 Looking at drop in ejection fraction or development of heart failure, they defined more than 2,000 patients from 14 studies in adult and pediatric patients treated with anthracyclines. In seven randomized controlled studies including 1,167 patients, cardiac events were significantly reduced with dexrazoxane (risk reduction =0.35 [95% confidence interval: 0.27–0.45], P<0.00001). Clinical data from randomized pediatric trials are far less solid, and short-term and long-term results from ongoing clinical trials are awaited.Citation43
Background: anthracycline extravasation
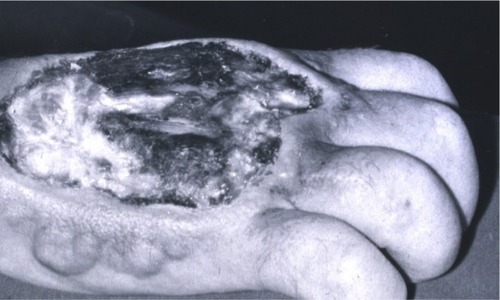
Extravasation is the unintentional leakage of a drug into the surrounding tissues. In cancer treatment, extravasation of the widely used anthracyclines such as doxorubicin, daunorubicin, and epirubicin may lead to severe, long-lasting tissue injuries. The clinical hallmarks of anthracycline extravasation are pain, swelling, and erythema at the infiltrated site that last for days, weeks, or even months.Citation44–Citation46 Symptoms of chest wall infiltration from a centrally placed catheter may have a delayed onset and may present as shoulder pain.Citation47 Over the following days, edema, blistering, and induration are often observed. The slowly growing ulceration, which may appear after several days or even weeks, has a propensity to invade deep structures such as tendons and joints irrespective of fascia.Citation48–Citation51 Even though small lesions may heal spontaneously, larger lesions can have a prolonged course lasting several months (). Long-term sequelae include pain and serious joint and nerve damage, permanent disfigurement, and other cosmetic changes. In severe cases of anthracycline extravasation, surgical debridement with subsequent skin grafting is necessary.Citation46–Citation49 Interruption or discontinuation of further scheduled cancer chemotherapy is an additional complication. Treatment options for anthracycline extravasation were limited and empirically based before dexrazoxane was found to be a potent and specific systemic antidote against the injuries.
Figure 2 Necrosis after an accidental anthracycline extravasation that was left untreated (before the discovery of dexrazoxane as an antidote).
Note: Copyright © 2014 by John Wiley & Sons, Inc. Reproduced from Langer SW. Extravasation reactions. In: Lacouture ME, editor. Dermatologic Principles and Practice in Oncology: Conditions of the Skin, Hair, and Nails in Cancer Patients. John Wiley & Sons Inc. Hoboken, New Jersey; 2014:295–300. All rights reserved.Citation16
Clinical efficacy in extravasation
Two larger prospective, multicenter clinical trials have been conducted: TT01 and TT02.Citation52 In addition, a number of case reports and a small pharmacokinetic study have has been published, as have two confirmative retrospective studies.Citation53–Citation59
The prospective trials are unique for all extravasation studies due to the eligibility criteria of histologically verified accidental anthracycline extravasations (positive fluorescence microscopy), and not on the suspicion of extravasation alone. Patients were entered from 24 different European oncology centers and had clinical extravasations of mainly doxorubicin and epirubicin.Citation52 Dexrazoxane was administered as an intravenous infusion over 1–2 hours: 1,000 mg/m2 was given within 6 hours after the extravasation injury; 1,000 mg/m2 24 hours later; and 500 mg/m2 another 24 hours later. Of 80 patients, 54 had positive biopsies and only one patient developed ulceration that required surgical intervention, corresponding to an overall efficacy rate of 98%. In addition, none of the additional 26 patients with suspected extravasations, but negative punch biopsies, developed ulceration. The secondary objectives of the studies were to avoid postponement of further planned cancer chemotherapy and to describe and evaluate subjective and objective symptoms and signs following dexrazoxane treatment, as well as evaluation of the tolerability and/or toxicity of dexrazoxane. More than two-thirds of the patients continued their planned chemotherapy without delay. One-third of the patients experienced a delay in planned chemotherapy of a median of 1 week. Few patients experienced mild, long-term sequelae as a result of the extravasation. No patients developed limitations of limb movement. In conclusion, dexrazoxane proved highly efficacious and tolerable in the management of accidental anthracycline extravasation. The treatment has also proved valuable in extravasations from central venous catheters.Citation60–Citation62
Side effects and matters of concern
When administered as a single agent, i.e. without concomitant anthracycline, Phase I studies showed dose-limiting myelotoxicity (neutropenia) at 1,500 mg/m2 per dose when administered for 3 or 5 consecutive days every 3 weeks, and with doses up to 7,400 mg/m2 over 60 minutes per week when administered weekly.Citation63–Citation67 Other side effects at near-maximum doses included reversible elevation of hepatic transaminases and increased urinary excretion of iron and zinc.Citation67 Nausea, alopecia, mucositis, and vomiting have also been reported in early-phase clinical studies.Citation63–Citation67 However, in Phase III cardioprotection studies comprising anthracycline-containing regimens, eg, fluorouracil, doxorubicin, and cyclophosphamide, the addition of dexrazoxane did not aggravate these side effects. The hematological toxicity was exacerbated but the incidence of neutropenic fever was not consistently exacerbated.Citation31,Citation40 In the prospective extravasation studies TT01 and TT02, dexrazoxane was administered on a 3-day schedule to patients experiencing anthracycline extravasation who were already receiving cycles of chemotherapy.Citation52 Consequently, the pattern of adverse events observed was therefore very similar to that of anthracyclines, and therefore it is hard to separate the adverse events due to dexrazoxane from those of anthracycline chemotherapy. In short, nausea/vomiting were reported in around one-third of patients, with approximately one-half of the patient population experiencing neutropenia and thrombocytopenia. Transient increases in liver enzymes (alanine aminotransferase/aspartate aminotransferase) were documented less commonly and reverted to normal within 10 days. Dexrazoxane may cause local injection-site reaction (pain, superficial phlebitis), and it is recommended that dexrazoxane be infused in a large vein.
In recent years, concern has been raised about the risk of long-term effects of dexrazoxane, particularly in pediatric patients who had repeated doses to prevent cardiotoxicity. Although very rare, a threefold increase in the incidence of second primary malignancies (myelodysplastic syndrome and acute myeloid leukemia) in dexrazoxane-treated pediatric patients compared with controls was reported in two randomized studies.Citation68,Citation69 On the other hand, no increase in secondary malignant neoplasms was observed after dexrazoxane cardioprotection in other large pediatric trials.Citation70,Citation71 Nonetheless, these findings have led to a restriction in the labeled indication for dexrazoxane in cardioprotection in Europe.
Conclusion
The story of dexrazoxane is a journey of 50 years through translational chemistry and medicine. It has moved dexrazoxane from the laboratory benches into clinical practice, where it may be used as a protective agent against anthracycline induced cardiotoxicity and extravasation injuries.
Disclosure
The author reports no conflicts of interest in this work.
References
- HasinoffBBKuschakTIYalowichJCCreightonAMA QSAR study comparing the cytotoxicity and DNA topoisomerase II inhibitory effects of bisdioxopiperazine analogs of ICRF-187 (dexrazoxane)Biochem Pharmacol19955079539587575679
- NitissJLTargeting DNA topoisomerase II in cancer chemotherapyNat Rev Cancer20099533835019377506
- GeigyJR UK Patent 978,7241964
- Eastman Kodak Co UK Patent 1,001,1571965
- CreightonAMHellmannKWhitecrossSAntitumour activity in a series of bisdiketopiperazinesNature196922251913843855782118
- HellmannKNewtonKAWhitmoreDNHanhamIWBondJVPreliminary clinical assessment of ICRF 159 in acute leukaemia and lymphosarcomaBr Med J1969156478228245251696
- HermanEHMhatreRMChadwickDPModification of some of the toxic effects of daunomycin (NSC-82,151) by pretreatment with the antineoplastic agent ICRF 159 (NSC-129,943)Toxicol Appl Pharmacol1974275175264212173
- HermanEHArdalanBBierCWaravdekarVKropSReduction of daunorubicin lethality and myocardial cellular alterations by pretreatment with ICRF-187 in Syrian golden hamstersCancer Treat Rep1979638992421236
- HermanEHFerransVJInfluence of vitamin E and ICRF-187 on chronic doxorubicin cardiotoxicity in miniature swineLab Invest198349169776408310
- HermanEHFerransVJYoungRSHamlinRLPretreatment with ICRF-187 allows a marked increase in the total cumulative dose of doxorubicin tolerated by beagle dogsDrugs Exp Clin Res19881495635703147886
- HermanEHFerransVJPreclinical animal models of cardiac protection from anthracycline-induced cardiotoxicitySemin Oncol1998254 Suppl 1015219768819
- LangerSWSehestedMJensenPBTreatment of anthracycline extravasation with dexrazoxaneClin Cancer Res200093680368610999761
- LangerSWSehestedMJensenPBDexrazoxane is a potent and specific inhibitor of anthracycline induced subcutaneous lesions in miceAnn Oncol200112340541011332155
- LangerSWThougaardAVSehestedMJensenPBTreatment of anthracycline extravasation in mice with dexrazoxane with or without DMSO and hydrocortisoneCancer Chemother Pharmacol200657112512816001176
- ThougaardAVLangerSWHainauBA murine experimental anthracycline extravasation model: pathology and study of the involvement of topoisomerase II alpha and iron in the mechanism of tissue damageToxicology20102691677220079798
- LangerSWExtravasation reactionsLacoutureMEDermatologic Principles and Practice in Oncology: Conditions of the Skin, Hair, and Nails in Cancer PatientsJohn Wiley & Sons IncHoboken, New Jersey2014295300
- GewirtzDAA critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicinBiochem Pharmacol199957772774110075079
- FortuneJMOsheroffNTopoisomerase II as a target for anticancer drugs: when enzymes stop being niceProg Nucleic Acid Res Mol Biol20006422125310697411
- HasinoffBBHellmannKHermanEHFerransVJChemical, biological and clinical aspects of dexrazoxane and other bisdioxopiperazinesCurr Med Chem1998511289481032
- ForceTIntroduction to cardiotoxicity review seriesCirc Res20101061192020056942
- YoussefGLinksMThe prevention and management of cardiovascular complications of chemotherapy in patients with cancerAm J Cardiovasc Drugs2005523324315984906
- HasinoffBBDaveyJPAdriamycin and its iron(III) and copper(II) complexes. Glutathione-induced dissociation; cytochrome c oxidase inactivation and protection; binding to cardiolipinBiochem Pharmacol19883719366336692845993
- De AngelisAPiegariECappettaDAnthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell functionCirculation2010121227629220038740
- FerransVJClarkJRZhangJYuZXHermanEHPathogenesis and prevention of doxorubicin cardiomyopathyTsitologiia199739109289379505340
- Von HoffDDRozencweigMLayardMSlavikMMuggiaFMDaunomycin-induced cardiotoxicity in children and adults. A review of 110 casesAm J Med1977622200208835599
- LipshultzSEColanSDGelberRDPerez-AtaydeARSallanSESandersSPLate cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhoodN Engl J Med1991324128088151997853
- KremerLCvan DalenECOffringaMOttenkampJVoûtePAAnthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up studyJ Clin Oncol200119119119611134212
- NielsenDJensenJBDombernowskyPEpirubicin cardiotoxicity: a study of 135 patients with advanced breast cancerJ Clin Oncol1990811180618102230869
- DardirMDFerransVJMikhaelYSCardiac morphologic and functional changes induced by epirubicin chemotherapyJ Clin Oncol1989779479582738625
- SpeyerJLGreenMDKramerEProtective effect of the bispiperazinedione ICRF-187 against doxorubicin-induced cardiac toxicity in women with advanced breast cancerN Engl J Med19883197457523137469
- SpeyerJLGreenMDZeleniuch-JacquotteAICRF-187 permits longer treatment with doxorubicin in women with breast cancerJ Clin Oncol1992101171271727913
- FeldmannJEJonesSEWeisbergSRAdvanced small cell lung cancer treated with CAV (cyclophosphamide + Adriamycin® + vincristine) chemotherapy and the cardioprotective agent dexrazoxane (ADR-529, ICRF-187, Zinecard®)Proc Ann Meet Am Soc Clin Oncol199211AA93
- VenturiniMMichelottiADel MastroLMulticenter randomized controlled clinical trial to evaluate cardioprotection of dexrazoxane versus no cardioprotection in women receiving epirubicin chemotherapy for advanced breast cancerJ Clin Oncol199614311231208955656
- WexlerLHAndrichMPVenzonDRandomized trial of the cardioprotective agent ICRF-187 in pediatric sarcoma patients treated with doxorubicinJ Clin Oncol1996143623728636745
- SwainSMWhaleyFSGerberMCEwerMSBianchineJRGamsRADelayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapyJ Clin Oncol1997154133313409193324
- SwainSMWhaleyFSGerberMCCardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancerJ Clin Oncol199715131813329193323
- LopezMViciPDi LauroKRandomized prospective clinical trial of high-dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomasJ Clin Oncol19981686929440727
- LipshultzSERifaiNDaltonVMThe effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemiaN Engl J Med200435114515315247354
- HensleyMLHagertyKLKewalramaniTAmerican Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectantsJ Clin Oncol200927112714519018081
- MartyMEspiéMLlombartAMonnierARapoportBLStahalovaVDexrazoxane Study GroupMulticenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapyAnn Oncol200617461462216423847
- SeymourLBramwellVMoranLAUse of dexrazoxane as a cardioprotectant in patients receiving doxorubicin or epirubicin chemotherapy for the treatment of cancer. The Provincial Systemic Treatment Disease Site GroupCancer Prev Control1999314515910474762
- KalamKMarwickTHRole of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysisEur J Cancer2013492900290923706982
- SepeDMGinsbergJPBalisFMDexrazoxane as a cardioprotectant in children receiving anthracyclinesOncologist2010151220122621051660
- LangerSWSehestedMJensenPBAnthracycline extravasation: a comprehensive review of experimental and clinical treatmentsTumori200995327328219688963
- SonneveldPWassenaarHANooterKLong persistence of doxorubicin in human skin after extravasationCancer Treat Rep19846868958966733702
- ReillyJJNeifeldJPRosenbergSAClinical course and management of accidental adriamycin extravasationCancer197740520532056922655
- MayoDJPearsonDCChemotherapy extravasation: a consequence of fibrin sheath formation around venous access devicesOncol Nurs Forum19952246756807675669
- LaughlinRALandeenJMHabalMBThe management of inadvertent subcutaneous adriamycin infiltrationAm J Surg1979137408412373478
- PreussPPartoftSCytostatic extravasationsAnn Plast Surg19871943233293688779
- LinderRMUptonJOsteenRManagement of extensive doxorubicin hydrochloride extravasation injuriesJ Hand Surg Am19838132386827049
- RudolphRSteinRSPattilloRSkin ulcers due to adriamycinCancer19763810871094953958
- MouridsenHTLangerSWButerJTreatment of anthracycline extravasation with Savene (dexrazoxane): results from two prospective clinical multicentre studiesAnn Oncol200718354655017185744
- LangerSWSehestedMJensenPBButerJGiacconeGDexrazoxane in anthracycline extravasationJ Clin Oncol20001816306410944144
- JensenJNLock-AndersenJLangerSWMejerJDexrazoxane-a promising antidote in the treatment of accidental extravasation of anthracyclinesScand J Plast Reconstr Surg Hand Surg200337317417512841619
- El-SaghirNOtrockZMufarrijADexrazoxane for anthracycline extravasation and GM-CSF for skin ulceration and wound healingLancet Oncol20045532032115120669
- BosAMvan der GraafWTWillemsePHA new conservative approach to extravasation of anthracyclines with dimethylsulfoxide and dexrazoxaneActa Oncol200140454154211504316
- MouridsenHTLangerSWTjoernelundJAnthracycline extravasation in breast cancer patients. Effective treatment with dexrazoxane in three multicenter trialsEuropean J Cancer (Suppl)200867197
- MuthuramalingamSGaleJBradburyJDexrazoxane efficacy for anthracycline extravasation: use in UK clinical practiceInt J Clin Pract201367324424923409691
- FontaineCNoensLPierrePDe GrèveJSavene® (dexrazoxane) use in clinical practiceSupport Care Cancer20122051109111222278308
- JordanKBehlendorfTMuellerFSchmollHJAnthracycline extravasation injuries: management with dexrazoxaneTher Clin Risk Manag20095236136619536310
- HünerlitürkoglouANTapprichCLangerSWSehestedMJensenPBHeintgesTSuccessful use of dexrazoxane in two cases of anthracycline containing polychemotherapyEuropean J Clin Med Oncology2009111315
- LangerSWTreatment of anthracycline extravasation from centrally inserted venous cathetersOncol Rev20082115117
- HolcenbergJSTutschKDEarhartRHPhase I study of ICRF-187 in pediatric cancer patients and comparison of its pharmacokinetics in children and adultsCancer Treat Rep19867067037093089595
- KoellerJMEarhartRHDavisHLPhase I trial of ICRF-187 by 48-hour continuous infusionCancer Treat Rep1981655–64594636786740
- LiesmannJBeltRHaasCHoogstratenBPhase I evaluation of ICRF-187 (NSC-169780) in patients with advanced malignancyCancer1981478195919626784914
- Von HoffDDHowserDLewisBJHolcenbergJWeissRBYoungRCPhase I study of ICRF-187 using a daily for 3 days scheduleCancer Treat Rep1981653–42492526786738
- VogelCLGorowskiEDavilaEPhase I clinical trial and pharmacokinetics of weekly ICRF-187 (NSC 169780) infusion in patients with solid tumorsInvest New Drugs1987521871983115912
- SalzerWLDevidasMCarrollWLLong-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984–2001: a report from the children’s oncology groupLeukemia20102435537020016527
- TebbiCKLondonWBFriedmanDDexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s diseaseJ Clin Oncol20072549350017290056
- KoppLMBernsteinMLSchwartzCLThe effects of dexrazoxane on cardiac status and second malignant neoplasms (SMN) in doxorubicin-treated patients with osteosarcoma (OS)J Clin Oncol201230Suppl abstr 9503
- BarryEVVroomanLMDahlbergSEAbsence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxaneJ Clin Oncol20082671106111118309945
