Abstract
The concept of cancer stem cells has gained considerable interest in the last few decades, partly because of their potential implication in therapy resistance. However, the lack of specific cellular surface markers for these cells has impeded their isolation, making the characterization of this cellular subpopulation technically challenging. Recent studies have indicated that leucine-rich repeat-containing G-protein-coupled receptor 4 and 5 (LGR4 and LGR5) expression in multiple organs may represent a global marker of adult stem cells. This review aims to give an overview of LGR4 and LGR5 as cancer stem cell markers and their function in development.
The concept of cancer stem cells
The clonal nature of most malignant tumors is well established. Several studies have shown, however, that as many as 1 million murine or human tumor cells are required to transplant a new tumor from an existing one, although exceptions have been described in melanoma, for example, from which one single cell can form a new tumor.Citation1,Citation2 The stochastic model proposes that every cell in the heterogeneous cancer cell population can equally form tumors ().Citation3 An alternative point of view is given by the hierarchical model describing a small cell population within the tumor capable of self-renewal and thereby forming tumors with a heterogeneous cell population ().Citation3 The complex model is the most recent idea explaining tumor progression. It assumes that several cancer stem cell (CSC) populations with genetic and epigenetic changes coexist. Genetic mutations would be able to produce completely new cell populations, while epigenetic changes might enable cells to form progeny with a limited fate ().Citation4–Citation6
Figure 1 Sources of heterogeneity within cancer. (A) The stochastic model postulates that all tumor cells are equally capable of self-renewal or differentiation, and are tumorigenic. Tumor heterogeneity is achieved through genetic and/or epigenetic alterations (indicated by flashes). (B) In the hierarchical model, also called the cancer stem cell model of tumor growth, only a subset of tumor cells, the so-called cancer stem cells, has the ability to self-renew, and these cells give rise to committed progenitors with limited proliferative potential, which eventually terminally differentiate. (C) Extending the two concepts, the complex model suggests that epigenetic changes potentially due to micro-environmental factors can influence the tumor cell phenotype and function and thereby can also affect tumor heterogeneity.
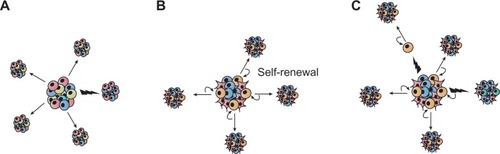
If the stochastic model is correct, then tumor cells are biologically homogeneous, and genetic or epigenetic programs that allow for tumorigenesis are operative in the majority of cells that comprise a tumor. The hierarchical model, however, predicts that the tumor cells are functionally heterogeneous and that quantitatively, the only cells capable of tumorigenesis are a small subpopulation within the tumor bulk. In the 1990s, studies provided strong evidence for the latter hypothesis in acute myeloid leukemia, demonstrating that these tumors evolve from stem cells in the bone marrow.Citation7–Citation9 This concept was then expanded to other hematopoietic malignancies, such as chronic myeloid leukemia,Citation10,Citation11 multiple myeloma,Citation12 and lymphoma.Citation13
At the apex of the hierarchy, so-called CSCs are defined by their ability to self-renew and differentiate. In the meantime, strong evidence suggests the existence of this rare and highly tumorigenic CSC population in several major solid cancers, including brain, colon, and breast carcinomas.Citation14 This subpopulation of cells has a capacity for self-renewal and multi-lineage differentiation, known features of non-CSCs. Technically, these characteristics have been demonstrated by transplantation assay using immunocompromised mice, combined with enrichment of a particular cell population, typically using cell surface marker expression such as cluster of differentiation (CD)44, EpCAM (epithelial cell adhesion molecule), and CD133.Citation15
Recently, stem cell-specific gene-expression signatures have helped to provide further insights into deregulated genes and pathways in CSCs, resulting in improved phenotypic characterization.Citation16
G-protein-coupled receptors and their role in cancer signaling
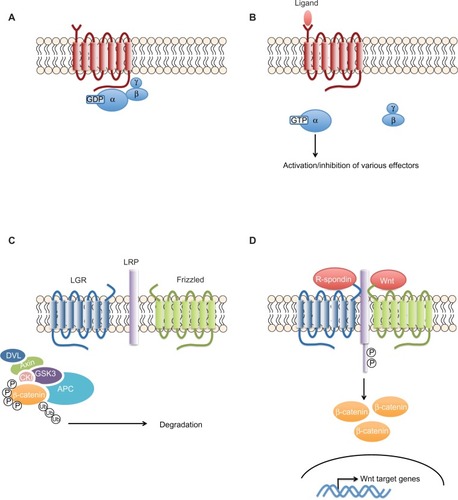
G-protein-coupled receptors (GPCRs) are transmembrane proteins, which, upon ligand binding, activate cytoplasmic G-proteins that stimulate several enzymes (). The G-protein-coupling specificity of each receptor defines the nature of the downstream signaling pathway.
Figure 2 The G-protein coupled receptors signaling pathway.
Notes: (A) The seven transmembrane domains of the GPCRs activate a panel of heterotrimeric G-proteins that differ at the level of their α-subunits. (B) Upon ligand stimulation of the GPCR, the α-subunit separates from the β- and γ-subunits and activates or inhibits different cytoplasmic enzymes. (C and D) In contrast to classical GPCRs, LGR4–6 do not activate heterotrimeric G-proteins to transduce the signal. Rather, upon R-spondin binding, they recruit the LRP–frizzled receptor complex, which binds to Wnt ligands, leading to the phosphorylation of LRP. The receptor complex then recruits the axin complex. β-catenin is no longer marked by phosphorylation for degradation, leading to its stabilization and accumulation and, ultimately, translocation into the nucleus. Based on Schuijers and Clevers with the permission of John Wiley and Sons. Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 2012;31(12):2685–2696.Citation91
Abbreviations: APC, adenomatous polyposis coli; CKI, casein kinase I; GDP, guanosine diphosphate; GPCR, G-protein-coupled receptor; GSK3, glycogen synthase kinase 3; GTP, guanosine triphosphate; LGR, leucine-rich repeat-containing GPCR; LRP, lipoprotein receptor-related protein; P, phosphate group; DVL, Dishevelled; Ub, Ubiquitin.
GPCRs contribute to many cellular functions, such as angiogenesis, tissue repair, inflammation, and indeed, cancer.Citation17 In fact, in addition to constitutively activating mutations of GPCRs and G-proteins, many tumors harbor overexpression of these receptors, which are activated by agonists released by stromal cells.Citation17
The GPCRs LGR5 and LGR4
LGR4 and LGR5 (leucine-rich repeat-containing GPCR 4 and 5), also known as GPR48 and GPR49, respectively, are members of the largest family of cell-surface molecules involved in signaling, the GPCRs.
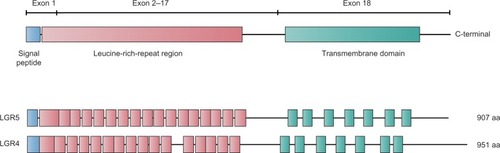
The LGR5 gene is ~144 kb long and is located on chromosome 12 at position 12q22–q23. LGR4 is ~107 kb long and is found on chromosome 11 at position 11p14–p13. Both proteins contain seven transmembrane domains (). Experimental evidence shows that the mature receptor proteins contain up to 17 leucine-rich repeats, each composed of 24 amino acids. These proteins were identified as putative glycoprotein receptors because they are 30%–40% homologous to the luteinizing hormone, follicle-stimulating hormone, and thyrotropin receptors, which contain only nine leucine-rich repeats.Citation18
Figure 3 Scheme depicting the structures of the LGR4 and LGR5 proteins.
Abbreviation: LGR, leucine-rich repeat-containing G-protein-coupled receptor.
For several years, the molecular functions and the ligands of LGR4 and LGR5 were unknown. Together with LGR6, they were considered to be “orphan” receptors. Recently, however, several groups have identified the R-spondins, soluble protein modulators of the canonical Wnt/β-catenin signaling pathway, as ligands for LGR5.Citation19 After binding R-spondin, LGR5 forms a protein complex with frizzled lipoprotein receptor-related proteins 5 and 6 (LRP5/6), which positively regulates Wnt signaling. Recent analysis of crystal structures of an R-spondin fragment and LGR5 ectodomain revealed the mode of binding as a pair of heterodimers.Citation20 In addition, anti-LGR5 antibody-mediated modulation of the Wnt reporter suggests a direct role of the R-spondin-LGR5 complex in Wnt pathway regulation, rather than indirectly via a tertiary protein complex together with LRPs or frizzled receptors. Thus, the R-spondin-LGR5 complex functions as a Wnt pathway regulator, probably forming a positive feedback loop, magnifying the Wnt pathway ().
“Blue ribbon visualization” is an in vivo lineage-tracing technique that uses the LacZ reporter gene under the activity of a promoter, allowing the identification of a cell of interest and its tracing in potential progeny. This method has revealed that LGR5 is a stem cell marker in various organs including the intestinal mucosa,Citation21 colon,Citation22 stomach,Citation23 hair follicle,Citation24 kidneyCitation25 and mammary gland.Citation26 In the small intestine, LGR5+ve cells at the bottom of crypts can be the cell of origin for all cell types in the epithelium. As such, a single LGR5+ve cell can generate a “mini-gut”-like organoid culture in vitro, recapitulating the crypt structure.Citation27 Intriguingly, LGR5+ve cells initially produce Paneth cells, which in turn provide niche factors such as Wnt3 for the LGR5+ve stem cell in the intestinal stem cell system.Citation28
In LGR4 knockout mice it was shown that LGR4 is critical for the development of various organs, including the male reproductive tract,Citation29 kidney,Citation30 eyelid,Citation31 erythropoiesis,Citation32 hair placode,Citation33 gallbladder and cystic duct,Citation34 and bones.Citation35 Notably, it has recently been reported that a c.376C<T nonsense mutation of human LGR4 results in termination at position 126. This mutation, leading to the complete loss of function of LGR4, has significant correlation with risks for osteoporosis, assessed by low bone mineral density, reduced testosterone levels, skin squamous cell carcinoma (SCC), and gallbladder/biliary tract cancer.Citation36 These results, identified by whole-genome sequencing in a large population, clearly show striking parallels with phenotypes observed in LGR4 knockout mice, and imply putative functions of LGR4 as a tumor suppressor gene.
Consistent with a potential role of LGR in morphogenesis, LGR5 knockout mice show 100% neonatal lethality with ankyloglossia, an abnormal craniofacial morphogenesis characterized by the fusion of the tongue to the floor of the oral cavity.Citation37 Since LGR4 was shown to be able to compensate LGR5 function in intestinal crypts, the process of the tongue’s development seems to be solely dependent on LGR5.
In the small intestine, LGR4 is expressed in all cell types within crypts, whereas LGR5 is restricted to crypt base columnar intestinal stem cells. It has been demonstrated that LGR4 is required for the “mini-gut” organoid culture in vitro, which is derived from LGR5+ve stem cells. Double knockout of both LGR4 and LGR5 completely abolished not only generation of the organoid, but also maintenance of crypts in vivo.Citation19 The abolishment of organoid formation by the LGR4/5 deletion could be rescued by Wnt activation. Using a Wnt reporter system in HEK293T (transfection of human embryonic kidney cell line. 293T) cells, LGR5 could compensate for the loss of Wnt signal activities caused by LGR4 knockdown. These findings suggest that LGR4 and LGR5 share the role of modulating the Wnt signaling pathway through R-spondin binding. Concordantly, immunoprecipitation assays demonstrated that all R-spondins (1–4) bind to LGR4–6,Citation19 and the contact residues of LGR4–6 at the binding sites of the R-spondins are strictly conserved.Citation20
Suggested role of LGR5 and LGR4 in CSCs
Recent advances using genetically modified mouse models have dramatically deepened our understanding of the adult tissue stem cell system harboring plasticity.
Indeed, the in vivo lineage tracing technique using the LacZ gene as a reporter revealed the plasticity in the early process of differentiation into Paneth cells, which can restore the LGR5+ve stem cell phenotype when regenerative machinery is switched on as a response to injury.Citation38 Significantly, this model provides explanations for the two biggest mysteries debated for a long time in the intestinal stem cell system field: ie, the controversy between the models of the rapidly cycling LGR5+ve crypt base columnar stem cells versus the BMI1+ve quiescent stem cells located at the base of the cryptCitation39 and the issue of why LGR5+ve stem cells in crypts are dispensable when LGR5 stem cells are sources for all types of cells found in the crypt.Citation40 The model proposes that the LGR5 positive stem cell-derived quiescent Paneth precursor cells at the base of the crypt, which are usually committed to becoming long-lived Paneth cells, can be a reservoir pool to restore the LGR5 stem cell population when needed in response to tissue damage.Citation38 Hence, these striking findings clearly impact the fields of both stem cell and CSC biology. A list of cancer entities in which LGR4 and LGR5 have been studied is summarized in .
Table 1 Summary of the involvement of LGR4 and LGR5 in different tumor entities
LGR5 in hepatocellular carcinoma
A decade ago, one of the pioneering studies on LGR5 in cancer reported the overexpression of LGR5 in hepatocellular carcinomas, pointing out the correlation with β-catenin mutations causing aberrant activation of the canonical Wnt pathway.Citation41 Recently, it was shown that in regenerative liver tissue induced by chemical injury, LGR5 marks liver stem cells, which can generate hepatocellular and cholangiocyte lineages.Citation42 However, the role of LGR5 in hepatocellular carcinoma stem cells still remains to be elucidated.
LGR5 in colon carcinoma
Given the specific expression of LGR5 in the stem cell compartment of the intestinal crypts, LGR5 is being intensively investigated in the field of colorectal cancer.
Many studies have defined LGR5 as a new stem cell marker, denoting the cell of origin for intestinal tumors similarly to prominin-1, also known as CD133.Citation43–Citation47 Indeed, several studies have demonstrated that both markers highlight the compartment of adult stem cells.Citation43,Citation44 However, it was shown that lineage tracing of prominin-1+ve cells using its promoter failed to mark restricted expression in intestinal stem cells,Citation45 suggesting that differences in the constructs in genetic mouse models can affect the results of such cell fate tracking. Moreover, the Wnt pathway has also been suggested to regulate prominin-1 expression,Citation46 concordantly with its central regulation for LGR5 expression in intestinal crypts.
Another study highlighted the capability of LGR5 to mark the long-lived intestinal stem cells and thereby the potential cells of origin. Indeed, specific deletion of the adenomatous polyposis coli gene, known to initiate intestinal cancer by Wnt-pathway-activating mutations, rapidly induced intestinal adenomas in LGR5+ve stem cells, but could not maintain adenomas when introduced to transit amplifying cells.Citation47
In colon cancer cells, LGR5 could function as a negative regulator of the Wnt pathway, which is contradictory to findings in intestinal stem cells, suggesting its cellular context-dependent functions.Citation48 Indeed, several regulatory mechanisms for LGR5 expression have been identified in various cellular contexts.Citation49–Citation53 ASCL2 (Achaete-scute complex homolog 2) is a basic helix-loop-helix transcription factor, which binds to the LGR5 promoter and is indispensible for maintenance of intestinal stem cells, exhibiting a restricted expression pattern in the compartment.Citation53 Inhibition of a proto-oncogene c-Jun, a component of the activator protein-1 (AP1) signaling pathway, decreases LGR5 expression in intestinal stem cells, together with Wnt pathway target genes.Citation52 c-Jun protein binds to the intronic region of the LGR5 gene locus and transactivates LGR5 expression upon phosphorylation-mediated activation of AP1 signaling.Citation49 This suggests the regulation of LGR5 expression due to the crosstalk of the Wnt and AP1 signaling pathways in intestinal stem cells.
In adenoma cells, a Wnt pathway enhancer, prostaglandin E2, increases LGR5 protein but not mRNA (messenger ribonucleic acid) levels independently of β-catenin, indicating post-translational regulation of LGR5 protein expression and conditional regulation of LGR5.Citation50 In addition, it was shown that promoter methylation can cause repression of LGR5 expression in some colon cancer cells.Citation51
LGRs in hair follicles and skin cancer
Hair follicle stem cells are also marked with restricted expression of LGR5. Using the lineage tracing system, it was shown that LGR5+ve cells in the bulge region of hair follicles represent the stem cell population, which could generate all cell lineages of hair follicles in vivo (but not of interfollicular epidermis and sebaceous glands) and efficiently generate hair follicles in transplanted nude mice.Citation24 LGR5+ve cells reside in the outer root sheath in the anagen phase, and actively cycle and migrate to generate progenies consisting of new hair follicles. Interestingly, expression profiles of the LGR5+ve hair follicle stem cells revealed the involvement of the sonic hedgehog signaling pathway, which was shown to regulate LGR5 in basal cell carcinoma (BCC).Citation54 In hair follicle progenitor cells, the Lim-homeodomain transcription factor Lhx2 (Lim homeobox 2) binds to the LGR5 promoter, shown by chromatin immunoprecipitation analysis, and negatively regulates its expression.Citation55
In an experiment combining the LGR5-lineage tracing system with a mouse model mimicking BCC with induced aberrant activation of sonic hedgehog signaling, it was shown that LGR5-traced hair follicle-derived cells are recruited in the early stages of BCC-like carcinogenesis in the interfollicle epidermis upon wounding.Citation56 Another group combined LGR5-lineage tracing and a skin SCC mouse model induced by E6/E7 oncogenes from human papilloma virus 16, driven by the K14 promoter. The animals developed SCC-like lesions, comprising K15+ve abnormal cells, which were traced as progenies of LGR5+ve cells.Citation57 Thus, these cells are suggested to be the cells of origin for the experimental SCC model. Intriguingly, an integrated analysis of germline polymorphism and expression profiling using a backcross of skin cancer resistant and susceptible mouse strains identified LGR5 as a potential master regulator gene of the hair follicle.Citation58
LGR4 and LGR5 in the mammary gland and breast cancer
One of the initial reports using LGR5-lineage tracing in the normal mammary gland showed that LGR5+ve cells represent lineage-committed mammary epithelial cells, which give rise only to myoepithelial cells, suggesting their limited potential.Citation59 It was also shown that LGR5+ve cells represent lineage-committed epithelial cells in physiological conditions, although LGR5+ve cells could be identified within the previously established CD45−CD24midCD49fhigh mammary gland stem cell fraction.Citation60 Under the stressed and regenerative conditions of the transplantation assay, LGR5+ve cells showed slightly enhanced capability to reconstitute mammary glands. However, the ability to reconstitute was also observed in LGR5−ve cells. Taken together, these results suggest LGR5 is expressed in lineage-committed cells that lack stem cell properties.
In contrast, more recently, it has been demonstrated that LGR5 clearly marks and characterizes the mammary gland stem cell fraction.Citation26 LGR5 expression was detected within selected populations in the basal cells of mammary glands. LGR5+ve basal cells exhibit higher activity to reconstitute new mammary glands in vivo.Citation26 These cells comprise a rare population within the Lin−CD24+CD49fhigh basal cell fraction that includes mammary stem cells.Citation61,Citation62 LGR5 enriches the mammary gland stem cells, which are capable of reconstitution from a single cell in serial transplantation assays to a much higher extent (one cell in four) than the other Lin−CD24+CD49fhigh basal cells. Moreover, the report demonstrated that a depletion assay of LGR5-expressing cells using the LGR5-DTR (diphtheria toxin receptor) mouse strain resulted in a significant decrease of the reconstruction potential of mammary glands, unlike in the case of intestinal stem cells.Citation40 These findings indicate that LGR5+ve cells represent indispensible mammary gland stem cells. Turning to breast cancer, it has been reported that an extracellular matrix component, tenascin, is a key regulator of lung metastasis of breast cancer.Citation63 In breast cancer cells, the expression level of tenascin regulates LGR5 expression, together with Msi1 (Musashi-1), a neural stem cell marker gene. Tenascin regulates spheroid formation derived from breast cancer cells. In spheroid cultures derived from breast cancer, LGR5 is more highly expressed than in adherent culture, in line with the observations in colorectal and glioblastoma spheroid cultures.Citation64 It was shown that LGR5 knockdown reduces lung metastases, suggesting a key role of LGR5 in CSC regulation, including metastasis-initiating potentials in breast cancer.
A recent study showed that LGR4 functions in mammary gland development and mammary stem cells by activating Sox2 (sex determining region Y-box 2) via the Wnt/β-catenin/Lef1 (lymphoid enhancer-binding factor 1) signaling pathway.Citation65
LGR5 in glioblastoma stem cells
In the 1990s, the localization of the expression of LGR5 was studied by in situ hybridization in the embryonic central nervous system and other tissues.Citation66 The authors reported that LGR5 is expressed in selected regions in the embryonic central nervous system, the developing face, the intervertebral disc anlagen, and the limb bud, at the active morphogenesis stage. In contrast, in adult mouse brain, LGR5 expression was not detected, except in the olfactory bulb in the central nervous system. These findings suggest that LGR5 is expressed in primitive neural cells during development only.
Recent findings showed that LGR5+ve cells isolated from cochlear sensory epithelium are capable of expansion, forming neurospheres, and could be induced to form differentiated hair sensory cells, suggesting that LGR5+ve cells are functional progenitors of hair cells.
Recently, two groups reported findings by lineage tracing in a particular kind of neural cell: the inner ear hair cell precursors/progenitors in cochlea tissue.Citation67,Citation68
Glioblastoma is the most frequent and malignant type of brain cancer. One of the pioneering studies on solid CSC research reported that CD133 marks glioblastoma stem cells. CD133 is a stem cell marker for hematopoietic and central nervous system and has been applied for the identification of CSCs in various types of malignancies, including colon cancer.Citation69 In parallel with colon CSCs, it has been reported that LGR5 is highly expressed in the CSC fraction of glioblastoma, and upon induced differentiation, the expression of LGR5 is decreased.Citation64,Citation70 Using meta-analysis of expression data, it has been shown that LGR5 is significantly more highly expressed in CD133-sorted cells from glioblastoma compared with the counterpart CD133−ve population.Citation64 In line with these observations, inhibition of LGR5 gene expression in glioblastoma stem-like cells causes cell death. In parallel to apoptosis induction, several stemness-related genes including Wnt or sonic-hedgehog pathway and cell cycle regulators such as L1CAM (human L1 cell adhesion molecule), FZD3 (frizzled homolog 3), PTCHD1 (patched domain containing 1), and CDKN1B (cyclin-dependent kinase inhibitor 1B) were significantly deregulated.Citation64 These findings imply that LGR5-mediated regulation of Wnt signaling is required for expansion of the glioblastoma stem cell population. Interestingly, inhibition of LGR5 causes a decrease in PFKFB4 (6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4) expression, which was recently identified as an indispensable gene for the glioblastoma stem-like cell survival.Citation71
In glioma, it was reported that the locus of 12q21 on which the LGR5 gene resides is frequently amplified. However, correlation of the mRNA expression of LGR5 with the genomic status could not be confirmed, possibly due to the small number of cases assessed by quantitative real-time polymerase chain reaction (n=5).Citation72
Additional tumor entities in which LGR5 involvement is suggested
A genome-wide study on soft tissue sarcoma samples identified the LGR5 gene locus as frequently amplified.Citation73 Another report on clinical sarcoma samples described a novel splice variant of LGR5 identified in soft tissue sarcoma, of which lower expression was shown to be associated with worse overall survival.Citation74 It has recently been reported that LGR5 is enriched in CD133+ve cells sorted from a Ewing’s sarcoma cell line.Citation75 In Ewing’s sarcoma patients, LGR5 is highly expressed in more aggressive cases with worse outcome.
In gastric cancer, it has been reported that post-therapeutic tissue expresses significantly higher levels of LGR5, NOTCH2, and POU5F1 (POU class 5 homeobox 1) mRNA, suggesting that LGR5+ve gastric cancer cells could be therapy resistant.Citation76 Upon analysis of a large cohort of 160 gastric adenocarcinoma samples and 99 normal mucosa samples, LGR5 could be detected at the bottom of the normal gastric gland unit along with the putative CSC markers CD26, CD44, ALDH1 (aldehyde dehydrogenase 1), and CD133, but was differentially expressed in gastric cancer tissues.Citation77 Low-differentiated gastric cancer showed lower expression of LGR5, in contrast to findings in glioblastoma. A recent report described that transforming growth factor beta-induced epithelial-mesenchymal transition in runt-related transcription factor 3 (Runx3)−/−p53−/− murine gastric epithelial cells accompanied with epidermal growth factor receptor/RAS (rat sarcoma viral oncogene) activation resulted in LGR5 induction, together with enhanced spheroid and colony formation activities.Citation78 Forced expression of KRAS (Kirsten rat sarcoma viral oncogene homolog) was also shown to be sufficient to induce LGR5 and sphere-forming activity in the murine gastric epithelial cells.
Furthermore, in lung adenocarcinoma clinical samples, LGR5 expression was shown to correlate with higher disease stage, larger tumor size, and shorter overall survival rate.Citation79 These findings imply the involvement of LGR5 in the pathogenesis of these tumor entities. Nonetheless, further analysis of its molecular function and usefulness as a CSC marker is required.
Potential clinical implications
The identification of the LGR5+ve bona fide intestinal stem cell population enabled the establishment of the expression profile signature specific for the compartment purified from the mouse intestine. This allowed the stratification of clinical colorectal cancer samples.Citation80 Importantly, the LGR5 intestinal stem cell signature was able to predict disease relapse in colorectal cancer patients. In the study, another Wnt target gene, the receptor tyrosine kinase EphB2 (ephrin type-B receptor 2), was used to sort intestinal cells as it is decreasingly expressed from the bottom of crypts toward the upper differentiated area. The EphB2-based enrichment of the colorectal CSCs clearly showed high expression of LGR5 in the fraction. The spheroid culture system maintained in serum-free medium supplemented with growth factors established from colorectal cancer specimens or cell lines has been shown to be highly tumorigenic and to highly express LGR5.Citation81,Citation82 However, technical difficulties have impeded the isolation of LGR5+ve cells from clinical cancer samples. Recently, originally generated antibodies were applied for the enrichment of the CSC fraction in colorectal cancer.Citation83 Moreover, it was shown that overexpression of LGR5 enhanced clonogenicity, whereas knockdown of LGR5 attenuated it, indicating the direct control of this colorectal CSC property by LGR5. Although it has been suggested that the R-spondin-LGR5 autocrine loop potentially regulates the LGR5+ve colorectal CSCs, further elucidation of the nature of LGR5+ve colorectal cancer cells is needed and may reveal novel therapeutic targets.
Interestingly, it was recently reported that the tissue microarray based immunostaining of a large cohort of nearly 300 glioma cases reveals a correlation of LGR5 expression with tumor malignancy. In addition, LGR5 was described as being associated with glioblastoma prognosis.Citation64
Potential application of LGR5 as a therapeutic target
Given that CSCs often utilize developmental signaling pathways, intensive efforts are being undertaken to develop Wnt signaling inhibitors aiming at selective therapeutics targeting CSCs.Citation84,Citation85 However, the complexity of Wnt signaling has rendered this a challenging task; the Wnt pathway comprising 19 Wnt ligands, 10 frizzled transmembrane receptors, LRP regulatory co-receptors, numerous modulating molecules involved in the downstream signaling cascades, including DVL, axin, and adenomatous polyposis coli, and transcription factor complex components such as CBP (CREB binding protein) and p300.Citation86 These components are currently being investigated preclinically and, in part, clinically, as therapeutic targets.Citation84,Citation85 Targeting LGR5 may offer a further strategy to block the amplification system of the Wnt signaling pathway for the stem cell compartment. The molecular structure of LGR5 offers potential options for therapeutic intervention. In fact, the majority of therapeutic drug targets currently in clinical use are actually GPCRs.Citation87 The recently unveiled binding mode of LGR5 and R-spondin may give hints to aid the development of small molecule blockers or antagonizing antibodies to attack the specific Wnt-enhancing machinery for the stem cell compartment.Citation88,Citation89 Indeed, the study demonstrated the potential of antibody-based modulation of the Wnt-enhancing function of R-spondin and LGR5 complex.Citation20 In addition, possibilities of LGR5 targeted antibody-based therapy mediated by complement-dependent cytotoxicity in vitro, and an antitumor effect in a xenograft model has been demonstrated.Citation90
Acknowledgments
We thank Dr Josephine Bageritz and Prof Dr Peter Lichter for their critical reading and their helpful suggestions.
Disclosure
The authors declare no conflicts of interest in this work.
References
- QuintanaEShackletonMSabelMSFullenDRJohnsonTMMorrisonSJEfficient tumour formation by single human melanoma cellsNature2008456722259359819052619
- RoeschAFukunaga-KalabisMSchmidtECA temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growthCell2010141458359420478252
- ReyaTMorrisonSJClarkeMFWeissmanILStem cells, cancer, and cancer stem cellsNature2001414685910511111689955
- LaksDRVisnyeiKKornblumHIBrain tumor stem cells as therapeutic targets in models of gliomaYonsei Med J201051563364020635435
- AndersonARWeaverAMCummingsPTQuarantaVTumor morphology and phenotypic evolution driven by selective pressure from the microenvironmentCell2006127590591517129778
- GreavesMMaleyCCClonal evolution in cancerNature2012481738130631322258609
- LapidotTSirardCVormoorJA cell initiating human acute myeloid leukaemia after transplantation into SCID miceNature199436764646456487509044
- HuntlyBJGillilandDGLeukaemia stem cells and the evolution of cancer-stem-cell researchNat Rev Cancer20055431132115803157
- BonnetDDickJEHuman acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cellNat Med1997377307379212098
- WangJCLapidotTCashmanJDHigh level engraftment of NOD/SCID mice by primitive normal and leukemic hematopoietic cells from patients with chronic myeloid leukemia in chronic phaseBlood1998917240624149516140
- SirardCLapidotTVormoorJNormal and leukemic SCID-repopulating cells (SRC) coexist in the bone marrow and peripheral blood from CML patients in chronic phase, whereas leukemic SRC are detected in blast crisisBlood1996874153915488608245
- HuffCAMatsuiWMultiple myeloma cancer stem cellsJ Clin Oncol200826172895290018539970
- JonesRJGockeCDKasamonYLCirculating clonotypic B cells in classic Hodgkin lymphomaBlood2009113235920592619188663
- VisvaderJELindemanGJCancer stem cells in solid tumours: accumulating evidence and unresolved questionsNat Rev Cancer200881075576818784658
- KlonischTWiechecEHombach-KlonischSCancer stem cell markers in common cancers – therapeutic implicationsTrends Mol Med2008141045046018775674
- KimJOrkinSEmbryonic stem cell-specific signatures in cancer: insights into genomic regulatory networks and implications for medicineGenome Med20113117522126538
- DorsamRTGutkindJSG-protein-coupled receptors and cancerNat Rev Cancer200772799417251915
- HsuSYLiangSGHsuehAJCharacterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane regionMol Endocrinol19981212183018459849958
- de LauWBarkerNLowTYLGR5 homologues associate with Wnt receptors and mediate R-spondin signallingNature2011476736029329721727895
- PengWCde LauWFornerisFStructure of stem cell growth factor R-spondin 1 in complex with the ectodomain of its receptor LGR5Cell Rep2013361885189223809763
- BarkerNvan EsJHKuipersJIdentification of stem cells in small intestine and colon by marker gene LGR5Nature200744971651003100717934449
- BakerMStem cells by any other nameNature2007449716138917898732
- BarkerNHuchMKujalaPLGR5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitroCell Stem Cell201061253620085740
- JaksVBarkerNKasperMLGR5 marks cycling, yet long-lived, hair follicle stem cellsNat Genet200840111291129918849992
- BarkerNRookmaakerMBKujalaPLGR5(+ve) stem/progenitor cells contribute to nephron formation during kidney developmentCell Rep20122354055222999937
- PlaksVBrenotALawsonDALGR5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesisCell Rep201331707823352663
- SatoTVriesRGSnippertHJSingle LGR5 stem cells build crypt-villus structures in vitro without a mesenchymal nicheNature2009459724426226519329995
- SatoTvan EsJHSnippertHJPaneth cells constitute the niche for LGR5 stem cells in intestinal cryptsNature2011469733041541821113151
- MendiveFLaurentPVan SchooreGSkarnesWPochetRVassartGDefective postnatal development of the male reproductive tract in LGR4 knockout miceDev Biol2006290242143416406039
- KatoSMatsubaraMMatsuoTLeucine-rich repeat-containing G protein-coupled receptor-4 (LGR4, Gpr48) is essential for renal development in miceNephron Exp Nephrol20061042e63e7516785743
- KatoSMohriYMatsuoTEye-open at birth phenotype with reduced keratinocyte motility in LGR4 null miceFEBS Lett2007581244685469017850793
- SongHLuoJLuoWInactivation of G-protein-coupled receptor 48 (Gpr48/LGR4) impairs definitive erythropoiesis at midgestation through down-regulation of the ATF4 signaling pathwayJ Biol Chem200828352366873669718955481
- MohriYKatoSUmezawaAOkuyamaRNishimoriKImpaired hair placode formation with reduced expression of hair follicle-related genes in mice lacking LGR4Dev Dyn200823782235224218651655
- YamashitaRTakegawaYSakumotoMDefective development of the gall bladder and cystic duct in LGR4- hypomorphic miceDev Dyn20092384993100019301403
- LuoJZhouWZhouXRegulation of bone formation and remodeling by G-protein-coupled receptor 48Development2009136162747275619605502
- StyrkarsdottirUThorleifssonGSulemPNonsense mutation in the LGR4 gene is associated with several human diseases and other traitsNature2013497745051752023644456
- MoritaHMazerbourgSBouleyDMNeonatal lethality of LGR5 null mice is associated with ankyloglossia and gastrointestinal distensionMol Cell Biol200424229736974315509778
- BuczackiSJZecchiniHINicholsonAMIntestinal label-retaining cells are secretory precursors expressing LGR5Nature20134957439656923446353
- SangiorgiECapecchiMRBmi1 is expressed in vivo in intestinal stem cellsNat Genet200840791592018536716
- TianHBiehsBWarmingSA reserve stem cell population in small intestine renders LGR5-positive cells dispensableNature2011478736825525921927002
- YamamotoYSakamotoMFujiiGOverexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutationsHepatology200337352853312601349
- HuchMDorrellCBojSFIn vitro expansion of single LGR5+ liver stem cells induced by Wnt-driven regenerationNature2013494743624725023354049
- ZhuLGibsonPCurrleDSProminin 1 marks intestinal stem cells that are susceptible to neoplastic transformationNature2009457722960360719092805
- SnippertHJvan EsJHvan den BornMProminin-1/CD133 marks stem cells and early progenitors in mouse small intestineGastroenterology2009136721872194. e119324043
- ShmelkovSVButlerJMHooperATCD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumorsJ Clin Invest200811862111212018497886
- MizrakDBrittanMAlisonMCD133: molecule of the momentJ Pathol200821413918067118
- BarkerNRidgwayRAvan EsJHCrypt stem cells as the cells-of-origin of intestinal cancerNature2009457722960861119092804
- WalkerFZhangHHOdorizziABurgessAWLGR5 is a negative regulator of tumourigenicity, antagonizes Wnt signalling and regulates cell adhesion in colorectal cancer cell linesPLoS One201167e2273321829496
- AguileraCNakagawaKSanchoRChakrabortyAHendrichBBehrensAc-Jun N-terminal phosphorylation antagonises recruitment of the Mbd3/NuRD repressor complexNature2011469732923123521196933
- Al-KharusiMRSmarttHJGreenhoughALGR5 promotes survival in human colorectal adenoma cells and is upregulated by PGE2: implications for targeting adenoma stem cells with NSAIDsCarcinogenesis20133451150115723349017
- de SousaEMFColakSBuikhuisenJMethylation of cancer-stem-cell-associated Wnt target genes predicts poor prognosis in colorectal cancer patientsCell Stem Cell20119547648522056143
- SanchoRNateriASde VinuesaAGJNK signalling modulates intestinal homeostasis and tumourigenesis in miceEmbo J200928131843185419521338
- van der FlierLGvan GijnMEHatzisPTranscription factor achaete scute-like 2 controls intestinal stem cell fateCell2009136590391219269367
- TaneseKFukumaMYamadaTG-protein-coupled receptor GPR49 is up-regulated in basal cell carcinoma and promotes cell proliferation and tumor formationAm J Pathol2008173383584318688030
- MardaryevANMeierNPoterlowiczKLhx2 differentially regulates Sox9, Tcf4 and LGR5 in hair follicle stem cells to promote epidermal regeneration after injuryDevelopment2011138224843485222028024
- KasperMJaksVAreAWounding enhances epidermal tumorigenesis by recruiting hair follicle keratinocytesProc Natl Acad Sci U S A2011108104099410421321199
- da Silva-DizVSole-SanchezSValdes-GutierrezAProgeny of LGR5-expressing hair follicle stem cell contributes to papillomavirus-induced tumor development in epidermisOncogene201332323732374322945646
- QuigleyDAToMDPerez-LosadaJGenetic architecture of mouse skin inflammation and tumour susceptibilityNature2009458723750550819136944
- Van KeymeulenARochaASOussetMDistinct stem cells contribute to mammary gland development and maintenanceNature2011479737218919321983963
- de VisserKECiampricottiMMichalakEMDevelopmental stage-specific contribution of LGR5(+) cells to basal and luminal epithelial lineages in the postnatal mammary glandJ Pathol2012228330030922926799
- StinglJEirewPRicketsonIPurification and unique properties of mammary epithelial stem cellsNature2006439707999399716395311
- ShackletonMVaillantFSimpsonKJGeneration of a functional mammary gland from a single stem cellNature20064397072848816397499
- OskarssonTAcharyyaSZhangXHBreast cancer cells produce tenascin C as a metastatic niche component to colonize the lungsNat Med201117786787421706029
- NakataSCamposBBageritzJLGR5 is a marker of poor prognosis in glioblastoma and is required for survival of brain cancer stem-like cellsBrain Pathol2013231607222805276
- WangYDongJLiDLGR4 regulates mammary gland development and stem cell activity through the pluripotency transcription factor Sox2Stem Cells20133191921193123712846
- HermeyGMethnerASchallerHCHermans-BorgmeyerIIdentification of a novel seven-transmembrane receptor with homology to glycoprotein receptors and its expression in the adult and developing mouseBiochem Biophys Res Commun199925412732799920770
- ShiFKempfleJSEdgeASWnt-responsive LGR5-expressing stem cells are hair cell progenitors in the cochleaJ Neurosci201232289639964822787049
- ChaiRKuoBWangTWnt signaling induces proliferation of sensory precursors in the postnatal mouse cochleaProc Natl Acad Sci U S A2012109218167817222562792
- O’BrienCAPollettAGallingerSDickJEA human colon cancer cell capable of initiating tumour growth in immunodeficient miceNature2007445712310611017122772
- MaoXGSongSJXueXYLGR5 is a proneural factor and is regulated by OLIG2 in glioma stem-like cellsCell Mol Neurobiol201333685186523793848
- GoidtsVBageritzJPuccioLRNAi screening in glioma stem-like cells identifies PFKFB4 as a key molecule important for cancer cell survivalOncogene201231273235324322056879
- FischerUKellerALeidingerPA different view on DNA amplifications indicates frequent, highly complex, and stable amplicons on 12q13-21 in gliomaMol Cancer Res20086457658418403636
- BarretinaJTaylorBSBanerjiSSubtype-specific genomic alterations define new targets for soft-tissue sarcoma therapyNat Genet201042871572120601955
- RotSTaubertHBacheMA novel splice variant of the stem cell marker LGR5/GPR49 is correlated with the risk of tumor-related death in soft-tissue sarcoma patientsBMC Cancer20111142921978106
- ScannellCAPedersenEAMosherJTLGR5 is Expressed by Ewing sarcoma and potentiates Wnt/beta-catenin signalingFront Oncol201338123596566
- BauerLLangerRBeckerKExpression profiling of stem cell-related genes in neoadjuvant-treated gastric cancer: a NOTCH2, GSK3B and beta-catenin gene signature predicts survivalPLoS One201279e4456622970250
- WuCXieYGaoFLGR5 expression as stem cell marker in human gastric gland and its relatedness with other putative cancer stem cell markersGene20135251182523664892
- VoonDCWangHKooJKEMT-induced stemness and tumorigenicity are fueled by the EGFR/Ras pathwayPLoS One201388e7042723950932
- RyugeSSatoYJiangSXThe clinicopathological significance of LGR5 expression in lung adenocarcinomaLung Cancer201382114314823915911
- Merlos-SuarezABarrigaFMJungPThe intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapseCell Stem Cell20118551152421419747
- VermeulenLTodaroMde Sousa MelloFSingle-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacityProc Natl Acad Sci U S A200810536134271343218765800
- KanwarSSYuYNautiyalJPatelBBMajumdarAPThe Wnt/betacatenin pathway regulates growth and maintenance of colonospheresMol Cancer2010921220691072
- KemperKPrasetyantiPRDe LauWRodermondHCleversHMedemaJPMonoclonal antibodies against LGR5 identify human colorectal cancer stem cellsStem Cells201230112378238622969042
- AnastasJNMoonRTWNT signalling pathways as therapeutic targets in cancerNat Rev Cancer2013131112623258168
- TakebeNHarrisPJWarrenRQIvySPTargeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathwaysNat Rev Clin Oncol2011829710621151206
- HollandJDKlausAGarrattANBirchmeierWWnt signaling in stem and cancer stem cellsCurr Opin Cell Biol201325225426423347562
- GeorgeSRO’DowdBFLeeSPG-protein-coupled receptor oligomerization and its potential for drug discoveryNat Rev Drug Discov200211080882012360258
- SalonJALodowskiDTPalczewskiKThe significance of G protein-coupled receptor crystallography for drug discoveryPharmacol Rev201163490193721969326
- JacobsonKACostanziSNew insights for drug design from the X-ray crystallographic structures of G-protein-coupled receptorsMol Pharmacol201282336137122695719
- SasakiYKosakaHUsamiKEstablishment of a novel monoclonal antibody against LGR5Biochem Biophys Res Commun2010394349850220197059
- SchuijersJCleversHAdult mammalian stem cells: the role of Wnt, Lgr5 and R-spondinsEMBO J201231122685269622617424
