Abstract
Purpose
No lung cancer xenograft model using non-obese diabetic (NOD)-scid Il2rg−/− mice has been reported. The purpose of this study is to select a suitable mouse strain as a xenogenic host for testing tumorigenicity of lung cancer.
Materials and methods
We directly compared the susceptibility of four immunodeficient mouse strains, c-nu, C.B-17 scid, NOD-scid, and NOD/LtSz-scid Il2rg−/− (NSG) mice, for tumor formation from xenotransplanted lung cancer cell lines. Various numbers (101–105 cells/head) of two lung cancer cell lines, A549 and EBC1, were subcutaneously inoculated and tumor sizes were measured every week up to 12 weeks.
Results
When 104 EBC1 cells were inoculated, no tumor formation was observed in BALB/c-nu or C.B-17 scid mice. Tumors developed in two of the five NOD-scid mice (40%) and in all the five NSG mice (100%). When 103 EBC1 cells were injected, no tumors developed in any strain other than NSG mice, while tumorigenesis was achieved in all the five NSG mice (100%, P=0.0079) within 9 weeks. NSG mice similarly showed higher susceptibility to xenotransplantation of A549 cells. Tumor formation was observed only in NSG mice after inoculation of 103 or fewer A549 cells (40% vs 0% in 15 NSG mice compared with others, respectively, P=0.0169). We confirmed that the engrafted tumors originated from inoculated human lung cancer cells by immunohistochemical staining with human cytokeratin and vimentin.
Conclusion
NSG mice may be the most suitable strain for testing tumorigenicity of lung cancer, especially if only a few cells are available.
Introduction
The study of cancer in humans is impeded by several reasons, such as inaccessibility to the tumor sites, difficulty in the assessment of tumor biology, and ethical concerns. To overcome these restrictions, mouse models have been generated. Immunodeficient mice that cannot reject xenotransplanted cells have been shown to be the best living recipients for developing xenograft models of human cancer.Citation1 Immunodeficient mice have also been important for investigating carcinogenesis, cancer therapy, and imaging of tumor growth and metastasis.Citation2 To date, the most extensively used immunodeficient mouse strains for developing lung cancer xenograft models include nude,Citation3 scid,Citation4 and non-obese diabetic (NOD)-scidCitation5–Citation7 mice.
The interleukin-2 receptor (IL-2R) γ-chain is known as the common cytokine receptor γ-chain. The IL-2R γ-chain is a crucial component of the high-affinity receptors for IL-2, IL-4, IL-7, IL-9, and IL-15, and is required for signaling through these receptors.Citation8,Citation9 Absence of the IL-2R γ-chain in mice leads to severe impairments in T- and B-cell development and function, and completely prevents natural killer (NK) cell development.Citation9–Citation11 The immunodeficient strains of Il2rg−/− mice include NOD · Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (abbreviated as NOD/LtSz-scid Il2rg−/− and often referred to as NSG mice), NOD · Cg-Prkdcscid Il2rgtm1Sug/Jic mice (abbreviated as NOD/Shi-scid Il2rg−/− and often referred to as NOG mice), C.Cg-Rag2tm1Fwa Il2rgtm1Sug mice (abbreviated as BALB/c-Rag2−/− Il2rg−/− mice) and Stock (H2d)-Rag2tm1Fwa Il2rgtm1Krf mice (referred to as H2d-Rag2−/− Il2rg−/− mice).Citation2
NOG mice have shown superiority in cancer xenoplantation systems compared with nude, scid, and NOD-scid mice, when human cervical cancer,Citation12 pancreatic cancer,Citation13 and multiple myelomaCitation14 cell lines were used. Similarly, higher tumorigenicity in NSG mice has been reported with neuroblastomaCitation1 and melanomaCitation15 cells. Acute leukemia cells injected in NSG mice generated faster and more efficient leukemic characteristics compared with other NOD-scid-related strains.Citation16 However, no lung cancer xenograft model using NOD-scid Il2rg−/− mice (neither NSG nor NOG) has been reported. To select a suitable mouse strain as a xenogenic host for testing tumorigenicity of lung cancer, we directly compared the susceptibility of nude, scid, NOD-scid, and NSG mice for tumor formation from xenotransplanted lung cancer cell lines.
Materials and methods
Animals
NSG mice were originally generated at The Jackson Laboratory (Bar Harbor, ME, USA). BALB/c-nu, C.B-17 scid, NOD-scid, and NSG mouse strains (50 female mice in each strain) were purchased from Charles River Laboratories Japan, Inc. (Yokohama, Japan) and maintained in the Division of Animal Experiments, Life Science Research Center, Kagawa University (Kagawa, Japan), according to the Institutional Regulations for Animal Experiments. The regulations included the best considerations on animal welfare and good practice of animal handling contributing to the replacement, refinement, and reduction of animal testing (3Rs). All animals were housed in polycarbonate cages with white wood chips for bedding. Animals were given free access to drinking water, and a basal diet, Oriental MF (Oriental Yeast Co, Ltd, Tokyo, Japan), under controlled conditions of humidity (60%±10%), lighting (12-hour light/dark cycle), and temperature (24°C±2°C).
Cells
Two human lung cancer cell lines (EBC1, squamous cell carcinoma, and A549, adenocarcinoma) were obtained from the Japan Cancer Research Bank (Tokyo, Japan). Cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum and passaged on reaching 80% confluence as reported previously.Citation17
Xenotransplantation in vivo
The protocols of the animal experiments were approved by the Animal Care and Use Committee for Kagawa University. For direct comparison of susceptibility to cancer cell engraftment, various numbers of EBC1 or A549 cells (101–105 cells/head, suspended in 0.1 mL of serum-free medium) were subcutaneously inoculated into five mice of each condition in each strain when mice were aged 6 weeks. The mice were monitored daily and tumor sizes were measured every week with calipers. The tumor volume (TV) was calculated using the formula TV = 1/2×A×B2 (where A= length in millimeters and B= width in millimeters) according to the previous study.Citation12 The criteria for successive engraftment were as follows: progressive nodule growth at the site of injection and TV values exceeding 10 mm3.Citation12 Mice were monitored up to 12 weeks after inoculation, and mice that developed a successive engraftment were euthanized.
Histology and immunohistochemistry
The engrafted tumors were fixed with 10% phosphate-buffered formalin, and paraffin-embedded sections were stained using hematoxylin and eosin. Anti-human vimentin (Clone V9; #M0725; Dako, Glostrup, Denmark) and anti-human pancytokeratin (AE1/AE3; #M3515; Dako) antibodies were used for the confirmation of human cell-derived tumors. A staining without primary antibodies was also prepared as a control for nonspecific effects of primary antibodies. Secondary antibodies (I-VIEW DAB Universal Kit, #518100032) were purchased from Roche Diagnostics KK (Tokyo, Japan).
Statistical analyses
The differences in incidence of each engraftment were tested by Fisher’s direct probability method with P<0.05 as the cutoff for significance. All statistical analyses were performed using Excel Statistic 2012 (Social Survey Research Information Co, Ltd, Tokyo, Japan).
Results
Tumorigenicity of lung cancer cell lines
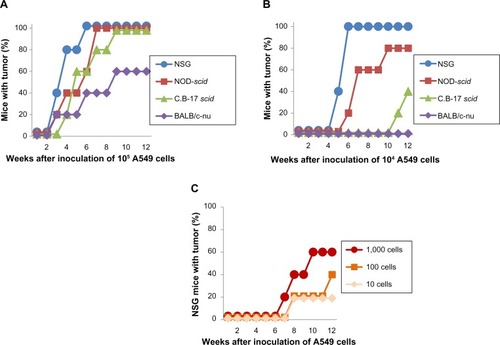
We directly compared the susceptibility of four immunodeficient mouse strains, such as nude (BALB/c-nu), scid (C.B-17 scid), NOD-scid, and NSG, to xenotransplanted human lung cancer cell lines. When 105 EBC1 cells were inoculated subcutaneously, tumors formed in all mice tested within 9 weeks after inoculation (). In NSG mice, all tumor formations had been completed in 3 weeks. When 104 EBC1 cells were inoculated, no tumor formation was observed in nude or scid mice (). Tumors developed in two of the five NOD-scid mice (40%), while tumors formed in all the five NSG mice (100%). The superiority of NSG mice to xenotransplantation was clearer after 103 cells were inoculated (, P=0.0079). No tumors developed in any strain other than NSG mice, in which 100% tumorigenesis was still observed in 9 weeks. All tumors in NSG mice grew progressively () and formed a large, hard, spheroid mass at 12 weeks (). Tumor formation developed in one of the five mice after inoculation of 102 EBC1 cells ().
Figure 1 Tumorigenicity in mice after subcutaneous inoculation of EBC1 cells.
Notes: Tumorigenicity after inoculation of 105 (A) and 104 (B) EBC1 cells. (C) Tumorigenicity in NSG mice after inoculation of 10–103 EBC1 cells. X-axis shows period after inoculation of cells (weeks). Y-axis shows mice with tumor formation (%). (D) Tumor sizes in NSG mice after inoculation of 103 EBC1 cells. Each symbol represents an individual mouse. (E) At 12 weeks after inoculation of 103 EBC1 cells, mice were euthanized. NOD-scid and NSG mice are shown.
Abbreviations: NSG, NOD/LtSz-scid Il2rg−/−; NOD, non-obese diabetic.
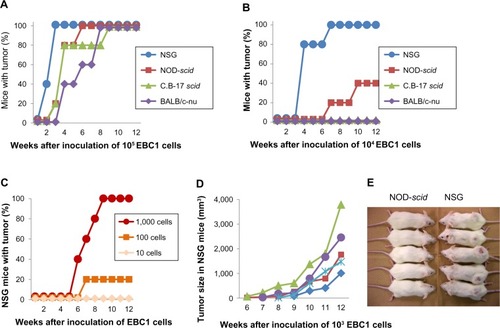
Similar to EBC1 cells, a higher xenotransplantability of A549 cells was observed in NSG mice when compared with other strains. Although there was no statistical significance in final tumor number after inoculation of 105 and 104 A549 cells (, respectively), tumorigenesis developed the fastest in NSG mice. After inoculation of 103 or fewer A549 cells, no tumor formation was observed in nude, scid, and NOD-scid mice. In NSG mice, however, tumors developed in 6 of 15 mice (40%) in conditions of less than 103 cells (), which is significantly higher when compared with other strains (P=0.0169). Unexpectedly, only ten A549 cells formed a tumor in one of the five NSG mice.
Pathology of the developed tumor
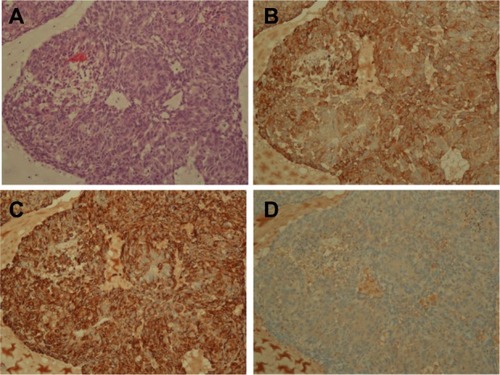
The pathologic findings of the developed tumor in the NSG mouse after inoculation of ten A549 cells showed, as expected, adenocarcinoma including glandular formation (). The tumor was positive for anti-human cytokeratin and anti-human vimentin (), suggesting that the developed tumor originated from a human source, that is, A549 cells. Tumors showed negative staining with an isotype control antibody, confirming accuracy of immunohistochemistry (). No metastatic tumor formation was observed in other organs including lungs and livers in all mice examined.
Discussion
This study demonstrates that the highest susceptibility to xenotransplantation of two lung cancer cell lines was observed in NSG mice. In NSG mice, tumorigenesis appeared faster after inoculation of lung cancer cells, even with fewer cells.
The first immunodeficient mouse model of cancer was developed in nude mice, which allowed the growth of human solid tumors, after inoculation of 1–20 million cells.Citation18 Thereafter, several kinds of immunodeficient mice have been generated with more efficient xenotransplantability. Among them, NOD-scid mice showed a greater susceptibility to lung cancer cell lines and primary cells.Citation5–Citation7 The non-small cell lung carcinoma (NSCLC) xenografts from patients undergoing surgery were implanted into NOD-scid mice within 24 hours of surgery and they were successfully engrafted in 40% of cases.Citation5 In this study, we demonstrated a dramatic improvement of xenotransplantation of fewer lung cancer cell lines in NSG compared with NOD-scid mice. When 103 or fewer cells were inoculated, tumors developed only in NSG mice and grew progressively, suggesting higher susceptibility of NSG than NOD-scid mice to xenotransplanted lung cancer cells. In addition, a subcutaneous tumor developed in one mouse that had been inoculated with only ten A549 cells.
The most commonly used procedure for diagnosing NSCLC is bronchoscopic examination,Citation19 and only 20%–25% of patients have resectable disease because of the diagnosis in an advanced stage.Citation20 However, the number of cancer cells obtained by a transbronchial biopsy (TBB) is usually quite few. Primary cell culture from patients with lung cancer has not fully been established, in particular, as the cell number is low, although many improvements in media used and culture technique have been reported for establishment of primary cell culture.Citation21 If humanized mice can be available by successful xenotransplantation of lung cancer cells obtained by TBB, it might be useful for establishment of the best therapy for individual tumor. In this regard, NSG mice could be the best candidate. In fact, human breast cancers were propagated in SCID/Beige and NSG mice models.Citation22 These models serve as a renewable, quality-controlled tissue resource for preclinical studies investigating metastasis and response to treatment.Citation22 Development of xenografts platform from patients with NSCLC for drug testing in NSG mice models is also explored.Citation23 In this study, tumorigenesis appeared faster after inoculation of lung cancer cells in NSG mice, which might also be attractive for rapid assessment of individual tumor biology.
Compared with NOD-scid mice, NSG mice lack host NK cell activity and cytokines signaling are impaired. The contribution of IL-2 is well known to induce NK cell cytotoxicity against various types of malignancy.Citation24–Citation26 NK cell cytotoxicity for lung cancer cells was markedly augmented by stimulation with IL-2.Citation27,Citation28 Compared with normal subjects, patients with lung cancer consistently had higher levels of IL-2 in their bronchoalveolar lavage fluid and this titer correlated with an increase in NK cell activity.Citation29,Citation30 A differential composition of the immune cell infiltration was also assessed in malignant and non-malignant lung tissue areas associated with NSCLC.Citation31 Interestingly, NK cells were almost absent in the malignant areas, while non-malignant counterparts were selectively populated by NK cells and those NK cells showed strong cytotoxic activity ex vivo.Citation31 The higher tumorigenicity in NSG mice observed in this study might be due to, in part, lack of IL-2-dependent NK cell cytotoxicity, although cytokines other than IL-2 might also have a role.
The relevance of mouse models would further increase when assessed in an orthotopic organ site, ie, the lungs for lung cancer models, rather than assessment of subcutaneous growth. Orthotopic models would have some advantages compared to subcutaneous models. In the previous reports, some lung cancer cells inoculated intravenously into immunodeficient mice induced multiple lung nodules and malignant pleural effusionCitation32 and murine lung cancer cells injected into the pleural space of mice developed malignant pleural effusion.Citation33 These models could make it possible to assess the therapeutic efficacy of cancer progression in a situation close to the actual target patients. On the other hand, in many previous studies, subcutaneous models have been used for assessment of lung cancer, because of some advantages in subcutaneous models. First, tumor formation is recognized clearly at a glance. Second, it is easier to confirm the course of tumor progression, without use of radiological techniques or euthanasia. Third, subcutaneous inoculation of cells is an easier experimental procedure than intravenous inoculation or injection into the pleural space. Based on these advantages, we selected subcutaneous models in this study.
Conclusion
In summary, we directly compared the ability of four immunodeficient mice to successfully engraft lung cancer cells. NSG mice were more susceptible to tumor formation than other strains including NOD-scid mice. NSG mice could be a good choice in cases of limited cell numbers such as samples obtained by TBB or identification of a weakly tumorigenic phenotype.
Acknowledgments
There was no funding source for this study. We thank Ms Takimi Tamaki for long-term assistance with the animal experiments.
Disclosure
The authors report no conflicts of interest in this work.
References
- SarteletHDurrieuLFontaineFNyalendoCHaddadEDescription of a new xenograft model of metastatic neuroblastoma using NOD/SCID/Il2rg null (NSG) miceIn Vivo2012261192922210712
- ShultzLDIshikawaFGreinerDLHumanized mice in translational biomedical researchNat Rev Immunol20077211813017259968
- WenQLMengMBYangBEndostar, a recombined humanized endostatin, enhances the radioresponse for human nasopharyngeal carcinoma and human lung adenocarcinoma xenografts in miceCancer Sci200910081510151919459845
- NavabRStrumpfDBandarchiBPrognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancerProc Natl Acad Sci U S A2011108177160716521474781
- JohnTKohlerDPintilieMThe ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage non-small cell lung cancerClin Cancer Res201117113414121081655
- DanielVCMarchionniLHiermanJSA primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitroCancer Res20096983364337319351829
- MerkJRolffJBeckerMLeschberGFichtnerIPatient-derived xenografts of non-small-cell lung cancer: a pre-clinical model to evaluate adjuvant chemotherapy?Eur J Cardiothorac Surg200936345445919502076
- SugamuraKAsaoHKondoMThe interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCIDAnnu Rev Immunol1996141792058717512
- OhboKSudaTHashiyamaMModulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chainBlood19968739569678562967
- CaoXShoresEWHu-LiJDefective lymphoid development in mice lacking expression of the common cytokine receptor gamma chainImmunity1995232232387697543
- DiSantoJPMüllerWGuy-GrandDFischerARajewskyKLymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chainProc Natl Acad Sci U S A19959223773817831294
- MachidaKSuemizuHKawaiKHigher susceptibility of NOG mice to xenotransplanted tumorsJ Toxicol Sci200934112312719182442
- SuemizuHMonnaiMOhnishiYItoMTamaokiNNakamuraMIdentification of a key molecular regulator of liver metastasis in human pancreatic carcinoma using a novel quantitative model of metastasis in NOD/SCID/gammacnull (NOG) miceInt J Oncol200731474175117786304
- MiyakawaYOhnishiYTomisawaMEstablishment of a new model of human multiple myeloma using NOD/SCID/gammac(null) (NOG) miceBiochem Biophys Res Commun2004313225826214684154
- QuintanaEShackletonMSabelMSFullenDRJohnsonTMMorrisonSJEfficient tumour formation by single human melanoma cellsNature2008456722259359819052619
- AglianoAMartin-PaduraIMancusoPHuman acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strainsInt J Cancer200812392222222718688847
- KanajiNBandohSFujitaJIshiiTIshidaTKuboACompensation of type I and type II cytokeratin pools in lung cancerLung Cancer200755329530217161499
- FoghJFoghJMOrfeoTOne hundred and twenty-seven cultured human tumor cell lines producing tumors in nude miceJ Natl Cancer Inst1977591221226327080
- JonesAMHansonIMArmstrongGRO’DriscollBRValue and accuracy of cytology in addition to histology in the diagnosis of lung cancer at flexible bronchoscopyRespir Med20019537437811392578
- DattaDLahiriBPreoperative evaluation of patients undergoing lung resection surgeryChest20031232096210312796194
- OieHKRussellEKCarneyDNGazdarAFCell culture methods for the establishment of the NCI series of lung cancer cell linesJ Cell Biochem Suppl19962424318806091
- ZhangXClaerhoutSPratAA renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft modelsCancer Res201373154885489723737486
- GandaraDRLiTLaraPNJrAlgorithm for codevelopment of new drug-predictive biomarker combinations: accounting for inter- and intrapatient tumor heterogeneityClin Lung Cancer201213532132522677432
- MetelitsaLSNaidenkoOVKantAHuman NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cellsJ Immunol200116763114312211544296
- AgahRMalloyBSherrodABeanPGirgisEMazumderATherapy of disseminated NK-resistant tumor by the synergistic effects of recombinant interleukin-2 and tumor necrosis factorJ Biol Response Mod1988721401513258905
- LalaPKElkashabMKerbelRSParharRSCure of human melanoma lung metastases in nude mice with chronic indomethacin therapy combined with multiple rounds of IL-2: characteristics of killer cells generated in situInt Immunol1990212114911582090199
- ItohKTildenABBalchCMLysis of human solid tumor cells by lymphokine-activated natural killer cellsJ Immunol198613610391039153084648
- RobinsonBWMorstynGNatural killer (NK)-resistant human lung cancer cells are lysed by recombinant interleukin-2-activated NK cellsCell Immunol198710622152223494538
- Stein-StreileinJGuffeeJRamosMPitchenikAESpontaneous lymphokine activated killer (LAK) activity in bronchoalveolar lavage cells from patients with bronchogenic carcinomaReg Immunol1989263703752485685
- PitchenikAEGuffeeJStein-StreileinJLung natural killer and interleukin-2 activity in lung cancer. A pulmonary compartment of augmented natural killer activity occurs in patients with bronchogenic carcinomaAm Rev Respir Dis19871366132713323500659
- EsendagliGBruderekKGoldmannTMalignant and non-malignant lung tissue areas are differentially populated by natural killer cells and regulatory T cells in non-small cell lung cancerLung Cancer2008591324017825949
- MatsumoriYYanoSGotoHZD6474, an inhibitor of vascular endothelial growth factor receptor tyrosine kinase, inhibits growth of experimental lung metastasis and production of malignant pleural effusions in a non-small cell lung cancer modelOncol Res2006161152616783964
- CuiRYoshiokaMTakahashiFIshidaHIwakamiSTakahashiKVinorelbine is effective for the malignant pleural effusion associated with lung cancer in miceAnticancer Res2008283A1633163918630520