Abstract
Purpose
Emphysema is an irreversible disease that is characterized by destruction of lung tissue as a result of inflammation caused by smoking. Resolvin D1 (RvD1), derived from docosahexaenoic acid, is a novel lipid that resolves inflammation. The present study tested whether RvD1 prevents smoking-induced emphysema and promotes lung tissue regeneration.
Materials and methods
C57BL/6 mice, 8 weeks of age, were randomly divided into four groups: control, RvD1 only, smoking only, and smoking with RvD1 administration. Four different protocols were used to induce emphysema and administer RvD1: mice were exposed to smoking for 4 weeks with poly(I:C) or to smoking only for 24 weeks, and RvD1 was injected within the smoking exposure period to prevent regeneration or after completion of smoking exposure to assess regeneration. The mean linear intercept and inflammation scores were measured in the lung tissue, and inflammatory cells and cytokines were measured in the bronchoalveolar lavage fluid.
Results
Measurements of mean linear intercept showed that RvD1 significantly attenuated smoking-induced lung destruction in all emphysema models. RvD1 also reduced smoking-induced inflammatory cell infiltration, which causes the structural derangements observed in emphysema. In the 4-week prevention model, RvD1 reduced the smoking-induced increase in eosinophils and interleukin-6 in the bronchoalveolar lavage fluid. In the 24-week prevention model, RvD1 also reduced the increased neutrophils and total cell counts induced by smoking.
Conclusion
RvD1 attenuated smoking-induced emphysema in vivo by reducing inflammation and promoting tissue regeneration. This result suggests that RvD1 may be useful in the prevention and treatment of emphysema.
Keywords:
Introduction
COPD is a major global health problem, with a prevalence of 5%–25% among adults worldwide.Citation1 COPD is among the top ten most common chronic health conditions and is associated with a marked restriction in daily activity and substantial utilization of health care resources.Citation2 COPD is commonly treated with bronchodilators, which have shown beneficial effects in several clinical trials;Citation3,Citation4 however, bronchodilators act on airways, not lung parenchyma, so the clinical response to treatment is the weakest, among COPD phenotypes, in emphysema, which mainly involves lung parenchyma.Citation5 To reduce hyperinflated lung, lung volume reduction therapy is currently undergoing trial; however, this approach can be applied only to a subset of patients.Citation6,Citation7 Furthermore, no treatment restores damaged lung parenchyma. Considering the paucity of treatments for emphysema, novel approaches are urgently needed.
The resolution of inflammation is now considered a dynamic program that is partially dependent on the equilibrium of leukocyte ingress and egress at inflamed sites.Citation8 Resolvin D1 (RvD1), a novel lipid derived from docosahexaenoic acid (DHEA), showed potent anti-inflammatory and proresolving properties in preclinical models of peritonitis,Citation9 colitis,Citation10 and dermal inflammation,Citation11 and recently in aspiration pneumoniaCitation12 and cigarette smoking-induced lung inflammation.Citation13
The main pathogenesis of emphysema is irreversible lung damage from chronic inflammation induced by smoking. Imbalance between protease and antiprotease is a dominant hypothesis.Citation14 Macrophages and neutrophils are main sources of proteases in lungs, and the degree of inflammation induced by these cells correlated with the severity of airflow obstruction.Citation15 According to a hypothesis from a UK study, repeated airway infection and hypersecretion of mucus are causes of COPD. In addition, respiratory tract infection is an important cause of acute exacerbation and progression of this disease.Citation16 We hypothesized that RvD1, which mediates the resolution of lung inflammation, may prevent emphysema and restore lung tissue by reducing irreversible injury. To test this hypothesis, the effect of RvD1 was assessed in a smoking-induced emphysema model.
Materials and methods
Emphysema mouse model
All the animals used in this study were specific-pathogen-free female C57BL/6 mice (Orient Bio, Seongnam, Republic of Korea) that were of age 8 weeks. All were kept on a 12-hour light and 12-hour dark cycle with free access to food and water. The lung damage from smoking and inflammation is the main pathogenesis of emphysema. Animal models of emphysema were induced by two well-established methods based on this pathogenesis. First, mice were exposed to cigarette smoke for 4 weeks with administration of 50 μg (1 μg/μL) of poly(I:C) via nasal aspiration at 3 weeks and 4 weeks (4-week model).Citation17 Second, mice were exposed to cigarette smoke for 24 weeks (24-week model). Cigarette smoke exposure was performed using 12 commercial cigarettes (8.0 mg of tar, 0.80 mg/cigarette, Eighty Eight Lights; KT&G, Daejeon, Republic of Korea) per day according to a protocol previously describedCitation18 with modification. In brief, ten to 12 mice were settled in an inhalation box (50 ×40 ×30 cm) connected to a pump and exposed for 10 minutes to mainstream cigarette smoke generated simultaneously from four cigarettes. The mice remained in the box for an additional 10 minutes after the cigarettes had burned. The box was then ventilated to remove the cigarette smoke, and the mice breathed normal room air for 5 minutes. Two additional exposures were performed in the same manner in a day. Control (CTL) or RvD1 only (RE) animals inhaled only clean room air in the cages. The Institutional Animal Care and Use Committee of the Asan Medical Center approved this study (2012-01-176), and all the experiments were performed in accordance with the committee guidelines. Animal care and treatment were guided according to the 8th edition of the Guide for the Care and Use of Laboratory Animals.
Experimental design
Four different administration protocols of RvD1 (Caymen, Ann Arbor, MI, USA; 100 ng or phosphate buffered saline [PBS] of the same volume) were performed. In the first protocol, RvD1 or PBS was injected in the tail vein 3 weeks and 4 weeks after the start of smoking exposure in the 4-week model. One day after the completion of the 4 weeks smoking exposure, mice were sacrificed (4-week prevention model; ). In the second protocol, RvD1 or PBS was injected in the tail vein 4 weeks and 5 weeks after the start of smoking exposure in the 4-week model. Two weeks after the completion of the 4-week smoking exposure, mice were sacrificed (4-week regeneration model; ). In the third protocol, RvD1 or PBS was injected at 18 weeks, 20 weeks, 22 weeks, and 24 weeks after the start of smoking exposure in the 24-week model. One day after the completion of the 24-week smoking exposure, mice were sacrificed (24-week prevention model; ). In the fourth protocol, RvD1 or PBS was injected at 0 week, 2 weeks, 4 weeks, and 6 weeks after the completion of smoking exposure in the 24-week model. Eight weeks after the completion of the 24-week smoking exposure, mice were sacrificed (24-week regeneration model; ). Each model included four groups: CTL, RE, smoking only (SM), and smoking exposure and RvD1 (SR).
Bronchoalveolar lavage
Mice were sacrificed using 40 mg/kg of Zoletil (Virbac, Carros, France) and 10 mg/kg of Rompun (Bayer AG, Leverkusen, Germany). The trachea was catheterized and perfused with two 0.6 mL and one 0.7 mL aliquot of cold saline. The cellular and liquid fractions of bronchoalveolar lavage (BAL) fluid were separated by centrifugation (2,200 rpm at 4°C for 5 minutes). The cell pellet was suspended, seeded onto a slide, and stained with Diff-Quick (Sysmex, Kobe, Japan). The BAL liquid fractions were stored in aliquots at −80°C pending cytokine measurement.
Quantification of cytokines in cell culture supernatant and BAL fluid
The levels of interleukin-6 (IL-6) in the culture supernatants and BAL fluids were measured with a commercially available ELISA kit (Merck Millipore, Billerica, MA, USA) using fluorescently labeled microsphere beads and a Luminex 100/200™ (Luminex Co., Austin, TX, USA) reader.
Histologic analysis
The left main bronchus was ligated, and the right lung was inflated with 0.5% low temperature melting agarose at a pressure of 15 cmH2O.Citation19 The lungs were then fixed with 3.5% formalin, embedded in paraffin, cut into 5 μm thick sections, and stained with hematoxylin and eosin. The mean linear intercept (MLI) was measured as described previously.Citation20 In brief, the average interalveolar septal wall distances were determined by the number of interruptions in 1 mm lines of alveolar wall. Any line that crossed a large vessel or bronchus was excluded from evaluation. Four or more lines were drawn in each field, and at least three random fields were examined per mouse. The degree of peribronchial and perivascular inflammations was scored on a subjective scale of 0 (no) to 4 (severe) in a blind manner. Each scoring was performed with the comparison of the standardized figures presenting grades. The inflammation score was defined as the sum of the peribronchial and perivascular scores (0–8).Citation21,Citation22 Histologic analyses were performed in different mice than those used for BAL.
Statistical analysis
All values were represented as mean ± standard deviation. Differences between multiple groups were analyzed by one-way analysis of variance with Tukey’s post tests or two-way analysis of variance with Bonferroni post tests. Significance was defined by a P-value of 0.05. All statistical calculations were conducted in GraphPad Prism Version 5.03 for Windows (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Inhibition of cigarette smoke induced emphysema development by RvD1
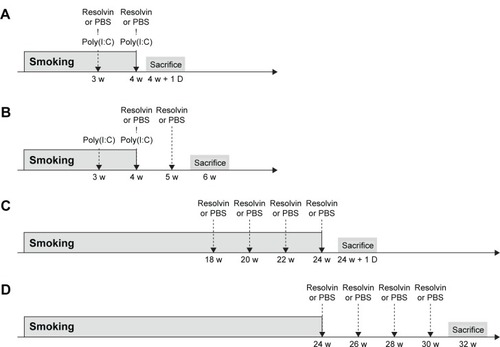
We first evaluated the effects of RvD1 using four different experimental protocols as described previously. In all the four models, cigarette smoke induced lung parenchymal destruction and airspace enlargement leading to increased MLI. RvD1 administration attenuated lung destruction significantly. In the 4-week prevention and regeneration models, the MLIs of the SM and SR groups were 50.75±4.09 μm vs 43.52±2.89 μm (P<0.05) and 49.42±2.82 μm vs 42.94±3.24 μm (P<0.01), respectively. In the 24-week prevention and regeneration models, the MLIs of the SM and SR groups were 52.46±4.17 μm vs 45.25±1.47 (P<0.01) and 49.22±4.51 μm vs 40.44±3.02 (P<0.01), respectively. The MLIs of the SR groups were not significantly different from those of the CTL groups in all of these protocols ().
Figure 2 Measurements of emphysema assessed by MLI.
Abbreviations: MLI, mean linear intercept; RvD1, resolvin D1; ANOVA, analysis of variance; CTL, control; RE, RvD1 only; SM, smoking; SR, smoking + RvD1.
Reduction of peribronchial and perivascular inflammatory cell infiltration by RvD1
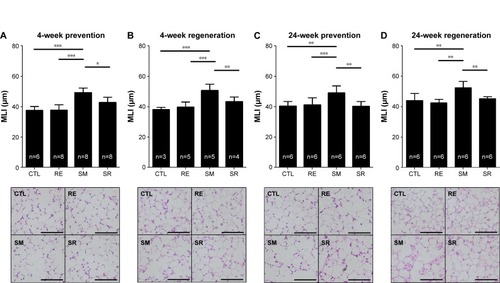
Inflammatory cell infiltration contributes to structural derangements in COPD via the release of proteases and reactive oxygen species.Citation23,Citation24 Therefore, we tested the effect of RvD1 on inflammatory cell infiltration induced by smoking. In all models, smoking caused inflammatory cell infiltration in perivascular and peribronchial areas. RvD1 inhibited inflammatory cell infiltration consistently in all the four models. Histologic score in the SR group showed a significant decrease in smoking-induced inflammatory cell infiltration. No difference in histologic scores was observed between the CTL and RE groups ().
Figure 3 Degree of inflammatory cell infiltration.
Abbreviations: ANOVA, analysis of variance; CTL, control; RE, RvD1 only; RvD1, resolvin D1; SM, smoking; SR, smoking + RvD1.
RvD1 reduced eosinophil counts and IL-6 in the BAL fluid in the 4-week prevention model
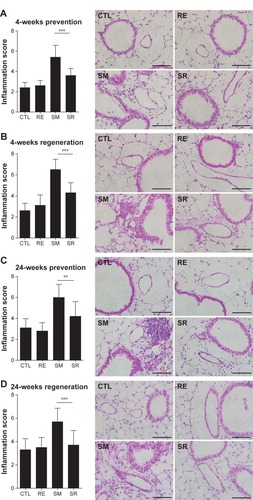
Chronic exposure to smoking significantly increased total cell counts, eosinophil counts, and neutrophil counts in the BAL fluid compared to the CTL group in the 4-week prevention model. RvD1 reduced eosinophil counts significantly in the SR group compared to the SM group (9.79±1.16 [×105]/mL vs 5.47±1.54 [×105]/mL, P<0.001). Neutrophil counts were also reduced in the SR group compared to the SM group without reaching statistical significance (2.32±1.00 [×105]/mL vs 1.63±0.80 [×105]/mL, P>0.05). No significant difference in total cell counts was observed between the SM and SR groups. In the 4-week prevention model, IL-6 was also increased in the BAL fluids of the SM group compared to those of the CTL group, and the IL-6 level was decreased significantly in the SR group compared to that in the SM group (1,008.02±247.65 pg/mL vs 598.51±161.94 pg/mL, P<0.01; ).
Figure 4 BAL fluid analysis in the 4-week prevention model.
Abbreviations: BAL, bronchoalveolar lavage; RvD1, resolvin D1; IL6, interleukin-6; ANOVA, analysis of variance; CTL, control; RE, RvD1 only; SM, smoking; SR, smoking + RvD1; Mac, macrophage; Lym, lymphocyte; Eos, eosinophil; Neu, neutrophil.
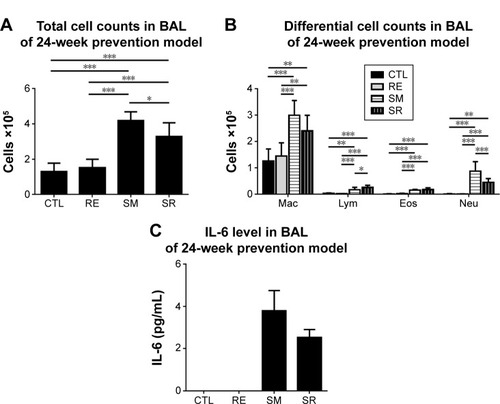
RvD1 reduced total cell counts and neutrophil counts in the BAL fluid in the 24-week prevention model
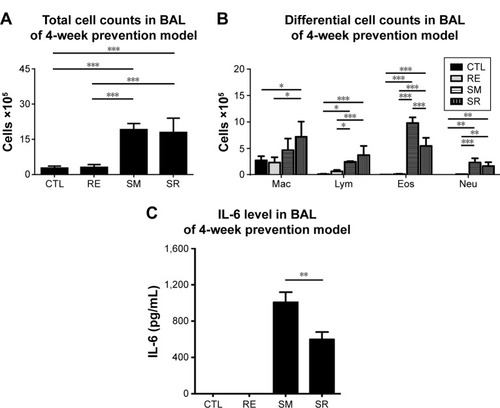
Chronic exposure to smoking also significantly increased total cell counts, eosinophil counts, and neutrophil counts in the BAL fluid compared to those in the CTL group in the 24-week prevention model. RvD1 reduced the total cell count and neutrophil count significantly in the SR group compared to those in the SM group (total cell count: 4.19±0.47 cells [×105]/mL vs 3.28±0.75 cells [×105]/mL, P<0.05; neutrophil count: 0.87±0.34 cells [×105]/mL vs 0.45±0.14 cells [×105]/mL, P<0.001). No significant difference in the eosinophil count was observed between the SM and SR groups (0.15±0.03 cells [×105]/mL vs 0.17±0.06 cells [×105]/mL, P>0.05). In the 24-week prevention model, IL-6 levels of the BAL fluid were reduced in the SR group compared to those in the SM group without reaching statistical significance (). IL-6 was not detected in the 24-week regeneration models (data not shown).
Figure 5 BAL fluid analysis in the 24 week prevention model.
Abbreviations: BAL, bronchoalveolar lavage; RvD1, resolvin D1; IL6, interleukin-6; ANOVA, analysis of variance; CTL, control; RE, RvD1 only; SM, smoking; SR, smoking + RvD1; Mac, macrophage; Lym, lymphocyte; Eos, eosinophil; Neu, neutrophil.
Discussion
This study showed that RvD1 can protect against the alveolar destruction induced by chronic exposure to smoking. RvD1 attenuated the inflammation caused by cigarette smoking. These results indicate that RvD1 can prevent smoking-induced lung damage and has possibility to regenerate lung tissue.
RvD1 is a lipid that promotes the resolution of inflammation, which is quite important to return to homeostasis. Unresolved chronic inflammation can cause tissue damage and fibrosis, followed by decreased function. RvD1 has already shown benefits in pulmonary infectious and allergic disease models by reducing inflammation. Resolvin E1 protects against acid-initiated acute lung injury and pneumonia by blocking neutrophil trafficking and proinflammatory mediator release.Citation12 RvD1 attenuates lung inflammation in lipopolysaccharide-induced acute lung injury by suppressing nuclear factor-kappa B activation.Citation25 Resolvin E1 inhibits bronchial hyperresponsiveness, mucus secretion, and eosinophil recruitment in an ovalbumin-sensitized asthma model.Citation26 A recent report showed that RvD1 resolves cigarette smoke-induced lung inflammation in the acute phase.Citation13 In human alveolar macrophages, resolvin decreased inflammatory cytokines and enhanced phagocytosis.Citation27 Considering this body of evidence, resolvin may provide therapeutic benefit in emphysema, an inflammatory disease induced by chronic exposure to smoking, and a recent study supports this.Citation28 Meanwhile, there were no reports of therapeutic resolvin administration in emphysema in human beings. The present study provides the novel evidence that the beneficial effect of resolvin on smoking-induced emphysema is consistent in various models, and it can be the basis for future human trials.
Although little is known about the role of resolvin in human beings, several indirect lines of evidence support beneficial effects in respiratory diseases. Enteric feeding of supplements enriched in omega-3 polyunsaturated fatty acids (a precursor of resolvin) improves clinical outcomes in acute respiratory distress syndrome by reducing time to liberation from mechanical ventilation and discharge from the intensive care unit.Citation29 A high intake of eicosapentaenoic acid (EPA) and DHEA was inversely correlated with the risk of COPD in smokers, suggesting a protective effect against smoking.Citation30 EPA and DHEA supplementation also significantly attenuates the allergen-induced asthmatic response.Citation31 Japanese workers, who have a higher fish consumption than their American counterparts, have a lower incidence of chronic bronchitis and a higher mean forced expiratory volume in 1 second than their American counterparts despite equivalent levels of cigarette smoking.Citation32
RvD1 attenuated the MLI increased by smoking in the 4-week and 24-week models and also decreased inflammatory cell counts in the BAL fluid; however, BAL fluids differed in their composition of inflammatory cells: differences in eosinophil counts were prominent in the 4-week models, whereas differences in neutrophil counts were observed in the 24-week models. New-onset smoking is a significant risk factor for acute eosinophilic pneumonia,Citation33 which is characterized by acute and organizing diffuse alveolar damage with eosinophil infiltration.Citation34 Acute eosinophilic pneumonia is the only well-established respiratory disease that develops in the initiation phase of smoking; therefore, eosinophil accumulation may be an important pathogenic feature of the inflammatory process in this phase. This suggests that the duration, time, and method of smoking can make a difference in alveolar cell composition. During chronic exposure to smoking, neutrophils are the main contributors to lung destruction, especially in emphysema.Citation35,Citation36 Cigarette smoke disrupts normal neutrophil apoptosis and efferocytosis. Impairment of neutrophil clearance can lead to irreversible local tissue damage via production of mediators, including neutrophil elastase and reactive oxygen species.Citation37 Considering the result of our study, resolvin can attenuate lung damage by reducing key inflammatory cells in both the initial and late phases of smoking. RvD1 also decreased the smoking-induced increase in IL-6; however, no statistical significance was reached in the 24-week model. Although smoking increases neutrophils in the BAL fluid over time,Citation38 the increase in inflammatory cytokines usually falls off after the acute phase of inflammation, which persists for several days.Citation39 The elevation in inflammatory cytokines such as IL-6 by chronic exposure to smoking does not seem high enough to reach statistical significance. Other cytokines such as interleukin-8 and interleukin-1 beta can have a role in the recruitment of neutrophils in chronic smoking. Meanwhile, the inflammation score suggested that chronic inflammatory change still persisted even after the recruitment of inflammatory cells has reduced. These histologic changes from chronic exposure to smoking might include fibrosis, bronchial wall thickening, or vascular remodeling.
To restore destroyed and injured lung is the ultimate goal of emphysema treatment, but this is not achieved by the current treatment. Pharmacologic treatment of COPD involves bronchodilators, which aim to decrease airway resistance; this treatment shows limited effectiveness against decreased elastic recoil and hyperinflation, which is the main pathogenesis of emphysema.Citation5 Smoking-related inflammation persists for several years even after smoking cessation, and COPD not only persists but may also continue to worsen.Citation40 A limited number of anti-inflammatory therapies, including a phosphodiesterase-4 inhibitor and a steroid, are available; however, long-term steroid use carries risks of adverse effects that potentially outweigh benefits,Citation41 and phosphodiesterase-4 inhibitors benefit mainly in chronic bronchitis.Citation42 In this context, our findings have implications for the treatment of emphysema and future clinical trials. Proresolving lipid mediators such as resolvin and its precursors, including EPA and DHEA, might be therapeutically useful to protect from and reduce lung injury in diseases induced by chronic smoke exposure such as COPD. Resolvin has a significant clinical potential because it promotes inflammation resolution and tissue repair without being immunosuppressive.
However, we should keep in mind that several kinds of molecules that improved emphysema in animal models failed to show efficacy in human beings. Simvastatin inhibits cigarette smoking-induced emphysema in rats,Citation22 and several retrospective studies suggested that statins could also be beneficial in human beings.Citation43,Citation44 Unfortunately, no reduction in COPD exacerbation was observed in a large randomized trial.Citation45 Several other treatments, including vitamin C,Citation46 angiotensin receptor blockade,Citation47 and stem cells,Citation48 attenuated smoke-induced emphysema in animals, but these effects have not been reproduced in human beings yet. Therefore, proresolving lipids should be tested in human trials. For a successful human study, the appropriate target population should be selected and the most effective administration protocols should be used. It is not clear whether nutritional supplementation with fish oil or omega-3 polyunsaturated fatty acids is enough or whether injections of more refined forms, such as resolvin or protectin, is necessary.
Conclusion
RvD1 attenuated smoking-induced emphysema and showed possibility to regenerate lung tissue by reducing inflammatory cells and cytokines. Although the exact mechanism should be elucidated further, these findings indicate that resolvin may play a role in the treatment of cigarette smoking-induced COPD.
Author contributions
All authors contributed toward data collection, statistical analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Acknowledgments
This study was supported by a grant (2013-553) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Republic of Korea.
Disclosure
The authors report no conflicts of interest in this work.
References
- GOLD [homepage on the Internet]Global Strategy for the Diagnosis, Management and Prevention of COPD2015 Available from: http://www.goldcopd.org/Accessed March 26, 2016
- MathersCDLoncarDProjections of global mortality and burden of disease from 2002 to 2030PLoS Med2006311e44217132052
- CalverleyPMAndersonJACelliBSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med2007356877578917314337
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med2008359151543155418836213
- LeeJHLeeYKKimEKResponses to inhaled long-acting beta-agonist and corticosteroid according to COPD subtypeRespir Med2010104454254919926461
- FishmanAMartinezFNaunheimKA randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysemaN Engl J Med2003348212059207312759479
- SciurbaFCErnstAHerthFJA randomized study of endobronchial valves for advanced emphysemaN Engl J Med2010363131233124420860505
- SerhanCNChiangNVan DykeTEResolving inflammation: dual anti-inflammatory and pro-resolution lipid mediatorsNat Rev Immunol20088534936118437155
- SunYPOhSFUddinJResolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivationJ Biol Chem2007282139323933417244615
- AritaMYoshidaMHongSResolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitisProc Natl Acad Sci U S A2005102217671767615890784
- SerhanCNResolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathwaysAnnu Rev Immunol20072510113717090225
- SekiHFukunagaKAritaMThe anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injuryJ Immunol2010184283684320007539
- HsiaoHMSapinoroREThatcherTHA novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammationPLoS One201383e5825823484005
- ShapiroSDProteolysis in the lungEur Respir J Suppl20034430s32s14582898
- SaettaMTuratoGMaestrelliPMappCEFabbriLMCellular and structural bases of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200116361304130911371392
- SethiSBacterial infection and the pathogenesis of COPDChest20001175 Suppl 1286S291S10843957
- KangMJLeeCGLeeJYCigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in miceJ Clin Invest200811882771278418654661
- HuhJWKimSYLeeJHBone marrow cells repair cigarette smoke-induced emphysema in ratsAm J Physiol Lung Cell Mol Physiol20113013L255L26621622846
- HalbowerACMasonRJAbmanSHTuderRMAgarose infiltration improves morphology of cryostat sections of lungLab Invest19947111491537518881
- ThurlbeckWMMeasurement of pulmonary emphysemaAm Rev Respir Dis19679557527645337140
- McKayALeungBPMcInnesIBThomsonNCLiewFYA novel anti-inflammatory role of simvastatin in a murine model of allergic asthmaJ Immunol200417252903290814978092
- LeeJHLeeDSKimEKSimvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungsAm J Respir Crit Care Med2005172898799316002570
- ChurgAWangRDTaiHMacrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha releaseAm J Respir Crit Care Med200316781083108912522030
- CarnevaliSPetruzzelliSLongoniBCigarette smoke extract induces oxidative stress and apoptosis in human lung fibroblastsAm J Physiol Lung Cell Mol Physiol20032846L955L96312547733
- LiaoZDongJWuWResolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARgamma/NF-kappaB pathwayRespir Res20121311023199346
- HaworthOCernadasMYangRSerhanCNLevyBDResolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammationNat Immunol20089887387918568027
- CroasdellAThatcherTHKottmannRMResolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophagesAm J Physiol Lung Cell Mol Physiol20153098L888L90126301452
- HsiaoHMThatcherTHColasRASerhanCNPhippsRPSimePJResolvin D1 reduces emphysema and chronic inflammationAm J Pathol2015185123189320126468975
- GadekJEDeMicheleSJKarlstadMDEffect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study GroupCrit Care Med19992781409142010470743
- ShaharEFolsomARMelnickSLDietary n-3 polyunsaturated fatty acids and smoking-related chronic obstructive pulmonary disease. Atherosclerosis Risk in Communities Study InvestigatorsN Engl J Med199433142282338015569
- ArmJPHortonCESpurBWMencia-HuertaJMLeeTHThe effects of dietary supplementation with fish oil lipids on the airways response to inhaled allergen in bronchial asthmaAm Rev Respir Dis19891396139514002543246
- ComstockGWStoneRWSakaiYMatsuyaTTonasciaJARespiratory findings and urban livingArch Environ Health19732731431504722870
- ShorrAFScovilleSLCersovskySBAcute eosinophilic pneumonia among US Military personnel deployed in or near IraqJAMA2004292242997300515613668
- TazelaarHDLinzLJColbyTVMyersJLLimperAHAcute eosinophilic pneumonia: histopathologic findings in nine patientsAm J Respir Crit Care Med199715512963029001328
- HoggJCChuFUtokaparchSThe nature of small-airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med2004350262645265315215480
- ShapiroSDGoldsteinNMHoughtonAMKobayashiDKKelleyDBelaaouajANeutrophil elastase contributes to cigarette smoke-induced emphysema in miceAm J Pathol200316362329233514633606
- AbboudRTVimalanathanSPathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysemaInt J Tuberc Lung Dis200812436136718371259
- D’HulstAIVermaelenKYBrusselleGGJoosGFPauwelsRATime course of cigarette smoke-induced pulmonary inflammation in miceEur Respir J200526220421316055867
- Perez-RialSdel Puerto-NevadoLTerron-ExpositoRGiron-MartinezAGonzalez-MangadoNPeces-BarbaGRole of recently migrated monocytes in cigarette smoke-induced lung inflammation in different strain of micePLoS One201389e7297524058452
- DecramerMJanssensWMiravitllesMChronic obstructive pulmonary diseaseLancet201237998231341135122314182
- JooMJAuDHFitzgibbonMLLeeTAInhaled corticosteroids and risk of pneumonia in newly diagnosed COPDRespir Med2010104224625219879745
- RennardSICalverleyPMGoehringUMBredenbrokerDMartinezFJReduction of exacerbations by the PDE4 inhibitor roflumilast – the importance of defining different subsets of patients with COPDRespir Res2011121821272339
- AlexeeffSELitonjuaAASparrowDVokonasPSSchwartzJStatin use reduces decline in lung function: VA Normative Aging StudyAm J Respir Crit Care Med2007176874274717673694
- ManciniGBEtminanMZhangBLevesqueLEFitzGeraldJMBrophyJMReduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary diseaseJ Am Coll Cardiol200647122554256016781387
- CrinerGJConnettJEAaronSDSimvastatin for the prevention of exacerbations in moderate-to-severe COPDN Engl J Med2014370232201221024836125
- KoikeKIshigamiASatoYVitamin C prevents cigarette smoke-induced pulmonary emphysema in mice and provides pulmonary restorationAm J Respir Cell Mol Biol201450234735724032444
- PodowskiMCalviCMetzgerSAngiotensin receptor blockade attenuates cigarette smoke-induced lung injury and rescues lung architecture in miceJ Clin Invest2012122122924022182843
- SchweitzerKSJohnstoneBHGarrisonJAdipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smokingAm J Respir Crit Care Med2011183221522520709815