Abstract
Introduction
Sputum eosinophilia occurs in approximately one-third of stable chronic obstructive pulmonary disease (COPD) patients and can predict exacerbation risk and response to corticosteroid treatments. Sputum induction, however, requires expertise, may not always be successful, and does not provide point-of-care results. Easily applicable diagnostic markers that can predict sputum eosinophilia in stable COPD patients have the potential to progress COPD management. This study investigated the correlation and predictive relationship between peripheral blood and sputum eosinophils. It also examined the repeatability of blood eosinophil counts.
Methods
Stable COPD patients (n=141) were classified as eosinophilic or noneosinophilic based on their sputum cell counts (≥3%), and a cross-sectional analysis was conducted comparing their demographics, clinical characteristics, and blood cell counts. Receiver operating characteristic curve analysis was used to assess the predictive ability of blood eosinophils for sputum eosinophilia. Intraclass correlation coefficient was used to examine the repeatability of blood eosinophil counts.
Results
Blood eosinophil counts were significantly higher in patients with sputum eosinophilia (n=45) compared to those without (0.3×109/L vs 0.15×109/L; P<0.0001). Blood eosinophils correlated with both the percentage (ρ=0.535; P<0.0001) and number of sputum eosinophils (ρ=0.473; P<0.0001). Absolute blood eosinophil count was predictive of sputum eosinophilia (area under the curve =0.76, 95% confidence interval [CI] =0.67–0.84; P<0.0001). At a threshold of ≥0.3×109/L (specificity =76%, sensitivity =60%, and positive likelihood ratio =2.5), peripheral blood eosinophil counts enabled identification of the presence or absence of sputum eosinophilia in 71% of the cases. A threshold of ≥0.4×109/L had similar classifying ability but better specificity (91.7%) and higher positive likelihood ratio (3.7). In contrast, ≥0.2×109/L offered a better sensitivity (91.1%) for ruling out sputum eosinophilia. There was a good agreement between two measurements of blood eosinophil count over a median of 28 days (intraclass correlation coefficient =0.8; 95% CI =0.66–0.88; P<0.0001).
Conclusion
Peripheral blood eosinophil counts can help identify the presence or absence of sputum eosinophilia in stable COPD patients with a reasonable degree of accuracy.
Introduction
Airway eosinophilia, a hallmark feature of asthma, is now a recognized inflammatory pattern in chronic obstructive pulmonary disease (COPD).Citation1–Citation3 Eosinophilic COPD, defined as sputum eosinophils ≥3%, is reported during acute exacerbations in up to 28% of cases,Citation4 and interestingly, in periods of disease stability, it is seen in approximately 34%Citation5 (or 38%Citation6) of COPD patients. Airway eosinophilia is a reliable predictor of responsiveness to inhaled and oral corticosteroid therapies in COPD.Citation6–Citation9
The detection and measurement of airway eosinophilia mostly require the assessment of induced sputum.Citation2 Although sputum induction is considered a direct and reliable method of assessing airway inflammation, it has a number of limitations.Citation10,Citation11 In addition to being unsuitable for point-of-care testing, it requires expertise and may not be always successful (failure rate of up to 30%).Citation10,Citation11 Due to these reasons, the search for minimally invasive and easily applicable diagnostic tools that can predict sputum eosinophilia in COPD and asthma has intensified.Citation4,Citation10,Citation12–Citation15 The use of peripheral blood cell counts as a potential alternative is attracting profound interest owing to its ease of application in clinical practice. The ability of blood eosinophils to predict sputum eosinophilia in patients with asthma has been reported, with promising results.Citation15–Citation18 In COPD, however, very few studies have addressed this, particularly during clinical stability. A recent report in 20 COPD patients and 21 healthy controls has demonstrated the association between bronchial and blood eosinophil counts.Citation19 Studies have also shown the potential ability of blood eosinophils to serve as a marker of response to corticosteroid treatments in exacerbatingCitation20 and stableCitation21,Citation22 COPD patients. The clinical characteristics of nonexacerbating COPD patients with persistently elevated levels of blood eosinophils (≥2%) and their longitudinal changes during a follow-up period of 3 years have also been investigated.Citation3 Nevertheless, studies examining the utility of blood eosinophils in detecting sputum eosinophilia in stable COPD are still lacking.
In this study, we hypothesized that peripheral blood eosinophils can serve as a promising surrogate marker for sputum eosinophilia in stable COPD. To test this hypothesis, a cross-sectional analytical study of 141 stable COPD patients was conducted with the aim of investigating the correlation and predictive relationship between peripheral blood and sputum eosinophils. In addition, the stability of peripheral blood eosinophil counts between two measurements over a median period of 28 days was examined.
Methods
Study design
A cross-sectional analytical study was conducted involving 141 patients with stable COPD (). The data for 71 participants were obtained from our previously published studies.Citation5,Citation23,Citation24 The remaining 70 participants were recruited from the respiratory ambulatory care clinics at John Hunter Hospital (Newcastle, Australia), the clinical research databases of the Priority Research Centre for Asthma and Respiratory Disease at the University of Newcastle and the Hunter Medical Research Institute (Newcastle, Australia), and through community advertisement. All participants provided written informed consent, and ethics approval was obtained from the Human Ethics Research Committees of the Hunter New England Local Health District (12/12/12/3.06) and the University of Newcastle (H-2013-0010).
Study participants
Adults (n=141) with stable COPD and paired blood and sputum cell counts, which were obtained from samples collected during the same visit, were included. COPD diagnosis was confirmed by incompletely reversible airflow limitation (post-bronchodilator forced expiratory volume in 1 second [FEV1] <80% predicted and FEV1 to forced vital capacity [FVC] ratio of <0.7). Stable COPD was defined as no increase in bronchodilator use, no use of oral corticosteroids or antibiotics, no unscheduled doctor’s visit, or no hospitalization due to COPD in the past 4 weeks. Participants were assessed for demographic features, lung function, airway and peripheral blood inflammatory cell counts, smoking history, body mass index, preceding year exacerbation history, medical history, dyspnea (modified Medical Research Council [mMRC]),Citation25 comorbidities (Charlson Comorbidity Index),Citation26 and health-related quality of life (St George Respiratory Questionnaire).Citation27 The BODEx (body mass index, airflow obstruction, dyspnea, severe exacerbation) index was also calculated.Citation28
Spirometry
Airflow limitation was assessed using spirometry (Medgraphics, CPFS/D™ USB Spirometer, BreezeSuite v7.1, MGC Diagnostics, Saint Paul, MN, USA) to measure pre- and postbronchodilator FEV1, FVC, and FEV1/FVC according to the standards of the American Thoracic Society.Citation29 The third National Health and Nutrition Examination Survey reference equations were used to calculate percent predicted.Citation30
Sputum induction and analysis
Sputum was induced using nebulized 4.5% saline in participants whose FEV1 was ≥1 L, using our previously described methods.Citation31 In those with FEV1 <1 L, 0.9% saline was used. Lower respiratory sputum portions were selected and dispersed using dithiothreitol, and total cell count viability was performed. Cytospins were prepared, stained (May-Grunwald–Giemsa), and a differential cell count was obtained from 400 nonsquamous cells. Sputum samples obtained from all participants had squamous cell contamination less than 80% and were deemed adequate for further analysis.Citation32 We defined eosinophilic COPD as sputum eosinophil count of ≥3%.Citation33
Blood collection and analysis
Peripheral venous blood was collected into Vacutainer® tubes (BD Worldwide, North Ryde, NSW, Australia). Full blood counts were performed using standardized methods on a Beckman Coulter LH series analyzer (Beckman Coulter Ltd, Brea, CA, USA), through Hunter Area Pathology Service (Newcastle, Australia). The stability of peripheral blood eosinophils between two measurements, approximately 28 days apart, was evaluated in 46 participants who had repeated measurements of blood cell counts.
Statistical analysis
Data were analyzed using Stata 13 (Stata Corporation, College Station, TX, USA). Results are reported as mean ± standard deviation (SD) for normally distributed data and as median and interquartile range for nonparametric data. Student’s t-test was used for comparisons of normally distributed data, and Wilcoxon rank sum test was used for skewed data. Comparison of categorical data was done using Fisher’s exact test. Spearman’s rank correlation coefficient was used to examine the association between absolute blood cell counts/ratios and sputum eosinophils. Receiver operating characteristic (ROC) curves were generated, and the area under the curve (AUC) was calculated to assess the predictive relationship between blood and sputum eosinophils. Intraclass correlation coefficient and Bland–Altman plot were used to determine the agreement between two measurements of blood eosinophil counts. All results were reported as significant when P<0.05.
Results
Clinical characteristics
presents the demographics and clinical characteristics of the 141 participants. Participants had a mean (SD) age of 69.8±7.7 years and mean postbronchodilator predicted FEV1 of 57.5%±17.9%. There were 89 (63.1%) males. Most of the participants (116, 82.3%) were ex-smokers with median pack-years of 37.5 (13.8, 62.5). In terms of GOLD (Global Initiative for Chronic Obstructive Lung Disease) grades, 11 (7.8%) patients were in GOLD I, 74 (52.5%) in GOLD II, 45 (31.9%) in GOLD III, and 11 (7.8%) in GOLD IV. With regard to the GOLD quadrants, using mMRC for symptom assessment, approximately half (53.9%) of the participants were in Quadrant D and 24.8% in Quadrant B. Over half (52.5%) were “frequent exacerbators”, having had two or more exacerbations in the past 12 months. Most (128, 90.8%) of the participants were prescribed maintenance inhaled corticosteroids (ICS) or ICS and long-acting β2 agonist (LABA) combination therapy (ICS/LABA), and of these, 102 (72.3% of the total population) participants were also taking long-acting muscarinic antagonists (LAMA).
Table 1 Demographics and clinical characteristics of the study population
Eosinophilic airway inflammation (sputum eosinophil count ≥3%) was present in 45 (31.9%) participants. Clinical characteristics were similar between those with sputum eosinophilia and those without, except for higher BODEx score (P=0.003) and more frequent high level of breathlessness (mMRC ≥2) (P=0.01) in the latter.
Peripheral blood cell counts/ratios
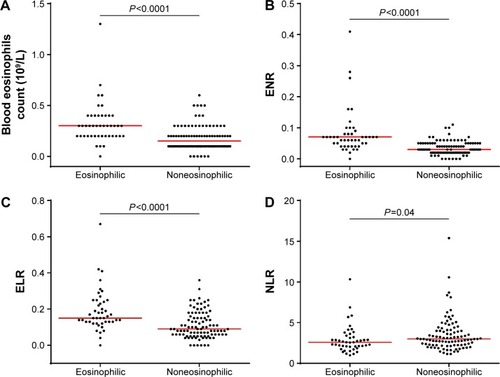
Both the absolute number and percentage proportion of blood eosinophils were significantly higher in eosinophilic COPD compared with noneosinophilic COPD (0.30×109/L vs 0.15×109/L, P<0.0001 and 3.95% vs 2.07%, P<0.0001, respectively) (, ). Noneosinophilic participants had significantly elevated blood neutrophil counts compared with the eosinophilic group (5.3×109/L vs 4.6×109/L, P=0.02). There was no difference in blood lymphocytes (P=0.84) and total white blood cell counts (P=0.32) between the two groups. Participants with sputum eosinophilia had significantly higher blood eosinophil/neutrophil ratio (ENR) and eosinophil/lymphocyte ratio (ELR), whereas those without had significantly higher neutrophil/lymphocyte ratio (, ).
Table 2 Blood cell count parameters and blood cell ratios
Figure 2 Scatter dot plot comparing.
Abbreviations: ENR, eosinophil/neutrophil ratio; ELR, eosinophil/lymphocyte ratio; NLR, neutrophil/lymphocyte ratio.
Correlation between blood and sputum eosinophils
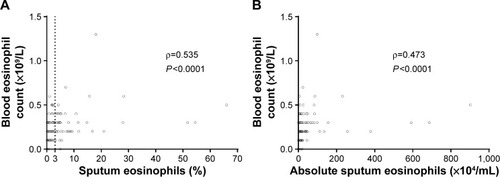
A significant correlation was found between blood eosinophil counts and the proportion (ρ=0.535; P<0.0001) () and number of sputum eosinophils (ρ=0.473; P<0.0001) (). Similarly, percentage sputum eosinophils correlated reasonably well with both blood ELR (ρ=0.488; P<0.0001) and blood ENR (ρ=0.592; P<0.0001). No significant association was observed between percentage sputum neutrophils and blood neutrophil/lymphocyte ratio (ρ=0.0287; P=0.7355).
Figure 3 Scatter plots for correlations between sputum and blood eosinophil counts.
Receiver operating characteristic (ROC) curve analysis
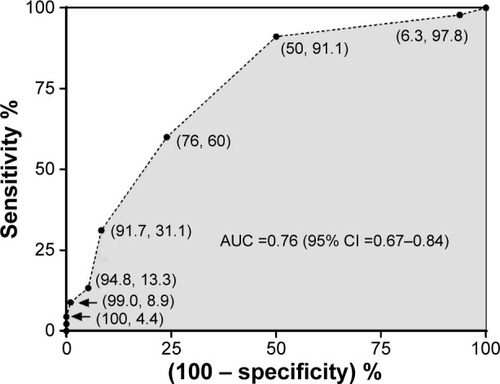
Absolute blood eosinophil count was predictive of sputum eosinophilia with AUC of 0.76 (95% confidence interval [CI] =0.67–0.84; P<0.0001) (). Percentage blood eosinophils, blood ELR, and blood ENR were also predictive of sputum eosinophilia with AUCs of 0.80 (95% CI =0.73–0.86), 0.74 (95% CI =0.65–0.83), and 0.81 (95% CI =0.73–0.89), respectively. The sensitivities and specificities of different cutoff points of absolute blood eosinophil counts were evaluated together with their ability to correctly classify patients (). A summary of sensitivity and specificity for percentage blood eosinophils and blood ENR of different cutoff points is provided in the Supplementary material (Tables S1 and S2). The absolute blood eosinophil count threshold that balanced sensitivity and specificity on the ROC curve was found to be ≥0.3×109/L (300/μL), with a sensitivity of 60%, specificity of 76%, and a positive likelihood ratio of 2.5. At this cutoff point, blood eosinophil counts enabled the correct identification of the presence or absence of sputum eosinophilia in 71 cases out of 100. A higher cutoff point of ≥0.4×109/L (400/μL) gave a greater specificity (91.7%), a higher positive likelihood ratio (3.7), and an essentially similar classifying ability. In contrast, a higher degree of sensitivity (91.1%) was achieved at a peripheral blood eosinophil cutoff point of 0.2×109/L (200/μL).
Table 3 Summary of sensitivity and specificity for different blood eosinophil cutoff points for detecting sputum eosinophilia
Figure 4 Receiver operating characteristic (ROC) curve for absolute blood eosinophil count to predict sputum eosinophilia (≥3%).
Based on the peripheral blood eosinophil threshold of ≥0.3×109/L, 76% of the noneosinophilic participants (73 out of 96) would be correctly characterized as not having sputum eosinophilia (true negatives) while the remaining 24% as false positives. On the other hand, 60% of the eosinophilic participants (27 out of 45) would be accurately identified as having sputum eosinophilia (true positives) while the remaining 40% as false negatives. Two-by-two contingency tables for the blood eosinophil cutoff points of ≥0.2×109/L and ≥0.4×109/L are provided in the Supplementary material (Tables S3 and S4).
Clinical characteristics of participants classified by blood eosinophil counts
Blood eosinophilia (blood eosinophil count ≥0.4×109/L) was present in 22 (15.6%) participants (). Patients with blood eosinophilia had a higher postbronchodilator FEV1% predicted and a lower BODEx score compared to those without (<0.4×109/L). All other clinical parameters were similar between the two groups. There were no differences in blood eosinophil counts between males and females (0.2 [0.1, 0.3] ×109/L and 0.2 [0.1, 0.3] ×109/L; P=0.9087), ex-smokers and never smokers (0.2 [0.1, 0.3] ×109/L and 0.2 [0.1, 0.3] ×109/L; P=0.8848), frequent and nonfrequent exacerbators (0.2 [0.1, 0.3] ×109/L and 0.2 [0.1, 0.3] ×109/L; P=0.96), or between patients taking ICS(/LABA) and those who did not (0.2 [0.1, 0.3] ×109/L and 0.1 [0.1, 0.2] ×109/L; P=0.221).
Table 4 Demographics and clinical characteristics of participants with and without blood eosinophilia
Stability study
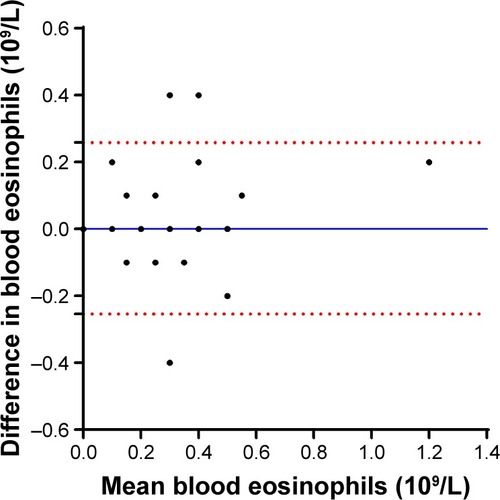
This study also assessed the stability of peripheral blood eosinophil counts in those participants who had repeated measurements of blood cell counts obtained from blood samples collected at two visits spaced a median of 28 (22.5, 35.5) days apart (n=46). There was a good agreement between the two measurements, with an intraclass correlation coefficient of 0.8 (95% CI =0.66–0.88; P<0.0001). The bias of measurement was negligible (0.002±0.13 [×109/L]), with equal scatter around the bias line, indicating no systematic measurement bias ().
Figure 5 Bland–Altman plot showing the difference between the absolute blood eosinophil counts of two measurements against the mean of the absolute blood eosinophil counts of the two measurements.
Abbreviation: SD, standard deviation.
Discussion
This study, which assessed the ability of peripheral blood eosinophils for detecting sputum eosinophilia in stable COPD, had three main findings. First, peripheral blood eosinophil count was shown to distinguish patients with sputum eosinophilia from those without, thereby indicating its potential use as a diagnostic biomarker for eosinophilic COPD. Second, we have shown that blood eosinophil counts and their ratios (ELR and ENR) are elevated in eosinophilic COPD and correlate reasonably well with sputum eosinophilia. Third, blood eosinophil counts between two measurements over a median period of 28 days were found to be stable.
The diagnostic performance of peripheral blood eosinophils in identifying eosinophilic airway inflammation in mild, moderate, and severe asthma has been previously investigated.Citation13–Citation18,Citation34 Studies examining the potential utility of blood eosinophils in COPD, particularly during stable conditions, are however few.Citation3,Citation19–Citation21 One study in COPD has shown that peripheral percentage blood eosinophil count (>2%) can serve as a sensitive biomarker to determine sputum eosinophilia (>3%) during exacerbations (AUC 0.85 [95% CI =0.78–0.93], sensitivity =90%, specificity =60%).Citation4 This AUC result is similar to our present AUC result in stable COPD.
As noted by Korevaar et al,Citation14 optimal cutoff points for diagnostic biomarkers of airway eosinophilia selected by balancing sensitivity and specificity on a ROC curve may not be clinically applicable, given that their sensitivity and/or specificity is often suboptimal compared to that of reference standard tests, such as bronchoalveolar lavage and sputum induction. From the clinical point of view, the choice of a cutoff point is also partly determined by the clinical question. In view of this, we have evaluated the sensitivity and specificity of blood eosinophil counts at different cutoff points (). According to our data, in patients with stable COPD, peripheral blood eosinophil counts can help correctly identify the presence or absence of sputum eosinophilia in 71 cases out of 100 at cutoff points of ≥0.3×109/L or ≥0.4×109/L. Nevertheless, the higher cutoff point had a higher positive predictive value (PPV) (number of true positives/[number of true positive + number of false positives]) and a much better specificity to rule in sputum eosinophilia. This implies that patients with blood eosinophil counts above the threshold of 0.4×109/L would most likely have COPD with eosinophilic airway inflammation. Akin to the suggestion of Fowler et al,Citation17 such patients may not necessarily need to undergo induction of sputum for the assessment of airway eosinophilia. Patients with blood eosinophil count of <0.4×109/L may however need further assessment because the proportion of false negatives at the cutoff point of 0.4×109/L is high (~69%). As a matter of interest, a similar blood eosinophil cutoff point of ≥0.45×109/L has been reported to help correctly identify sputum eosinophilia in patients with severe asthma with a specificity of 97%, sensitivity of 49.3%, and PPV of 89.2.Citation17 Another recent study has also reported a blood eosinophil cutoff point of ≥0.41×109/L (specificity of 95%, sensitivity of 36%, and PPV of 79) for detecting sputum eosinophilia in a population of asthmatic patients of different phenotypes.Citation15
For a clinician who wants to rule out sputum eosinophilia, on the other hand, a peripheral blood eosinophil cutoff point with a high sensitivity would be of interest. Based on our results, patients are unlikely to have sputum eosinophilia if their blood eosinophil count is below 0.2×109/L because the sensitivity at the cutoff point of 0.2×109/L is 91.1%.
It is interesting to note that in our study, there was discordance between sputum and blood eosinophils in approximately 40% of the patients with eosinophilic COPD at a peripheral blood eosinophil cutoff point of 0.3×109/L. This observation is similar to that of a recent study in patients with uncontrolled asthma where one-third of the participants exhibited discordance between blood and sputum eosinophils.Citation35 In order to gain a better insight into our true-positive (27 patients) and false-negative (18 patients) cohorts, their demographic features, clinical characteristics, and sputum cell counts were compared. The results nevertheless revealed no significant difference between the two groups (Table S5). However, it should be highlighted that this analysis may have been underpowered due to small sample size.
Plausible causes for the discordance between sputum and blood eosinophils may include the imbalance between the production and subsequent clearance of eosinophils by airway macrophagesCitation36 and variations in the process of recruitment of eosinophils into the airways.Citation35 The fact that only a proportion of the eosinophilic COPD patients had blood eosinophilia may suggest the involvement of more than one distinct biological mechanisms underlying eosinophilic airway inflammation within this COPD phenotype.Citation37 As in the case of asthma, Th2 cytokines could be responsible for inflammation in eosinophilic COPD.Citation37 Nevertheless, eosinophilic airway inflammation in the absence of elevated levels of Th2 has also been reported in some COPD patients.Citation38 One potential mechanism for the non-Th2 eosinophilic inflammation in COPD could be the epithelial-innate lymphoid cell type 2 (ILC2) pathway, which has been suggested to play a similar role in severe nonallergic asthma.Citation39 Obviously, further investigation is warranted to understand the mechanism underlying the different endotypes of the eosinophilic COPD phenotype.
Our data demonstrated that blood eosinophils and their ratios (ELR and ENR) were elevated in patients with sputum eosinophilia compared with those without. Absolute blood eosinophil counts correlated reasonably well with both the absolute sputum counts and percentage sputum eosinophils, which is in agreement with the findings of Wagener et al,Citation18 Zhang et al,Citation16 and Fowler et alCitation17 in asthma, but not with those of Hastie et alCitation34 and Amorim et al.Citation40 In accordance with a previous finding in asthma,Citation16 in our study, both blood ENR and ELR also correlated with percentage sputum eosinophils and were predictive of eosinophilic COPD with AUCs of 0.81 and 0.74, respectively. Recently, Khatry et alCitation41 have suggested that ratios of blood cell types, such as ELR and ENR, may minimize variations associated with measurement, sample processing, and therapies and yield a more accurate diagnostic performance over actual blood cell counts. This certainly will be a topical issue for future studies in COPD.
According to Price et al,Citation42 blood eosinophilia (defined as ≥0.5×109/L) occurs in 10% of stable COPD patients and is associated with higher rate of exacerbations, particularly in nonsmokers receiving maintenance therapy. Elevated levels of blood eosinophils in COPD patients have also been associated with increased risk of mortality from exacerbations.Citation43 Nevertheless, our analysis indicated no significant difference in blood eosinophil counts between frequent and nonfrequent exacerbators. Similarly, there was no difference in the number of severe exacerbations in the past 12 months between patients with blood eosinophilia (≥0.4×109/L) and those without (<0.4×109/L). It is worth noting here that up to 90% of our study population was using either ICS/LABA alone or in combination with LAMA, which are known to reduce the risk of exacerbation. All things considered, the aforementioned discrepancies may possibly be due to differences in the characteristics of study populations.
In our study, the stability of peripheral blood eosinophil counts between two measurements over a median period of 28 days was found to be acceptable. This implies that a single measurement of blood eosinophil count may provide indicative information about eosinophilic airway inflammation status in stable COPD. The stability of peripheral blood eosinophil counts in repeated measurements in COPD population has been reported in other studies as well.Citation20,Citation21
Conclusion
In this study, we found a predictive relationship between blood and sputum eosinophils in stable COPD. Peripheral blood eosinophils were stable between two measurements, suggesting that a single blood eosinophil count may potentially serve as a reliable marker for eosinophilic COPD. A potential limitation of this study may be the fact that we did not have bronchoalveolar lavage or endobronchial biopsy samples for the assessment of airway eosinophilia.
The clinical relevance of our work lies in the fact that a simple blood test may allow the clinician to positively diagnose eosinophilic COPD. In relation to this, the question of when to use ICS in COPD is of high clinical importance. Biomarkers that allow for the identification of patients who are most likely to respond to ICS, and consequently minimize harm arising from inappropriate treatment, have the potential to significantly progress COPD management. In this regard, it will be interesting and a worthwhile endeavor to examine ICS response at different blood eosinophil thresholds in future prospective studies.
Acknowledgments
This study was supported by National Health and Medical Research Council (NHMRC), Australia, Grant ID: 1045230 (PGG, VMM, JLS, PABW); Ramaciotti Foundation (VMM); Lung Foundation of Australia (VMM); National Health and Medical Research Council (NHMRC), Australia, Grant ID: 455508 (2007–2010); and Priority Research Centre for Asthma and Respiratory Diseases PhD Scholarship and Emlyn and Jennie Thomas Postgraduate Medical Research Scholarship through the Hunter Medical Research Institute (NAN). The authors wish to acknowledge Kelly Steel, Amber Smith, Hayley Lunn, Penny Baines, Gabrielle LeBrocq, Brooke Emmett, Clare Powell, and Hayley Candler for their role in data collection; Kellie Fakes, Bridgette Donati, and Michelle Gleeson for their role in sample processing; and Heather Powell for statistical advice.
Disclosure
VMM has received grants from Ramaciotti Foundation, NHMRC, Lung Foundation of Australia during the conduct of the study; personal fees and honoraria for education and advisory boards from Menarini and personal fees from GSK, Novartis, and Astra Zeneca outside this work. KJB reports research fellowship from The Thoracic Society of Australia and New Zealand/National Asthma council. PGG is supported by a NHMRC practitioner fellowship and has received speakers fees from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, and Novartis outside this work. PWJ reports being employed as a global medical expert by GSK. The authors report no other conflicts of interest in this work.
References
- SahaSBrightlingCEEosinophilic airway inflammation in COPDInt J Chron Obstruct Pulmon Dis200611394718046901
- BrightlingCEClinical applications of induced sputumChest200612951344134816685028
- SinghDKolsumUBrightlingCELocantoreNAgustiATal-SingerRfor ECLIPSE investigatorsEosinophilic inflammation in COPD: prevalence and clinical characteristicsEur Respir J20144461697170025323230
- BafadhelMMcKennaSTerrySAcute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkersAm J Respir Crit Care Med2011184666267121680942
- McDonaldVMHigginsIWoodLGGibsonPGMultidimensional assessment and tailored interventions for COPD: respiratory utopia or common sense?Thorax201368769169423503624
- LeighRPizzichiniMMMorrisMMMaltaisFHargreaveFEPizzichiniEStable COPD: predicting benefit from high-dose inhaled corticosteroid treatmentEur Respir J200627596497116446316
- BrightlingCEMonteiroWWardRSputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trialLancet200035692401480148511081531
- BrightlingCEMcKennaSHargadonBSputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary diseaseThorax200560319319815741434
- PizzichiniEPizzichiniMMGibsonPSputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitisAm J Respir Crit Care Med19981585 Pt 1151115179817701
- PavordIDBafadhelMExhaled nitric oxide and blood eosinophilia: independent markers of preventable riskJ Allergy Clin Immunol2013132482882924001802
- BainesKJPavordIDGibsonPGThe role of biomarkers in the management of airways diseaseInt J Tuberc Lung Dis201418111264126825299856
- PavordIDGibsonPGInflammometry: the current state of playThorax201267319119222344396
- YapEChuaWMJayaramLZengIVandalACGarrettJCan we predict sputum eosinophilia from clinical assessment in patients referred to an adult asthma clinic?Intern Med J2013431465221790924
- KorevaarDAWesterhofGAWangJDiagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: a systematic review and meta-analysisLancet Respir Med20153429030025801413
- WesterhofGAKorevaarDAAmelinkMBiomarkers to identify sputum eosinophilia in different adult asthma phenotypesEur Respir J201546368869626113672
- ZhangXYSimpsonJLPowellHFull blood count parameters for the detection of asthma inflammatory phenotypesClin Exp Allergy20144491137114524849076
- FowlerSJTavernierGNivenRHigh blood eosinophil counts predict sputum eosinophilia in patients with severe asthmaJ Allergy Clin Immunol20151353822824.e82225445828
- WagenerAHde NijsSBLutterRExternal validation of blood eosinophils, FE (NO) and serum periostin as surrogates for sputum eosinophils in asthmaThorax201570211512025422384
- EltboliOMistryVBarkerBBrightlingCERelationship between blood and bronchial submucosal eosinophilia and reticular basement membrane thickening in chronic obstructive pulmonary diseaseRespirology201520466767025645275
- BafadhelMMcKennaSTerrySBlood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trialAm J Respir Crit Care Med20121861485522447964
- PascoeSLocantoreNDransfieldMTBarnesNCPavordIDBlood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trialsLancet Respir Med20153643544225878028
- SiddiquiSHGuasconiAVestboJBlood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2015192452352526051430
- SimpsonJLPowellHBainesKJThe effect of azithromycin in adults with stable neutrophilic COPD: a double blind randomised, placebo controlled trialPLoS One201498e10560925148049
- McDonaldVGibsonPGScottHShould we treat obesity in COPD? The effects of diet and exercise trainingRespirology201610.1111/resp.12746
- BestallJCPaulEAGarrodRGarnhamRJonesPWWedzichaJAUsefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary diseaseThorax199954758158610377201
- CharlsonMEPompeiPAlesKLMacKenzieCRA new method of classifying prognostic comorbidity in longitudinal studies: development and validationJ Chronic Dis19874053733833558716
- JonesPWQuirkFHBaveystockCMLittlejohnsPA self-complete measure of health status for chronic airflow limitation. The St George’s respiratory questionnaireAm Rev Respir Dis19921456132113271595997
- Soler-CatalunaJJMartinez-GarciaMASanchezLSTorderaMPSanchezPRSevere exacerbations and BODE index: two independent risk factors for death in male COPD patientsRespir Med2009103569269919131231
- Standardization of Spirometry, 1994 Update. American Thoracic SocietyAm J Respir Crit Care Med19951523110711367663792
- HankinsonJLOdencrantzJRFedanKBSpirometric reference values from a sample of the general US populationAm J Respir Crit Care Med199915911791879872837
- GibsonPGWlodarczykJWHensleyMJEpidemiological association of airway inflammation with asthma symptoms and airway hyperresponsiveness in childhoodAm J Respir Crit Care Med1998158136419655704
- FahyJVBousheyHALazarusSCSafety and reproducibility of sputum induction in asthmatic subjects in a multicenter studyAm J Respir Crit Care Med200116361470147511371420
- PavordIDBrightlingCEWoltmannGWardlawAJNon-eosinophilic corticosteroid unresponsive asthmaLancet199935391712213221410392993
- HastieATMooreWCLiHBiomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjectsJ Allergy Clin Immunol20131321728023706399
- SchleichFNChevremontAPaulusVImportance of concomitant local and systemic eosinophilia in uncontrolled asthmaEur Respir J20144419710824525441
- KulkarniNSHollinsFSutcliffeAEosinophil protein in airway macrophages: a novel biomarker of eosinophilic inflammation in patients with asthmaJ Allergy Clin Immunol201012616169.e6320639010
- WoodruffPGAgustiARocheNSinghDMartinezFJCurrent concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: making progress towards personalised managementLancet201538599791789179825943943
- GhebreMABafadhelMDesaiDBiological clustering supports both “Dutch” and “British” hypotheses of asthma and chronic obstructive pulmonary diseaseJ Allergy Clin Immunol201513516372.e1025129678
- BrusselleGBrackeKTargeting immune pathways for therapy in asthma and chronic obstructive pulmonary diseaseAnn Am Thorac Soc201411Suppl 5S322S32825525740
- AmorimMMFernandesPBCaetanoLBDracoulakisSSantoroILFernandesALNasal lavage is better than blood count in predicting sputum eosinophiliaClin Exp Allergy20154551006100825675973
- KhatryDBGossageDLGebaGPDiscriminating sputum- eosinophilic asthma: accuracy of cutoffs in blood eosinophil measurements versus a composite index, ELENJ Allergy Clin Immunol20151363812814.e81225913197
- PriceDRigazioAPostmaDBlood eosinophilia and the number of exacerbations in COPD patients [abstract]ERJ201444Suppl 584416
- HospersJJSchoutenJPWeissSTRijckenBPostmaDSAsthma attacks with eosinophilia predict mortality from chronic obstructive pulmonary disease in a general population sampleAm J Respir Crit Care Med199916061869187410588599