Abstract
Background
It is generally accepted that people with chronic obstructive pulmonary disease (COPD) are at increased risk of vascular disease, including venous thromboembolism (VTE). While it is plausible that the risk of arterial and venous thrombotic events is greater still in certain subgroups of patients with COPD, such as those with more severe airflow limitation or more frequent exacerbations, these associations, in particular those between venous events and COPD severity or exacerbation frequency, remain largely untested in large population cohorts.
Methods
A total of 3,594 patients with COPD with a first VTE event recorded during January 1, 2004 to December 31, 2013, were identified from the Clinical Practice Research Datalink dataset and matched on age, sex, and general practitioner practice (1:3) to patients with COPD with no history of VTE (n=10,782). COPD severity was staged by degree of airflow limitation (ie, GOLD stage) and by COPD medication history. Frequent exacerbators were defined as patients with COPD with ≥ 2 exacerbations in the 12-month period prior to their VTE event (for cases) or their selection as a control (for controls). Conditional logistic regression was used to estimate the association between disease severity or exacerbation frequency and VTE.
Results
After additional adjustment for nonmatching confounders, including body mass index, smoking, and heart-related comorbidities, there was evidence for an association between increased disease severity and VTE when severity was measured either in terms of lung function impairment (odds ratio [OR]moderate:mild =1.16; 95% confidence intervals [CIs] =1.03, 1.32) or medication usage (ORsevere:mild/moderate =1.17; 95% CIs =1.06, 1.26). However, there was no evidence to suggest that frequent exacerbators were at greater risk of VTE compared with infrequent exacerbators (OR =1.06; 95% CIs =0.97, 1.15).
Conclusion
COPD severity defined by airflow limitation or medication usage, but not exacerbation frequency, appears to be associated with VTE events in people with COPD. This finding highlights the disconnect between disease activity and severity in COPD.
Introduction
Chronic obstructive pulmonary disease (COPD), which is currently ranked fourth in the list of causes of global mortality,Citation1 represents a considerable and potentially growing burden on health services worldwide. As it is largely a disease of middle-age, patients with COPD frequently present with other age- and smoking-related chronic diseases: comorbidities that are common in COPD thus include cardiovascular disease (CVD), diabetes, skeletal muscle weakness, lung cancer, osteoporosis, and depression. Of these, CVD is a leading cause of morbidity and mortality among patients with COPD, especially among those with mild-to-moderate disease.Citation2,Citation3
The close association between CVD and COPD has received much attention over the past 10–15 years. Although common risk factors (eg, smoking and aging) are undoubtedly important, COPD has been established as an independent risk factor for selected CVD outcomes, in particular myocardial infarction (MI), with low-grade, chronic systemic inflammation proposed as the common denominator linking the two mechanistically.Citation2,Citation4 Moreover, several researchers have suggested that the increased risk of CVD is greater still in those with more severe diseaseCitation5,Citation6 and around the time of an exacerbation, findings which are in keeping with the systemic inflammation hypothesis.Citation7
Although systemic inflammation has been identified as a pathologic feature of COPD at all stages of disease severity, experimental evidence suggests that it intensifies as lung function deteriorates and disease progresses.Citation8 Similarly, exacerbations of COPD have been associated with acutely increased periods of both lung and systemic inflammation, thereby inducing a prothrombotic state. Furthermore, patients who have more “active” disease and experience frequent exacerbations of COPD tend to have increased airway and systemic inflammation, even during periods when their disease is stable.Citation7 Collectively, these findings provide support for an increased CVD risk with increasing disease severity and a mechanism by which frequent exacerbators could have an increased risk of CVD relative to infrequent exacerbators.Citation4
Although attention has focused on arterial cardiovascular outcomes, and on MI in particular, patients with COPD are believed to also be at increased risk of a range of other vascular outcomes, including venous thromboembolism (VTE), which manifests as either deep vein thrombosis (DVT), or as pulmonary embolism (PE). Given that the risk factors for VTE are broadly similar to those for arterial thrombosis, an increased risk of VTE events among people with COPD is consistent with the aforementioned systemic inflammation mechanism. Although this association has been fairly well established in small-scale studies involving patients hospitalized with an acute exacerbation of COPD (AECOPD),Citation9,Citation10 relatively few large-scale, population based studies of this type have been undertaken. Moreover, the relationships between VTE risk and disease severity and between VTE risk and exacerbation frequency have not been described in detail in a large population cohort.Citation5,Citation6,Citation11
Using data from the UK Clinical Practice Research Datalink (CPRD), we therefore undertook a matched case–control study to investigate the profile of VTE risk in COPD, specifically to determine whether the risk of VTE disease varies according to disease severity (as measured by degree of airflow limitation or medication usage) or exacerbation frequency.
Methods
Data sources
The CPRD database contains research standard, anonymized longitudinal primary health care data for ~ 14 million patients, equating to ~79 million person-years of follow-up,Citation12 and as such is one of the world’s largest e-health datasets. Records comprise coded diagnostic and clinical information, as well as complete prescribing information and details of referrals and test results. Currently, ~5.5 million people (8% of the population) from over 660 primary care practices located throughout the UK contribute data to CPRD. Patients enrolled in CPRD have been shown to be broadly representative of the UK population in terms of age, sex, and geographical distribution.Citation12
Study population
The study population comprised active CPRD patients with a diagnosis of COPD, aged at least 35 years on the date of their diagnosis. COPD was identified in CPRD by means of a validated list of Read codes.Citation13
Case definition
Cases were all individuals in the study population who experienced a first VTE during the chosen period of study, from January 1, 2004 to December 31, 2013. Diagnostic Read codes were used to define VTE.Citation14 The primary outcome was created by grouping any event with a diagnostic Read code for DVT or PE.
Patients not registered with CPRD at the time of their first VTE event (the index date), or who had <1 year of research standard CPRD registration prior to their index date, were excluded. Individuals with a previous diagnosis of either DVT or PE were also excluded as the risk of a second or recurrent event is likely to be different from the first, as were any patients whose first VTE was given a prevalent code (). Other exclusion criteria are noted in .
Figure 1 Patient flow: selection of cases and matched controls from a source population of patients contributing data to CPRD with a diagnosis of COPD.
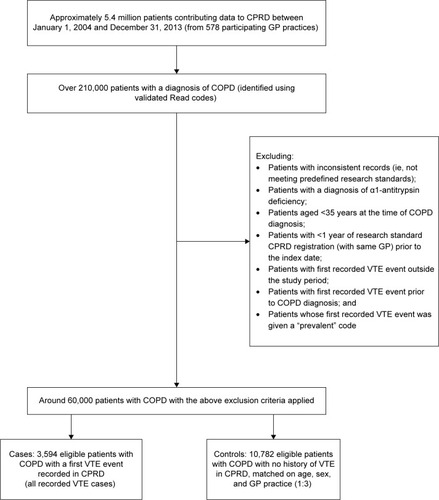
Each identified case (n=3,594) was matched to three controls (patients with COPD but without VTE); controls were selected at random from the pool of eligible patients with COPD on the date of the VTE in the case (index date). Controls were matched to cases (1:3) on the basis of their sex, index age (within 5 years), and general practitioner (GP) practice. Inclusion and exclusion criteria for the controls were the same as those for the cases. Since the sampling of controls allowed replacement, controls could later become a case and could be selected as a control for more than one case.
Exposure definitions
Two different measures of disease severity were employed, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging system (which ranks patients according to their degree of airflow limitation) and a surrogate marker based on prescribing history of COPD medications. These include short- and long-acting β2-agonists and muscarinic antagonists, as well as inhaled and oral corticosteroids. The latter measure, which also categorized patients as having mild, moderate, severe, or very severe disease, relied on the NICE treatment guidelines for COPD that recommend different combinations of medications depending on disease severity.Citation15 The derivation of the medications-based measure of disease severity from GPs’ prescribing data for individual patients is detailed in Supplementary material. Given our study objectives – to define at least one of our main exposures solely in terms of lung function impairment rather than as a composite measure which combines several aspects of disease – the classical spirometry-only GOLD staging classification was used in preference to the revised (2011) version.Citation16
Acute exacerbation events were also identified from patient records using a recently validated definition: in order to be classified as having had an AECOPD, patients had to have an exacerbation code and be prescribed a prespecified antibiotic or oral prednisolone, or both.Citation17 Individuals were then categorized as either frequent or infrequent exacerbators depending on the number of exacerbations recorded in the 12-month period prior to their index date. Those recording two or more exacerbations in the year preceding the index date were coded as frequent exacerbators and as infrequent exacerbators otherwise.Citation18
Additional information was extracted from CPRD on demographic factors (ethnicity and socioeconomic status), comorbid CVD (atrial fibrillation, heart failure, peripheral arterial disease [PAD], acute coronary syndrome [ACS], MI, and angina), risk factors (body mass index [BMI categorized according to the standard World Health Organization classification], hypertension, diabetes, dyslipidemia, family history of CVD, smoking history [current, ex-, or never]), drug prescriptions (for antiplatelets, anticoagulants, statins, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, nitrates, calcium channel blockers, and β-blockers, as well as a range of COPD medications), and coronary interventions (percutaneous coronary intervention or coronary artery bypass graft). For comorbid CVDs, their risk factors (BMI excepting) and medications, exposures were defined as binary variables. However, in order to be coded as being on a given medication, patients had to have been prescribed the same medication on at least two occasions prior to their index date.
Statistical analysis
Conditional logistic regression was used to estimate odds ratios (ORs) for the associations between aspects of COPD disease and VTE. In the first instance, regression models were constructed to explore the impact of COPD severity and various potential confounders on the odds of developing VTE. Similar analysis strategies were used to estimate the odds of experiencing a VTE event in frequent and infrequent exacerbators.
In the interests of avoiding problems of collinearity, we adjusted for the presence of selected risk factors (BMI, smoking, family history of heart disease, history of hypertension, dyslipidemia, or diabetes) and also for various common CVD comorbidities (atrial fibrillation, heart failure, PAD, angina, MI, and ACS), but not for prescribed medications for these conditions.
For the purposes of this analysis, the traditional change-in-effect method for selecting confounders was complemented with a causal inference-based approach involving the use of directed acyclic graphs.Citation19 Accordingly, the regression analyses were – in addition to the matching variables – adjusted for smoking and BMI, and also for heart failure and a composite measure representing presence of pathologically similar CVD comorbidities (categorized as no CVDs, any one of angina, MI, PAD, and ACS or two or more of angina, MI, PAD, and ACS).
All analyses were conducted using STATA, version 13 (StataCorp LP, College Station, TX, USA).
Missing data
For all variables, except GOLD stage, the proportion of missing records was <3%. A detailed evaluation of the nature of missingness in GOLD stage data was conducted, comparing patients with and without spirometry measurements in their primary health record to determine if they differed with respect to GP practice, exposure, outcome, or potential confounders and whether or not these data were missing at random. Despite some evidence that missingness (18% in cases and 14% in controls) was skewed in favor of older, nonsmoking women from practices with a lower number of patients with COPD, it was concluded that the degree and patterns of missingness were unlikely to bias our effect estimates, and therefore a complete case analysis would be appropriate.
Power calculations
Given that VTE is a relatively rare occurrence, power calculations were carried out to verify that the study would be sufficiently powered to detect a significant difference in the odds of experiencing such an event in frequent exacerbators relative to infrequent exacerbators. For the purposes of this calculation, it was assumed (on the basis of UK prevalence data) that the CPRD dataset would have 306 patients who experienced the outcome of interest (a VTE) and who would be classed as a frequent exacerbator. For a 1:1 matching of cases and controls and an α of 5% (type 1 error), the minimum and maximum detectable ORs would be 0.369 (highest OR < 1) and 2.036 (smallest OR > 1), respectively.
Ethics
Ethical approval was obtained from the LSHTM ethics committee (reference: 8140), as well as the CPRD’s Independent Scientific Advisory Committee (ISAC) (Protocol number: 14_108R). Written informed patient consent was deemed not necessary as routinely-collected anonymized electronic healthcare records were used for this study.
Results
A total of 3,594 subjects (1,870 men and 1,724 women) experienced a VTE (as either a DVT or a PE) within the study period (cases). Age at first VTE ranged from 42.6 to 96.1 years; the mean age at VTE was 74.1 years. On average, men experienced their first VTE slightly earlier than women (mean age at VTE in women, 74.8 years; mean age at VTE in men, 73.4 years).
The characteristics of 3,594 cases and 10,782 matched controls (case:control ratio, 1:3) are summarized in . In this cohort of patients with COPD, being obese and having at least one heart-related comorbidity increased the risk of a first VTE, independently of age, sex, and GP practice.
Table 1 Characteristics of cases and matched controls, and ORs for first VTE (univariate analyses adjusted for age, sex, and GP practice by matching)
Approximately half the control group (n=10,119) were classified as having “moderate” COPD (GOLD stage 2). A further 27% had “severe” disease (GOLD stage 3), 7% had “very severe” disease (GOLD stage 4), while the remaining 16% were classed as having “mild” disease (GOLD stage 1) (Table S1). These proportions are largely similar in the cases. When patients’ COPD was staged according to their medication history, a greater proportion were categorized as having severe and very severe disease (Table S2); whereas spirometry placed a third (34%) of the controls in the severe and very severe categories (GOLD stages 3+4), the medications-based measure put just over a half (54%) in these two categories. In addition, the medications-based staging ranked proportionally more women than men in the severe/very severe COPD disease categories (55.9% versus 53.3%).
Twenty-eight percent of the control group (and 29% of cases) were classed as frequent exacerbators (). Among the controls, women and ex-smokers were more likely to be frequent exacerbators. The proportion of frequent exacerbators did not appear to vary markedly across the BMI categories, but those with underlying conditions such as diabetes, heart failure, and CVDs were more likely to experience a greater number of exacerbations. Broadly, similar patterns are observed among the cases (). Interestingly, patients with hypertension appeared to suffer fewer exacerbations of their COPD symptoms than those recording a blood pressure within the normal range.
Table 2 Characteristics of frequent and infrequent exacerbators, separately by cases and controls
Among the controls classed as having “severe” and “very severe” disease (GOLD stages 3 and 4), 31.3% and 33.3% were coded as frequent exacerbators, respectively, whereas 28.5% and 27.6% of those classed as having “mild” or “moderate” disease were coded as infrequent exacerbators, respectively (). Similarly, when staged according to medication history, frequent exacerbators accounted for 37.4% of those classed as having “very severe” disease compared with 22.1% of those classed as having “mild” COPD.
Table 3 Percentage of frequent exacerbators with mild, moderate, severe, and very severe disease (based on the control group only)
The results of the regression analyses are presented in . After adjusting for BMI and smoking habits (in addition to the matching variables – age, sex, and GP practice), the odds of having a VTE event in those with GOLD stage 2 (moderate) disease were increased by 17% compared with those with GOLD stage 1 disease (mild) (OR =1.17; 95% confidence interval [CI] =1.03, 1.33). Additionally adjusting for heart-related comorbidities (CVDs and heart failure) did not materially affect the result (OR =1.16; 95% CI =1.03, 1.32). The odds of experiencing a VTE did not increase further with increasing airflow obstruction (ORGOLD3+4:GOLD1=1.16; 95% CI =1.02, 1.33).
Table 4 Summary of main results
Ignoring the fact that medication history tends to rank patients higher in terms of their disease severity than GOLD staging, the effect estimates for the alternative measure of disease severity were of the same order of magnitude (“crude” ORsevere:mild/moderate=1.16; 95% CI =1.07, 1.26). Again, the fully adjusted ORs were very similar to the “crude” estimates (ie, those adjusted for the matching variables only) (adjusted ORsevere:mild/moderate=1.17; 95% CI =1.06, 1.26). However, in contrast to GOLD staging, there is evidence of a dose–response relationship in the association between VTE risk and increasing disease severity ().
The OR for the association between exacerbation frequency and VTE, adjusted for the matching variables only, was 1.06 (95% CIs =0.97, 1.15). This result was not altered by adjusting for smoking and BMI (OR =1.06; 95% CIs =0.97, 1.15) or additionally for heart-related comorbidities (OR =1.05; 95% CIs =0.96, 1.14). There was no evidence of effect modification by either sex, age, BMI, or smoking.
Discussion
This study investigated the effect of both COPD severity and exacerbation frequency on VTE risk using a large population-based cohort of GP-diagnosed patients with COPD. Our analyses provided good evidence that VTE risk increases with worsening disease, independently of age, sex, BMI, smoking status, and selected comorbid CVDs. We would anticipate that people with more severe disease, which we defined according to airflow limitation (GOLD stage) and also by medication history, would be more susceptible to VTE, and thus our finding is not unexpected. Whether this is due to higher levels of systemic inflammation or increased immobility, or a combination of both, is not clear from these data.
As staging people according to their history of use of COPD medications captures slightly different aspects of COPD disease than the classical spirometry-based GOLD system, we would not necessarily expect to see close correspondence in terms of the magnitude of the effect estimates. However, aside from the fact that medication-based staging tends to rank patients higher in terms of their disease severity than GOLD staging, the effect estimates for our two alternative measures of disease severity were in broad agreement, strengthening our support for the hypothesis that having more severe, ie, more advanced, COPD increases risk of VTE.
Conversely, we found no evidence of an association between exacerbation frequency and VTE in either a crude matched analysis (matching on age, sex, and GP practice) or after adjusting for additional confounders, including BMI, smoking status, and selected heart-related comorbidities.
That we observe an increased VTE risk in patients with more advanced disease but not in those with a more active disease profile (frequent exacerbators) is somewhat surprising and contrary to current thinking about the mechanisms linking COPD and vascular disease risk. The period during and immediately after an exacerbation has been identified as especially high risk for acute cardiovascular events such as MI and stroke.Citation7 It follows that VTE risk may also be heightened during this time. Studies have repeatedly demonstrated that VTE prevalence, and that of PE in particular, is relatively high among those hospitalized for AECOPD, as high as one in four according to Rizkallah et al.Citation10 This observation, in combination with a plausible biological mechanism linking AECOPD (an acute inflammatory event in the lungs) with endothelial dysfunction, atherosclerotic plaque instability, and acute vascular events (both venous and arterial), has lead several authors to hypothesize that frequent exacerbators (relative to infrequent exacerbators) are at increased risk of VTE.
Our apparently counterintuitive result draws attention to a concept that in recent years has gained ground in the COPD literature, namely, the need to distinguish more clearly between the “severity” and “activity” of disease.Citation20 Several studies have demonstrated the disconnect between activity and severity in COPD: for instance, Calverley et alCitation21 showed that, on average, individuals with “moderate” disease (GOLD stage 2) had higher rates of forced expired volume in 1 second (FEV1) decline than those classed as having “severe” or “very severe” disease (GOLD stage 3+4). Thus, among the COPD population, there will be patients who have active but not necessarily severe disease and vice versa. This is reflected in our study population, as in others,Citation16,Citation18 where it is noticeable that a substantial proportion of frequent exacerbators are found across all categories of disease severity, irrespective of the method of staging disease ().
In light of our findings, we posit that disease severity – as opposed to activity – is likely to be the more important determinant and a better predictor of VTE risk in COPD. Moreover, in view of the strong evidence of associations between the cardiovascular comorbidities and disease severity, we suggest that the presence of cardiovascular comorbidities coupled with increased immobility, a common manifestation of advanced COPD and a known risk factor for VTE, are the key contributors – as opposed to frequent exacerbations – to the elevated VTE risk in COPD
Comparison with other studies
Many previous studies have tended to focus on prevalent disease rather than incidence of new cases of CVD in COPD, and by concentrating on the latter, our study offers a different perspective. In one of the first studies to investigate VTE in a large cohort of patients with COPD, Kim et alCitation6 report an increased prevalence of VTE among COPDGene study participants with GOLD stage 2–4 disease relative to those who were classed as having normal or near normal lung function. In addition, among their GOLD stage 2–4 participants, those with a higher BMI, a shorter 6-minute walk distance, congestive heart failure, PAD, or pneumothorax were more likely to experience a VTE, a finding that adds weight to our conclusion. However, as the authors themselves acknowledge, their diagnosis of VTE was based on self-report, whereas we relied on GP-recorded diagnoses. Interestingly, the number of exacerbations was greater in the group of COPDGene study participants with a self-reported history of VTE than in those without.
Schneider et alCitation5 have also conducted a comprehensive analysis of CVD outcomes in COPD, again using the UK-based CPRD dataset. PE and DVT were among the outcomes investigated, and the authors reported strong evidence of an association between COPD and the risk of developing PE but not DVT. They also investigated the effect of disease severity (as defined by treatment history and categorized as mild, moderate, and severe) on the risk of developing PE and DVT in a nested case–control analysis. Although the authors report a significant increase in PE risk across all categories of severity, especially in those in the most severe disease category (ie, on oxygen therapy), the conclusions that may be drawn were compromised by the small numbers involved (less than ten cases).
In our analysis, we found that the increase in VTE risk between patients with GOLD stage 2 versus GOLD stage 1, and GOLD stage 3+4 versus GOLD stage 1 was of the same order of magnitude (~17%), implying the existence of a threshold value, a certain degree of lung function deterioration that is associated with increased VTE risk but beyond which risk does not increase (as opposed to a dose–response relationship). Our alternative analysis, which relied on medication history to stage disease severity – as did Schneider et al’s studyCitation5 – did not replicate this finding. The reason for this difference may well be medication-related: it is plausible that some patients with COPD, especially those with more symptomatic and advanced disease are being prescribed medications (for the relief of their COPD symptoms and management of their comorbidities) that carry an inherent risk of clotting and/or arrhythmias, which may in themselves lead to a progressive increased risk of VTE. Alternatively, the difference in the VTE risk relationships might be a reflection of the disconnect between impaired lung function and symptomatic disease; medication-based measures of disease severity will tend to place those with more symptomatic disease (eg, pronounced cough and breathlessness) in progressively higher disease severity categories as their disease progresses and their symptoms worsen. These highly medicated, symptomatic individuals will probably also have multiple comorbidities and limited exercise capacity, putting them at increased risk of VTE. On the other hand, not all individuals with pronounced lung function impairment will have highly symptomatic disease (lung function and symptoms are not always highly correlated) and may not necessarily be at high risk of VTE.
Strengths and weaknesses
The main strength of this analysis is its large sample size; the use of electronic health records provides a large cohort of nationally representative patients for whom detailed, validated health data are available. The identification of patients with COPD in CPRD has been validated in a previous study,Citation13 as has AECOPD.Citation17 Coding of VTE events is also likely to be relatively free from problems caused by misclassification, nor do we have any reason to suspect that VTE events will be captured any more or less reliably among the groups of individuals defined by the main exposure categories. However, it is possible that patients with mild COPD, and who less likely to seek health care, are underrepresented in our study cohort.
The potential for residual or unmeasured confounding due to variables not included in the analysis is perhaps a more significant weakness. Matching on GP practice provides a means, at least partially, of controlling for some unmeasured and hard-to-measure risk factors, such as GP attitudes to treatment and management of COPD, socioeconomic status, and exposure to environmental factors. However, there are a number of potential confounders that we were unable to adjust for in this study. Among those of primary concern are additional risk factors for VTE, including the presence of cancer, hospitalization for various types of surgery, prescription of hormone replacement therapy, and pregnancy. Given the age of the study population (predominantly aged 60 years and over), it is unlikely that any subjects are pregnant. The number of women on hormone replacement therapy is likely to be low, but any distributional differences between cases and controls will possibly be captured by controlling for GP practice. If it can be assumed that the proportion of subjects with cancers is the same in both the cases and the controls (more likely to be true of lung cancers than other cancers given the study population all have COPD), then the presence of cancer will not confound the association. However, as this is far from certain, the ability to control for cancer would be desirable, as would be controlling for various types of surgery known to be high risk of VTE. Factors that would also merit further investigation in future studies involving this patient population include use of other medications that may potentially influence the risk of developing VTE and the role of the long-term management of atrial fibrillation.
Conclusion
The key finding to emerge from this study is that while risk of VTE in COPD increases with increasing disease severity, it does not appear to be associated with exacerbation frequency.
Disclosure
The authors report no conflicts of interest in this work.
References
- WHOHealth Statistics and Information Systems: Projection of Mortality and Causes of Death, 2015 and 2030 [webpage on the Internet]Geneva, SwitzerlandWorld Health Organization2015
- RoversiSRoversiPSpadaforaGRossiRFabbriLMCoronary artery disease concomitant with chronic obstructive pulmonary diseaseEur J Clin Invest20144419310224164255
- SinDDAnthonisenNRSorianoJBAgustiAGMortality in COPD: role of comorbiditiesEur Respir J20062861245125717138679
- ManSFVan EedenSSinDDVascular risk in chronic obstructive pulmonary disease: role of inflammation and other mediatorsCan J Cardiol201228665366122902150
- SchneiderCBothnerUJickSSMeierCRChronic obstructive pulmonary disease and the risk of cardiovascular diseasesEur J Epidemiol201025425326020191376
- KimVGoelNGangarJRisk factors for venous thromboembolism in chronic obstructive pulmonary diseaseChronic Obstr Pulm Dis (Miami)20141223924925844397
- DonaldsonGCHurstJRSmithCJHubbardRBWedzichaJAIncreased risk of myocardial infarction and stroke following exacerbation of COPDChest201013751091109720022970
- SinDDManSFWhy are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary diseaseCirculation2003107111514151912654609
- GunenHGulbasGInEYetkinOHacievliyagilSSVenous thromboemboli and exacerbations of COPDEur Respir J20103561243124819926740
- RizkallahJManSFSinDDPrevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and meta-analysisChest2009135378679318812453
- BertolettiLQuenetSMismettiPClinical presentation and outcome of venous thromboembolism in COPDEur Respir J201239486286821885395
- HerrettEGallagherAMBhaskaranKData resource profile: Clinical Practice Research Datalink (CPRD)Int J Epidemiol201544382783626050254
- QuintJKMüllerovaHDiSantostefanoRLValidation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD)BMJ Open201447e005540
- LawrensonRToddJCLeydonGMWilliamsTJFarmerRDValidation of the diagnosis of venous thromboembolism in general practice database studiesBr J Clin Pharmacol200049659159610848723
- NICEChronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care [update]London, UKNational Institute for Health and Clinical Excellence2010
- GOLDGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease [updated 2015]Global Initiative for Chronic Obstructive Lung Disease2015
- RothnieKJMüllerováHHurstJRValidation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare recordsPLoS ONE2016113e015135726959820
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- GreenlandSPearlJRobinsJMCausal diagrams for epidemiologic researchEpidemiology199910137489888278
- VestboJRennardSChronic obstructive pulmonary disease biomarker(s) for disease activity needed – urgentlyAm J Respir Crit Care Med2010182786386420884938
- CalverleyPMAndersonJACelliBSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med2007356877578917314337
