Abstract
Purpose
This study aimed to investigate whether the prevalence of postoperative pulmonary complications (PPCs) in patients with non-small-cell lung cancer (NSCLC) is even higher in the early stages of COPD than in such patients with normal lung function and to verify the usefulness of symptom- or quality of life (QoL)-based scores in predicting risk for PPCs.
Patients and methods
Patients undergoing pulmonary resection for NSCLC between July 2012 and October 2014 were prospectively enrolled. Preoperative measurements of lung function, dyspnea, and QoL, operative characteristics, PPCs, duration of postoperative hospitalization, and in-hospital mortality were assessed.
Results
Among 351 consecutive patients with NSCLC, 343 patients with forced expiratory volume in 1 second (FEV1) ≥70% of predicted value were enrolled. At least one PPC occurred in 57 (16.6%) patients. Prevalence of PPC was higher in patients with COPD (30.1%) than in those with normal spirometry (10.0%; P<0.001). However, in patients with COPD, the prevalence of PPC was not different in patients with FEV1 ≥70% compared to those with FEV1 <70% and between group A (low risk and less symptoms) and group B (low risk and more symptoms) patients with COPD, based on the new Global initiative for chronic Obstructive Lung Disease 2011 guidelines. In patients with COPD, body mass index (odds ratio [OR]: 0.80, P=0.007), carbon monoxide diffusing capacity of the lung (DLCO), % predicted value (OR: 0.97, P=0.024), and operation time (OR: 1.01, P=0.003), but not COPD assessment test or St George Respiratory Questionnaire scores, were significantly associated with PPCs.
Conclusion
Even in patients with early-stage COPD, the prevalence of PPCs is higher than in patients with NSCLC with normal spirometry. However, this rate is not different between group A and group B patients with COPD. In accordance with this, scores based on symptoms or QoL are not predictors of risk of PPCs in patients with early-stage COPD.
Introduction
Lung cancer is a common fatal disease, and <40% of all patients with lung cancer are candidates for a curative resection. Despite the improvement in surgical techniques and perioperative management, postoperative pulmonary complications (PPCs) still occur in 12%–40% of patients with lung cancer who have undergone surgical resection.Citation1–Citation6 PPC is one of the major causes of mortality following lung resection, accounting for up to 84% of all deaths. Other major significant clinical and economic impacts of PPCs include a prolonged hospital stay and the need for admission to the intensive care unit.Citation7–Citation9 Many previous studiesCitation8,Citation10–Citation22 have suggested that the patient’s health status, age, sex, body mass index (BMI), history of smoking, chronic pulmonary disease, and preoperative pulmonary function tests (PFTs) are predictors of PPC. However, they have not been adopted widely in clinical practice, because none of them has been proven to be a sufficiently reliable predictor of PPCs,Citation8,Citation10–Citation22 and further controlled trials are required before a consensus regarding these predictors can be established.
In recent years, lung cancer in never-smokers with normal lung function has increasingly been detected, owing to the routine health examination now widely performed in Korea and other countries.Citation23,Citation24 In patients who have been surgically resected, well-known risk factors, such as poor PFT or smoking status, have not been useful for predicting PPCs. It is unclear whether the prevalence of and risk factors for PPCs in patients with lung cancer are different between patients with early-stage COPD and those with a normal PFT. In addition, the difference in the prevalence between group A (low risk and less symptoms) and group B (low risk and more symptoms) patients with COPD, based on the new Global initiative for chronic Obstructive Lung Disease (GOLD) 2011 guidelines, has not been studied yet.
To address these problems, we have prospectively enrolled patients with non-small-cell lung cancer (NSCLC) undergoing curative surgery and we have compared the prevalence of and risk factors for PPCs between patients with COPD with forced expiratory volume in 1 second (FEV1) ≥70% and patients with a normal PFT. In addition, we also evaluated whether dyspnea or quality of life (QoL)-based scores, such as the COPD assessment test (CAT) or St George Respiratory Questionnaire (SGRQ) scores, play a role in the prediction of PPCs.
Patients and methods
Study design and patients
All patients undergoing thoracic surgery for lung cancer were prospectively enrolled between July 2012 and October 2014 in the Department of Thoracic and Cardiovascular Surgery at the Seoul National University Hospital (SNUH). The following patients were excluded: those without available PFT results prior to surgery, those with an FEV1 value <70% of the predicted value, those with histological diagnosis of small cell carcinoma, those with incomplete data, and those who had not given informed consent. Emergency procedures were also excluded from the analysis.
All surgical procedures were performed under general anesthesia with single lung ventilation. Patients underwent lung resection for NSCLC by either an open approach or a thoracoscopic approach. The surgical approach was decided by experienced thoracic surgeons. Postoperative pain control was achieved by continuous thoracic epidural anesthesia or systemic opioids (parenteral administration or intravenous patient-controlled administration). All patients received daily physiotherapy from the first postoperative day, comprising deep breathing exercises, incentive spirometry, supported coughing, and mobilization. The study was approved by the Institutional Review Board and Ethics Committee of SNUH (IRB number H-1308-084-514) and was conducted in compliance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
Preoperative evaluation
Preoperative evaluation for all patients included obtaining demographic information and detailed medical history, a physical examination, blood and urine examinations, a 12-lead electrocardiogram, and a chest radiograph (CXR). The five grades of the American Society Anesthesiology classification were used as a composite index of each patient’s overall health status. All participating patients completed the following questionnaires: the Modified Medical Research Council (mMRC) scale, the CAT, and the SGRQ. Preoperative PFTs with bronchodilator responses were conducted in all patients.
Definitions
COPD diagnoses were confirmed by spirometry, defined by a postbronchodilator FEV1/functional vital capacity (FVC) ratio of <70%, and early stage of COPD was defined as FEV1 ≥70% of the predicted value.Citation25
Patients with COPD were classified into the following four groups based on symptoms, airflow obstruction, and exacerbation history:Citation26 group A, low risk and low symptom burden; group B, low risk and higher symptom burden; group C, high risk and low symptom burden; group D, high risk and higher symptom burden. High risk was defined as FEV1 <50% predicted value and high exacerbation risk (≥2/year), and a higher symptom burden was defined by mMRC ≥2 or CAT ≥10. Posttuberculosis (TB) lesions on CXR were defined as follows: a previous history of anti-TB treatments, a negative acid-fast bacilli smear and mycobacterial culture for TB on the sputum sample, and the presence of scarring, fibrosis, cavitation, emphysema, or other destructive lung changes on CXR.
PPCs were defined as follows:Citation10 atelectasis requiring bronchoscopy; bacterial pneumonia (confirmed by infiltrative shadows on CXR, positive sputum culture, body temperature ≥37.5°C, and white blood cell count >10,000/µL); empyema (positive bacterial infection with pleural effusion); acute interstitial pneumonia (aggravation of dyspnea upon exertion, deterioration of arterial blood gases, and diffuse interstitial abnormalities compatible with acute interstitial pneumonia); mechanical ventilation ≥3 days; reintubation within 48 hours; tracheostomy; bronchial stump dehiscence; and persistent air leak (air leak for >5 days or patients undergoing an intervention for a large-volume air leak prior to Day 6).
Data collection
All patients were followed up after surgery and all complications occurring prior to discharge were recorded. Data collected included demographic characteristics, operative procedure and time, pathologic diagnosis, hospital length of stay (LOS), in-hospital mortality, and PPCs.
Statistical analysis
On the basis of the results of previous studies,Citation1–Citation6,Citation27 we assumed that the average rate of PPCs would be 31% in the patients with COPD and 14% in those without COPD. A study design with a 1:2 allocation of COPD group: non-COPD group, with a significance level of 0.05 and using a two-sided, two-sample t-test, with a power of 90%, would require 100 patients in the COPD group and 200 patients in the non-COPD group. Allowing for a 15% dropout rate over the study period, the total sample size required for the study is 345 individuals.
Unless otherwise specified, results are expressed as mean (standard deviation) or median (range) for continuous variables and as a percentage for categorical variables. Student’s t-test was used to compare continuous variables, and the chi-square or Fisher’s exact tests were used to compare categorical variables. The variables with P<0.01 in univariate analysis were entered into a multivariate logistic regression analysis to identify independent predictors of PPCs. The model selection for the regression analyses was based on a stepwise procedure, which alternates between dropping the least significant variable from the model and then reconsidering all potential variables for reintroduction into the model until no further variables can be added. Group analysis was also performed to investigate different risk factors according to COPD status. Unless otherwise noted, all tests were two-sided and were performed at the 0.05 significance level. Analyses were performed using SPSS 20.0 (IBM Corporation, Armonk, NY, USA).
Results
Preoperative patient characteristics
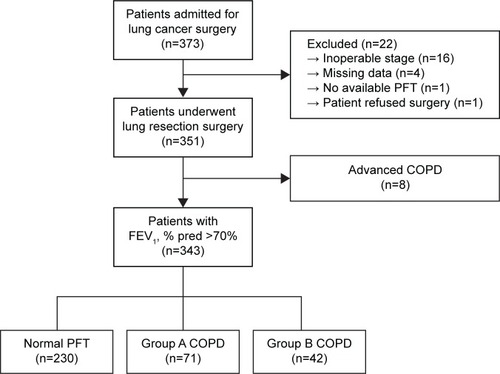
Three hundred and seventy-three patients diagnosed with NSCLC between July 2012 and October 2014 at Seoul National University Hospital, a university-affiliated tertiary care hospital in Korea, were eligible for curative resection. Thirty patients were excluded from the study due to unexpected inoperable stage, eg, only an “open and closure” operation could be performed, because of pleural nodules found during surgery (n=16); incomplete data available in questionnaires (n=4); no available preoperative PFT (n=1); refusal of operation (n=1); and FEV1 <70% predicted value (n=8) (). Baseline characteristics of the 343 patients are shown in . Of the 343 patients, 236 (68.8%) patients were male, and the median age of the cohort was 66.2 (27−84) years. The mean BMI was 23.1 (standard deviation: 3.0) kg/m2. The American Society Anesthesiology score of most (95.0%) of the patients was one or two. Among 343 patients, 113 (32.9%) patients had COPD with FEV1 ≥70%. The median duration of COPD from the diagnosis was 3.4 (1–24) months, and no patients used oral or inhaled steroids. Five patients had a short-acting beta-2-agonist inhaler on an as-needed basis. Only one patient experienced acute exacerbation of COPD ~18 months ago. They were older (P<0.001) and had a higher proportion of ever-smokers (P<0.001). However, preoperative comorbidities, including diabetes, hypertension, chronic liver disease, and chronic kidney disease, were not significantly different between patients with and those without early-stage COPD. shows the results of preoperative evaluation of lung function, dyspnea, and QoL. The percent predicted values for FEV1, FVC, FEV1/FVC, and carbon monoxide diffusing capacity of the lung (DLCO) were significantly lower in the COPD group than in the non-COPD group (P<0.05). Both mMRC and CAT scores were not significantly different between patients with and those without early-stage COPD, but patients with early-stage COPD had a higher SGRQ symptom score (P<0.001). A higher trend in activity scores and total scores of SGRQ was observed in the COPD group than in the non-COPD group, but the differences were not statistically significant.
Table 1 Demographic characteristics of study patients
Table 2 Preoperative evaluation: PFT, mMRC, CAT, and SGRQ questionnaire
Operative characteristics
Two hundred and thirty-four (68.2%) patients underwent a video-assisted thoracic surgery and 109 (31.8%) underwent thoracotomy. The proportion of patients who underwent thoracotomy was significantly higher in the COPD group than in the non-COPD group (P=0.001). The extent of lung resections was as follows: wedge resections or segmentectomies (6.7%), lobectomies or bilobectomies (88.0%), and pneumonectomies (5.3%). These were not different between the COPD and non-COPD groups. The median operative time was 170.7 (53–410) minutes, and patients with COPD had significantly longer operative times (P<0.001). Adenocarcinoma was the most common histological type of cancer (62.4%), followed by squamous cell carcinoma (32.4%). As expected, adenocarcinoma was the most frequent histological type in the non-COPD group, while squamous cell carcinoma was more frequent than adenocarcinoma in the COPD group. The pathological stage in more than half of the patients was stage I, and this was not different between the COPD and non-COPD groups (all patients: stage I: 56.3%; stage II: 19.5%; stage III: 22.7%; stage IV: 1.5%).
COPD grades and groups
Among the 113 patients with early-stage COPD, 87 (77.0%) patients had a mild grade and the other 26 (23.0%) patients had a moderate grade of COPD. The patient distribution according to the newly defined COPD groups revealed that 71 (62.8%) patients belonged to group A and 42 (37.2%) patients to group B. None of the patients in this study belonged to group C or D. The demographic, preoperative, and operative characteristics were similar in patients with mild and those with moderate COPD grades, except for the PFT values (FEV1, % pred, FVC, % pred, FEV1/FVC (%), all P<0.001) and the proportion of patients with mMRC scale ≥2 (P=0.018). In the COPD groups, differences were noted only in questionnaires, such as the mMRC scale, CAT score, and SGRQ score (all P<0.001; ).
Postoperative outcomes
The postoperative outcomes are demonstrated in . PPCs occurred in 57 (16.6%) patients. Persistent air leak was the most frequent PPC, followed by bacterial pneumonia and atelectasis requiring bronchial toileting by bronchoscopy. PPCs were more commonly seen in patients with early-stage COPD (34 patients, 30.1%) than in those without COPD (23 patients, 10.0%; P<0.001). In contrast, the prevalence of PPCs was not different between patients with FEV1 ≥70% and those with FEV1 <70% or between group A patients and group B patients. Among the 34 patients with COPD with PPCs, six (17.6%) patients had moderate COPD grades and 17 (50.0%) patients were included in COPD group B. The degree of airflow limitation in patients with COPD was not significantly associated with PPCs (P=0.374), but the COPD group tended to have more PPCs, although this did not reach statistical significance (P=0.064). Although PPCs were prevalent in patients with early-stage COPD than in those with a normal PFT, hospital LOS, and in-hospital mortality were not significantly different between these groups. In contrast, LOS was significantly longer in patients with PPCs (median: 14.1 days) than in patients without PPCs (median: 7.3 days; P<0.001).
Table 3 Postoperative outcomes
Risk factors for PPCs
We evaluated the variables that were significantly associated with PPCs in univariate analyses (P<0.05). Although some of these variables are clinically related to each other (eg, smoking, COPD, and post-TB lesions on CXR), none were sufficiently collinear by Spearman correlation coefficients to exclude as candidates for multivariable analysis. Multivariate logistic regression analysis was used to identify preoperative variables independently associated with PPCs (). Eligible variables were limited to those significantly associated with PPCs at P≤0.01. The perioperative variables significantly independently associated with PPCs included the following: COPD (odds ratio [OR]: 3.42; 95% CI: 1.775–6.596; P<0.001); post-TB lesion on CXR (OR: 2.73; 95% CI: 1.178–6.345; P=0.019); BMI (OR: 0.77; 95% CI: 0.688–0.870; P<0.001), DLCO, % pred (OR: 0.97; 95% CI: 0.957–0.991; P=0.003), and operative time (OR: 1.01; 95% CI: 1.001–1.012; P=0.004).
Table 4 Multivariate analysis of risk factors for PPCs
Then, we evaluated risk factors for PPCs in patients with or without COPD, separately. In the COPD group, BMI (P=0.016), COPD subgroup (P=0.064), DLCO, % pred (P=0.064), CAT score (P=0.059), SGRQ total score ≥25 (P=0.086), and operation times (P=0.002) were associated with PPCs in univariate analyses (Table S1). Upon multivariate analysis, BMI (OR: 0.80; 95% CI: 0.681–0.941; P=0.007), DLCO, % pred (OR: 0.97; 95% CI: 0.944–0.996; P=0.024), and operation time (OR: 1.01; 95% CI: 1.004–1.018; P=0.003), but not COPD group, remained significant predictors of PPCs for patients with COPD (). In patients without COPD, male sex (P=0.030), BMI (P<0.001), post-TB lesion on CXR (P=0.003), SGRQ symptom (P=0.004), activity (P=0.034), impact (P=0.053), total score (P=0.010), and SGRQ total score ≥25 (P=0.008) were associated with higher PPC rates in univariate analysis (Table S1). On multivariate analysis, post-TB lesion (OR: 4.43; 95% CI: 1.248–15.690; P=0.021), male sex (OR: 3.39; 95% CI: 1.059–10.858; P=0.040), BMI (OR: 0.71; 95% CI: 0.589–0.851; P<0.001), and DLCO, % pred (OR: 0.97; 95% CI: 0.943–0.990; P=0.005) were independent predictors of PPCs after lung resection in patients without COPD ().
Discussion
These days, routine health examinations are undertaken widely, and this has resulted in identification of increased proportions of patients with lung cancer with relatively preserved lung function. In this study, 343 patients undergoing thoracic surgery for lung cancer were prospectively enrolled over a period of 28 months. Most of them had normal or slightly compromised lung function, and only 26 (7.6%) patients had a moderate degree of airflow obstruction. The eight patients with FEV1 <70% of predicted value who were excluded also had a moderate degree of airflow limitation; no patients with severe or very severe airflow obstruction underwent thoracic surgery during the study period. This trend was parallel to our aim to find new risk factors for PPCs in patients with relatively preserved lung function.
Previously published studiesCitation8,Citation28–Citation31 have shown COPD to be a well-known risk factor for PPCs, probably because of impairment of gas exchange and mucociliary clearance of aspirated bacteria. However, no prior studies have been conducted in patients with relatively preserved lung function. Therefore, we evaluated whether the prevalence of and risk factors for PPCs were different between patients with early COPD with FEV1 ≥70% and those with a normal PFT. Compared to patients without COPD, patients with early COPD with FEV1 ≥70% had a higher rate of PPCs (30.1% vs 10.0%, P<0.001). The prevalence of PPCs according to the GOLD criteria for classification of COPD has not been evaluated. Among patients with early COPD, PPCs were more commonly seen in patients belonging to group B than in those belonging to group A (40.5% vs 23.9%, P=0.064). Bacterial pneumonia occurred more frequently in group B than in group A patients (23.8% vs 9.9%, P=0.045). However, neither COPD grades nor the degrees of airflow obstruction were significantly associated with PPCs (P=0.374). Therefore, we evaluated the utility of symptom-based scoring systems, such as mMRC, CAT, or SGRQ, in the prediction of PPCs. The proportion of patients with CAT score ≥10 or SGRQ total score ≥25 was higher in patients with PPCs (P=0.059 and P=0.086, respectively), and COPD group B patients had a higher PPC rate than group A patients (P=0.064), which indicates the importance of the preoperative symptom burden. However, these were not significant in multiple regression analyses. There are several potential explanations for this finding. Our study population included a high proportion of patients with asymptomatic lung cancer. They were diagnosed because of a solitary pulmonary nodule on routine CXR screening, and COPD was diagnosed in the process of the lung cancer workup. Furthermore, these patients were typically functionally independent and had fewer comorbidities.
Our study demonstrated four independent perioperative risk factors for PPCs after lung resection surgery, other than COPD: old TB lesions on CXR, low DLCO, low BMI, and longer operation times. Low BMI and DLCO were common risk factors for PPCs in both the COPD group and the non-COPD group. In this study, preoperative values and predicted values of FEV1, FEV1/FVC, and DLCO were all significantly lower in the PPC group (P<0.05). However, once COPD is taken into account, those PFT values were no longer independent factors for predicting PPCs, except DLCO. Patients with preoperative FEV1 and DLCO <60% predicted value have been shown to have increased risk of postoperative complications in previous studies.Citation32,Citation33 This study revealed that DLCO was the most useful spirometric parameter for assessing the risk of PPCs even in patients with FEV1 and DLCO >70%. A lower BMI was also significantly associated with higher rates of PPCs in both groups of patients. A number of studies have shown that a low BMI is associated with a poor prognosis in patients with COPD.Citation34–Citation37 However, this study revealed that low BMI was an independent risk factor for PPCs even in patients without COPD. Lower BMI is often associated with protein depletion, which in turn is associated with impairment of respiratory muscle strength, reduction in diaphragmatic muscular mass, and maximum voluntary ventilation, predisposing the patient to more PPCs.Citation38,Citation39
Longer operation time was a specific risk factor for patients with COPD, whereas post-TB lesions on CXR and male sex were important factors that increased the risk of PPCs in patients without COPD. Licker et alCitation40 showed that prolonged surgery was independently associated with an increased risk of postoperative complications. Surgery time can be influenced by the patient’s status, the complexity of the surgery, and the surgeon’s skill level. In addition, patients with severe adhesions would require longer operating time.Citation41 We also found that the duration of surgery was another independent significant risk factor for complications, particularly in patients with COPD. Therefore, we suggest that lung resection for patients with COPD should be performed by an experienced surgical team and that the surgery duration should be limited to the shortest possible time. Post-TB lesions on CXR were found to be an important risk factor for PPCs in patients without COPD. We attempted to restrict smoking status when defining post-TB lesions, because a smoking history could potentially have biased the estimated effect of TB on loss of lung function and on PPCs. Even after adjustment for both smoking status and pack-years, post-TB lesions were still significantly associated with increased risk of PPCs (P=0.002). This is the first prospective study to show that post-TB lesions on CXR is an independent risk factor for PPCs. Previously, Lawrence et alCitation12 found that abnormal CXR was associated with a three-fold increase in PPCs compared with the occurrence in the absence of this finding. However, abnormal CXR could be nonspecific, because it is affected by various cardiopulmonary diseases.Citation12 Recently, a cohort study, based on a nationwide representative sampling of Korean subjects, reported that previous TB lesions on CXR comprise a risk factor for obstructive lung disease and even a minimal TB lesion was also a strong risk factor in never-smokers.Citation42 Our findings supported those of previous reports. In this study, patients with post-TB lesions showed a higher percentage of COPD than patients without such findings (P<0.040). This result can be explained by considering the destructive and fibrosing properties of pulmonary TB, causing pulmonary overdistension.Citation43 Male sex was another important risk factor for PPCs in patients without COPD. There were 131 (57%) male patients in the non-COPD group, which was significantly lower than the number in the COPD group. After adjustment for both smoking status and pack-years, male sex was still significantly associated with an increased rate of PPCs (P=0.030). Some recent studies showed that male patients have a higher risk of complications in open and laparoscopic colorectal surgery,Citation44 but no study has suggested that male sex is a specific risk factor for PPCs after thoracic surgery, especially in patients with normal lung function. Genetic differences or compliance with a daily physiotherapy from the first postoperative day comprising deep breathing exercise, incentive spirometry, supported coughing, and mobilization might be plausible explanations for this, but further study is needed to confirm these findings.
There are several limitations to this study. First, this study was conducted in only one tertiary referral hospital, and the results may have limited generalizability to other populations. A prospective multicenter study with an appropriate spectrum of patients with COPD and normal patients would be necessary to validate predictors identified in our study and to define the best predictors of PPCs more rigorously. However, serious PPCs, even in patients with severe COPD, are infrequent.Citation45 This low event rate, combined with the small number of patients with severe COPD undergoing lung resection surgery, would necessitate a large sample size for any prospective study. Second, we followed up our patients during their hospitalization, and thus we evaluated only the short-term impact of the various factors on PPCs. Longitudinal studies are needed to evaluate the long-term clinical impact of PPCs, such as the number of readmissions and all-cause mortality after PPCs.
Conclusion
In patients with NSCLC, the prevalence of PPCs is higher even in early stages of COPD than in such patients with normal spirometry. Symptom- or QoL-based scores, such as CAT or SGRQ scores, are not significant risk predictors for PPCs in patients with early-stage COPD.
Author contributions
Conception and design: ESK, C-GY, YTK, CHK, IKP; analysis and interpretation of the data: ESK, C-HL, C-GY; drafting of the article: ESK, C-GY; critical revision of the article for important intellectual content: ESK, C-HL, C-GY, YWK, SKH; final approval of the article: ESK, YTK, CHK, IKP, WB, SMC, JL, YSP, C-HL, S-ML, J-JY, YWK, SKH, C-GY. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors acknowledge the patients with NSCLC who allowed us to conduct clinical research studies in an effort to improve the lives of patients undergoing lung cancer surgery.
Supplementary material
Table S1 Perioperative variables significantly associated with PPCs on univariate analysis
Disclosure
The authors report no conflicts of interest in this work.
References
- WangJOlakJUltmannREFergusonMKAssessment of pulmonary complications after lung resectionAnn Thorac Surg19996751444144710355428
- HazelriggSRLandreneauRJBoleyTMThe effect of muscle-sparing versus standard posterolateral thoracotomy on pulmonary function, muscle strength, and postoperative painJ Thorac Cardiovasc Surg19911013394400 discussion 400–4011999932
- BuschEVerazinGAntkowiakJGDriscollDTakitaHPulmonary complications in patients undergoing thoracotomy for lung carcinomaChest199410537607668131538
- BolligerCTJordanPSolerMExercise capacity as a predictor of postoperative complications in lung resection candidatesAm J Respir Crit Care Med19951515147214807735602
- EpsteinSKFalingLJDalyBDCelliBRInability to perform bicycle ergometry predicts increased morbidity and mortality after lung resectionChest199510723113167842753
- OkitaAYamashitaMAbeKVariance analysis of a clinical pathway of video-assisted single lobectomy for lung cancerSurg Today200939210410919198986
- LawrenceVAHilsenbeckSGMulrowCDDhandaRSappJPageCPIncidence and hospital stay for cardiac and pulmonary complications after abdominal surgeryJ Gen Intern Med199510126716788770719
- GuptaHRamananBGuptaPKImpact of COPD on postoperative outcomes: results from a national databaseChest201314361599160623287892
- AgostiniPCieslikHRathinamSPostoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors?Thorax201065981581820805178
- SekineYSuzukiHYamadaYKohEYoshinoISeverity of chronic obstructive pulmonary disease and its relationship to lung cancer prognosis after surgical resectionThorac Cardiovasc Surg201361212413022535670
- Lopez-EncuentraAAstudilloJCerezalJBronchogenic Carcinoma Cooperative Group of the Spanish Society of Pneumology and Thoracic Surgery (GCCB-S)Prognostic value of chronic obstructive pulmonary disease in 2994 cases of lung cancerEur J Cardiothorac Surg200527181315736303
- LawrenceVADhandaRHilsenbeckSGPageCPRisk of pulmonary complications after elective abdominal surgeryChest199611037447508797421
- MyrdalGGustafssonGLambeMHorteLGStahleEOutcome after lung cancer surgery. Factors predicting early mortality and major morbidityEur J Cardiothorac Surg200120469469911574210
- PatelRLTownsendERFountainSWElective pneumonectomy: factors associated with morbidity and operative mortalityAnn Thorac Surg199254184881610259
- BernardADeschampsCAllenMSPneumonectomy for malignant disease: factors affecting early morbidity and mortalityJ Thorac Cardiovasc Surg200112161076108211385374
- WahiRMcMurtreyMJDeCaroLFDeterminants of perioperative morbidity and mortality after pneumonectomyAnn Thorac Surg198948133372764597
- KearneyDJLeeTHReillyJJDeCampMMSugarbakerDJAssessment of operative risk in patients undergoing lung resection. Importance of predicted pulmonary functionChest199410537537598131537
- PierceRJCoplandJMSharpeKBarterCEPreoperative risk evaluation for lung cancer resection: predicted postoperative product as a predictor of surgical mortalityAm J Respir Crit Care Med199415049479557921468
- StephanFBoucheseicheSHollandeJPulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factorsChest200011851263127011083673
- BrunelliAAl RefaiMMonteverdeMBorriASalatiMFianchiniAStair climbing test predicts cardiopulmonary complications after lung resectionChest200212141106111011948039
- LickerMJWidikkerIRobertJOperative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trendsAnn Thorac Surg20068151830183716631680
- KroenkeKLawrenceVATherouxJFTuleyMRHilsenbeckSPostoperative complications after thoracic and major abdominal surgery in patients with and without obstructive lung diseaseChest19931045144514518222804
- YanoTMiuraNTakenakaTNever-smoking nonsmall cell lung cancer as a separate entity: clinicopathologic features and survivalCancer200811351012101818618510
- SubramanianJGovindanRLung cancer in ‘never-smokers’: a unique entityOncology2010241293520187318
- Standards for the diagnosis and care of patients with chronic obstructive pulmonary diseaseAmerican Thoracic SocietyAm J Respir Crit Care Med19951525 pt 2S77S1217582322
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- SekineYYamadaYChiyoMAssociation of chronic obstructive pulmonary disease and tumor recurrence in patients with stage IA lung cancer after complete resectionAnn Thorac Surg200784394695017720404
- SmetanaGWLawrenceVACornellJEAmerican College of PhysiciansPreoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of PhysiciansAnn Intern Med2006144858159516618956
- WongDHWeberECSchellMJWongABAndersonCTBarkerSJFactors associated with postoperative pulmonary complications in patients with severe chronic obstructive pulmonary diseaseAnesth Analg19958022762847818113
- ArozullahAMDaleyJHendersonWGKhuriSFMultifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement ProgramAnn Surg2000232224225310903604
- GuptaPKGuptaHKaushikMPredictors of pulmonary complications after bariatric surgerySurg Obes Relat Dis20128557458121719358
- FergusonMKLittleLRizzoLDiffusing capacity predicts morbidity and mortality after pulmonary resectionJ Thorac Cardiovasc Surg19889668949003193801
- Richter LarsenKSvendsenUGMilmanNBrenoeJPetersenBNExercise testing in the preoperative evaluation of patients with bronchogenic carcinomaEur Respir J1997107155915659230247
- PrescottEAlmdalTMikkelsenKLToftengCLVestboJLangePPrognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart StudyEur Respir J200220353954412358326
- WilsonDORogersRMWrightECAnthonisenNRBody weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing TrialAm Rev Respir Dis19891396143514382658702
- Gray-DonaldKGibbonsLShapiroSHMacklemPTMartinJGNutritional status and mortality in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med199615339619668630580
- LandboCPrescottELangePVestboJAlmdalTPPrognostic value of nutritional status in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med199916061856186110588597
- AroraNSRochesterDFRespiratory muscle strength and maximal voluntary ventilation in undernourished patientsAm Rev Respir Dis19821261587091909
- AroraNSRochesterDFEffect of body weight and muscularity on human diaphragm muscle mass, thickness, and areaJ Appl Physiol198252164707061279
- LickerMSpiliopoulosAFreyJGDe PerrotMChevalleyCTschoppJMManagement and outcome of patients undergoing thoracic surgery in a regional chest medical centreEur J Anaesthesiol200118854054711473561
- PeiGZhouSHanYLiuZXuSRisk factors for postoperative complications after lung resection for non-small cell lung cancer in elderly patients at a single institution in ChinaJ Thorac Dis2014691230123825276365
- LeeSWKimYSKimDSOhYMLeeSDThe risk of obstructive lung disease by previous pulmonary tuberculosis in a country with intermediate burden of tuberculosisJ Korean Med Sci201126226827321286020
- SniderGLDoctorLDemasTAShawARObstructive airway disease in patients with treated pulmonary tuberculosisAm Rev Respir Dis197110356256405579906
- KirchhoffPDinclerSBuchmannPA multivariate analysis of potential risk factors for intra- and postoperative complications in 1316 elective laparoscopic colorectal proceduresAnn Surg2008248225926518650636
- KroenkeKLawrenceVATherouxJFTuleyMROperative risk in patients with severe obstructive pulmonary diseaseArch Intern Med199215259679711580723