Abstract
Background and objective
The association between TNF-α −308 G/A polymorphism and COPD remains controversial due to insufficiently strict study designs and small group sizes among different studies. In the present study, a meta-analysis update which followed a stricter procedure was performed to obtain a clearer understanding of this association.
Methods
A comprehensive database search was conducted to identify the case–control studies published up to July 2015 which reported an association between the TNF-α −308 G/A polymorphism and COPD risk. Data were extracted to calculate pooled odds ratios with 95% confidence intervals under the most appropriate genetic and allelic models. Sensitivity was analyzed, and heterogeneity as well as publication bias was assessed.
Results
Thirty-eight eligible studies, comprising 3,951 COPD cases and 5,110 controls, were included in this study, among which 22 studies comprising 2,067 COPD cases and 2,167 controls were performed in Asians, and 16 studies comprising 1,884 COPD cases and 2,943 controls were in non-Asians. The overall result showed that TNF-α −308 G/A polymorphisms were significantly associated with increased COPD risk in both the codominant genetic and allelic models. Individuals with the GA or AA genotype were more susceptible to COPD development than those with the GG genotype. In addition, individuals with the AA genotype were more susceptible to developing COPD than those with the GA genotype. The subgroup analysis stratified by ethnicity supported the results in Asians but not in non-Asians. However, no association was found between TNF-α −308 G/A polymorphisms and COPD susceptibility either in Asians or in non-Asians in the meta-analysis conducted with restriction to former/current smokers.
Conclusion
The present meta-analysis suggested that the TNF-α −308 G/A polymorphism was associated with an increased risk of COPD among Asians but not in non-Asians. Furthermore, individuals with the AA genotype of TNF-α −308 were more susceptible to developing COPD.
Introduction
COPD is characterized by the progressive development of airflow limitation that is not fully reversible.Citation1 COPD has been estimated to become the third leading cause of death in the world by 2020.Citation2 According to statistics, COPD is ranked as the third and fourth leading cause of death in rural and urban areas of the People’s Republic of China, respectively.Citation3 Cigarette smoking is considered to be a major environmental factor contributing to the development of COPD. However, only 25%–40% of cigarette smokers develop COPD,Citation4 indicating that other components may be involved in COPD development.Citation5–Citation7
Accumulated evidence indicates that genetic factors influence COPD susceptibility. A number of studies have demonstrated that TNF-α is relevant to the pathogenesis of COPD, including involvement in neutrophil release from the bone marrow and neutrophil activation.Citation8 Increased levels of TNF-α have been found in the sputum,Citation9 bronchoalveolar lavage fluid, bronchial biopsies, and circulationCitation10 of COPD patients. Genetic polymorphism analyses have identified several single-nucleotide polymorphisms in the TNF-α gene associated with COPD risk, including −238 G/A, −308 G/A, −376 G/A, −863 C/A, −857 T/C, −1031 T/C, and +489 G/A.Citation11–Citation14 Among these, the −308 G/A polymorphism is the best studied; however, a consistent association has not yet been found.Citation15 Studies in AsiansCitation16,Citation17 and non-AsiansCitation18 have demonstrated that the TNF-α −308 G/A polymorphism is associated with an increased risk of COPD. However, other studies in both AsiansCitation19,Citation20 and non-AsiansCitation21–Citation25 have showed opposite results.
A limited number of meta-analyses have been performed to further clarify the association between the TNF-α −308 polymorphisms and COPD risk;Citation26–Citation29 however, a firm conclusion has not been achieved because of several limitations in the previous meta-analyses including 1) failure to check the Hardy–Weinberg equilibrium (HWE), 2) lack of quality assessment, 3) inappropriate genetic model, and 4) a limited number of included studies. All these factors have led to considerable argument regarding the studies’ paradoxical conclusions. Additionally, only studies published up to 2010 were included in the most recent meta-analysis.Citation29 In the present study, we conducted a meta-analysis update with studies published up to July 2015. Additionally, we followed a stricter procedure: 1) only studies in accordance with HWE were included, 2) all included studies had a quality score no less than 5 because studies with quality scores ≤4 are considered as low-quality studies,Citation30 and 3) the most appropriate genetic model was employed. Thus, our report presented more detailed information which will not only help obtain a clearer understanding of the association between TNF-α −308 polymorphisms and COPD but also help pave the way for individualized treatment of COPD patients.
Materials and methods
The current meta-analysis was conducted according to the guidelines presented in the review by Sagoo et al.Citation31
Search strategy for publication
A comprehensive search was conducted using the terms “TNF”, “tumor necrosis factor”, “polymorphism”, and “COPD” in several electronic databases (PubMed, EMBASE, ISI Web of Science, Cochrane Central Register of Controlled Trials, China National Knowledge Infrastructure, Database of Chinese Scientific and Technical Periodicals, China Biology Medicine disc database, and WANFANG databases) to identify studies that examined the association of TNF-α −308 (rs1800629) G/A polymorphisms with COPD published up to July 2015. Additional studies were identified by manually reviewing the bibliographies of relevant articles as well as relevant review articles. The search was performed without restriction regarding race, ethnicity, or geographic area. Only published studies with full text in English or Chinese were included. Concerning duplicate populations included in several publications, only the most recent or complete study was included in this meta-analysis.
Eligibility criteria
Eligible studies were required to meet the following inclusion criteria: 1) evaluation of the TNF-α −308 polymorphism and COPD risk, 2) employment of a case–control design, 3) inclusion of adult subjects within the case group or control group, 4) disclosure of the number of individual genotypes with COPD in cases and controls, and 5) congruency of the distribution of genotypes among controls with HWE. Studies were excluded if 1) they contained overlapping data with another study, 2) the number of wild-type genotypes or alleles was not stated, and 3) they reviewed only editorials, reviews, and abstracts. All articles were reviewed to determine eligibility by two independent investigators. A consensus with a third reviewer was needed if there was any disagreement between the two investigators.
Data extraction
Data were checked and extracted from each study by two independent investigators. Data inconsistencies or discrepancies were resolved by consensus of all investigators before being standardized into a unified dataset. The following information was extracted from each study: first author’s name, publication year, country/territory, numbers of cases and controls, ethnicity of the study population, source of control subjects, smoking status in cases and controls, and genotype and allele distribution.
Quality assessment
The Newcastle–Ottawa quality assessment scaleCitation32 was applied to assess the quality of each study by two investigators. The quality was evaluated with three major components: 1) selection of cases and controls, 2) comparability of cases and controls, and 3) ascertainment of exposure. Any disagreement was resolved by a third investigator. Only studies with a quality score ≥5 were included in the current study.
Statistical analysis
Statistical analysis was performed according to standard procedures.Citation30 Pooled odds ratios (ORs) were calculated with the Mantel–Haenszel (M–H) mean of the logarithm with a 95% confidence interval (CI). First, an allele comparison was conducted to determine the allele risk. Second, OR1, OR2, and OR3 were explored for the genotypes (GG vs AA [OR1], GG vs GA [OR2], and GA vs AA [OR3]) to identify the most appropriate genetic model. When OR1 = OR3 ≠1 and OR2 =1, a recessive model was suggested. When OR1 = OR2 ≠1 and OR3 =1, then a dominant model was suggested. When OR1 > OR2 >1 and OR1 > OR3 >1 (or OR1 < OR2 <1 and OR1 < OR3 <1), then a codominant model was suggested.Citation33 Lastly, the most appropriate genetic model was used to pool the results.
Heterogeneity was assessed by using the chi-square-based Cochran Q-test, which was considered significant if P<0.10, and the I2 statistic. If I2>50%, the random-effect model was adopted as the pooling method; otherwise, the fixed-effect model was used. To explore the source of the heterogeneity, subgroup analyses were performed with respect to ethnicity and smoking status.
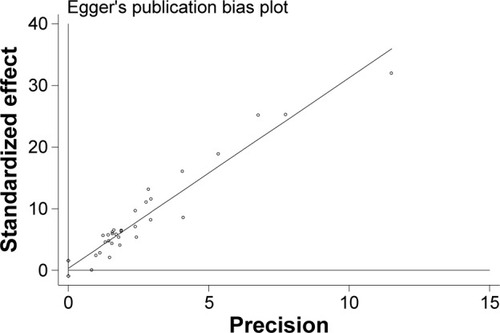
A sensitivity analysis was conducted to assess the stability of the results. One study at a time was excluded to evaluate how robust the pooled estimator was. Publication bias was estimated by using Egger’s test.
All statistical analyses were performed with STATA version 11.0. A P-value <0.05 was considered statistically significant.
Results
Study characteristics
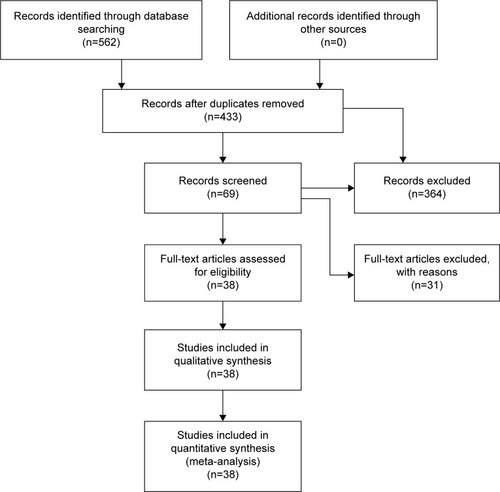
The flow diagram in summarizes the selection process carried out for this meta-analysis. A total of 38 eligible articles were included in the current meta-analysis, comprising 3,951 COPD cases and 5,110 controls.Citation11–Citation13,Citation16,Citation18,Citation19,Citation22,Citation24,Citation34–Citation63 Twenty articles were published in English and 18 in Chinese. There were 16 studies performed in non-Asians which comprised 1,884 COPD cases and 2,943 controls and 22 studies in Asians which comprised 2,067 COPD cases and 2,167 controls. Fifteen studies contained sufficient information for subgroup analysis by smoking status. All of the cases were confirmed by the diagnostic criteria of COPD.Citation64–Citation68 The genotype distributions of the TNF-α −308 polymorphism were in accordance with HWE in the controls of all the studies. Based on the quality assessment scale for case–control studies, two studies scored 5 points, 22 studies scored 6 points, eleven studies scored 7 points, and the other three scored 8 points. The characteristics of these studies are shown in . The detailed genotype, allele information, and HWE results are listed in .
Table 1 Characteristics of the included studies
Table 2 Genotype distribution of the TNF-α −308 G/A polymorphism in case and control
Meta-analysis results
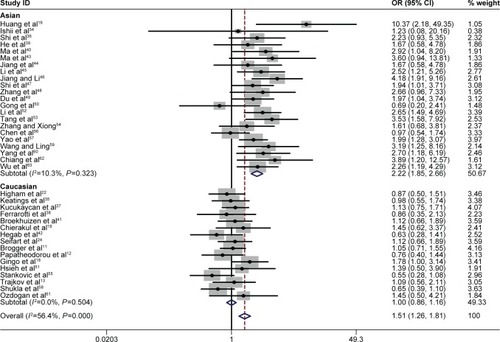
A summary of the meta-analysis results concerning association between TNF-α −308 polymorphism and COPD risk is provided in . The A allele was associated with an increased COPD risk in the overall population (OR =1.56, 95% CI 1.29–1.89, P=0.000 for heterogeneity, I2=70.8%) (). The estimated OR1, OR2, and OR3 were 1.776 (P=0.000), 1.513 (P=0.211), and 1.216 (P=0.000), respectively, suggesting a codominant model as the most appropriate genetic model. Then, the pooled ORs were calculated under the codominant genetic model. OR was 1.78 for GG vs AA and 1.51 for GG vs GA ( and ), demonstrating a significant association between TNF-α −308 polymorphism and COPD in the overall population. Individuals with AA genotype were more susceptible to develop COPD than those with GA genotype. To identify the origin of heterogeneity, a subgroup analysis stratified by ethnicity was conducted. As shown in –, a stronger correlation of the polymorphism with COPD risk was found in Asians under the genetic model (OR =3.25 for GG vs AA and OR =2.22 for GG vs GA), and similar results were observed in the allelic model. Interestingly, the AA genotype carriers had a higher risk of developing COPD than GA carriers in Asian patients. Conversely, no association was found in non-Asians in the genetic model (OR =1.05 for GG vs AA and OR =1.00 for GG vs GA). Notably, heterogeneity was significantly decreased when stratified analysis was performed by ethnicity status in both models, indicating the ethnicity contributed partly to heterogeneity, and similar results were found in the allelic model.
Table 3 Summary ORs for relationship between the TNF-α −308 polymorphism and COPD risk
Figure 2 Forest plot for the association between TNF-α –308 polymorphism and COPD in all subjects using allelic model (G vs A).
Note: Weights are from random effects analysis.
Abbreviations: OR, odds ratio; CI, confidence interval.
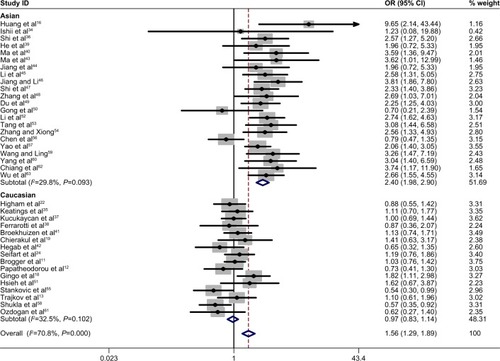
Figure 3 Forest plot for the association between TNF-α −308 polymorphism and COPD in all subjects using codominant genetic model (G/G vs A/A genotype).
Abbreviations: OR, odds ratio; CI, confidence interval.
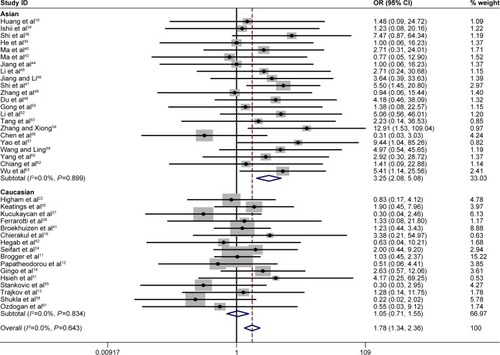
Figure 4 Forest plot for the association between TNF-α −308 polymorphism and COPD in all subjects using codominant genetic model (G/G vs G/A genotype).
Abbreviations: OR, odds ratio; CI, confidence interval.
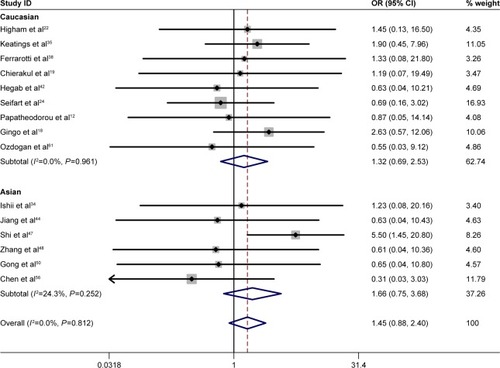
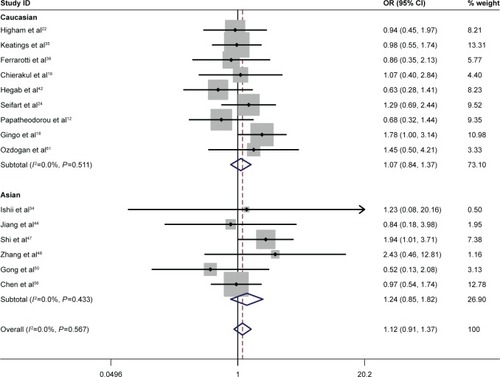
Specific environmental factors, such as smoking, may contribute to the distribution of genetic polymorphisms.Citation69 Moreover, there was a difference in the TNF-α −308 polymorphism between smoking and nonsmoking COPD patients.Citation45 To minimize the effect of cigarette smoking on the association between the TNF-α −308 G/A polymorphism and COPD risk, a second meta-analysis was conducted with studies in which the COPD cases and the controls were current/former smokers. Interestingly, no significant association was found between the TNF-α −308 polymorphism and COPD risk either in Asian smokers or in non-Asian smokers. The statistics in non-Asians included OR =1.32 for GG vs AA and OR =1.07 for GG vs GA. The statistics in Asians included OR =1.66 for GG vs AA and OR =1.24 for GG vs GA ( and ). Our study indicated that the A allele was not a risk factor for the development of COPD in smoking populations.
Sensitivity analysis
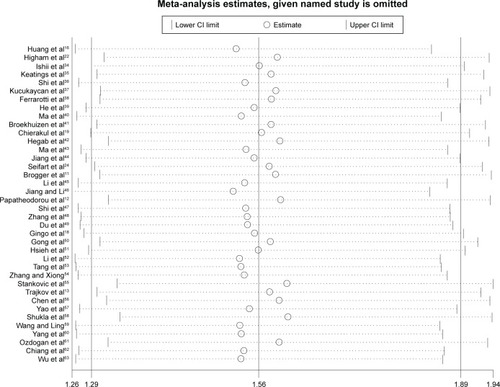
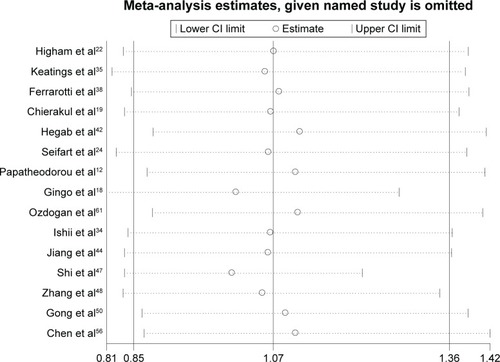
Sensitivity analysis was performed by sequentially excluding each study to assess the stability of the results in this meta-analysis. The corresponding pooled ORs were not materially altered in the overall meta-analysis (). In the meta-analysis with restriction to smokers, two studiesCitation18,Citation47 were found to be the source of heterogeneity in Asian smokers (). After excluding these two studies from the analysis, the pooled OR did not vary significantly, indicating that the results were relatively reliable (data not shown).
Discussion
In the present meta-analysis update, we conducted a comprehensive database search for potential articles published up to July 2015 to evaluate the association between TNF-α −308 polymorphism and COPD risk, and several of the articles were not included in the previous meta-analysis. To our knowledge, this is the first report to analyze the association between TNF-α −308 polymorphism and COPD risk under the codominant genetic model. Thus, more detailed information can be achieved under this genetic model. Finally, a total of 38 studies with 3,951 patients and 5,110 controls were included in the meta-analysis. The results showed a significant association between the TNF-α −308 polymorphism and COPD susceptibility in the overall population. Individuals with the A allele (GA or AA) were more susceptible to developing COPD than those with the GG genotype. Additionally, we further clarified that individuals with the AA genotype had a higher risk of developing COPD than those with the GA genotype (77.6% vs 51.3%). The previous meta-analysis investigating the current question employed a dominant genetic model, and did not provide detailed information about the AA and GA genotypes separately. Here, for the first time, our report indicated that carriers of the AA genotype of TNF-α −308 were the most vulnerable to COPD development.
To identify the origin of heterogeneity, a subgroup analysis stratified by ethnicity was conducted. In our study, significant associations were shown in Asians but not in non-Asians, which is consistent with the previous meta-analysis.Citation28,Citation29 Our data reconfirmed that the TNF-α −308 G/A polymorphism was associated with COPD risk even under a stricter study design and procedure. Furthermore, our study further identified a stronger correlation between the TNF-α −308 G/A polymorphism and COPD risk in Asians with the AA genotype compared with those with the GA genotype. Notably, heterogeneity was significantly decreased when the analysis stratified by ethnicity was performed. We speculated that it may be because that the A allele is more important for COPD susceptibility in Asians than in non-Asians.
To minimize the effect of smoking status on the association, a second meta-analysis restricted to smokers was conducted. Interestingly, no correlation was found between the TNF-α −308 G/A polymorphism and the risk of COPD in either Asian smokers or non-Asian smokers. This result was contrary to the previous meta-analysis which showed an obvious correlation between the TNF-α –308 G/A polymorphism and the risk of COPD in smokers. Although moderate heterogeneity was observed in Asian smokers in the allelic model, which may distort the result, the pooled OR did not vary significantly after the removal of two studies that were considered the origin of the heterogeneity. This indicated that the results of this meta-analysis in the smokers were reliable. The opposite results may be attributed to the following: 1) the codominant model was adopted in the current study, which was different from the previous study (dominant model); and 2) due to stricter inclusion criteria, several studies were excluded from the current meta-analysis. Based on the results of our study, it seems that other factors may contribute to COPD development in smokers, and we speculated that the A allele may be a risk factor in nonsmokers; however, a firm conclusion should not be drawn until a larger number of studies with a sufficient number of nonsmokers can be included in the meta-analysis.
There are several limitations of the present meta-analysis that should be considered when explaining the results: 1) Even though we followed a strict procedure for data collection and data analysis to minimize the heterogeneity, several pooled ORs were obtained from heterogeneous studies. 2) There were not enough nonsmokers in the case and control groups to conduct a subgroup analysis to ascertain whether the A allele of TNF-α –308 was associated with the risk of COPD development in the nonsmoking population. 3) The numbers of studies were limited for this meta-analysis; some studies were excluded from the study. This selection bias may have an effect on the genotyping publication bias. What’s more, more studies are needed to further improve the power of the study. 4) The genotyping methods in the studies included are different, which may cause some bias on the result.
In conclusion, this meta-analysis update suggested that the A allele of TNF-α −308 is a risk factor for developing COPD. Additionally, individuals with the AA genotype appeared to be more susceptible to developing COPD than those with the GA genotype. Additionally, the subgroup analysis in Asians (but not in non-Asians) supported the results. The data presented in the current report may provide insight for COPD treatment based on patients’ genotype. In future, larger and more strictly controlled studies are needed to evaluate the relationship between gene polymorphisms and COPD. What’s more, relationship between gene polymorphisms and COPD in nonsmoking populations should be explored to further elucidate if gene polymorphism is an independent risk factor associated with the development of COPD, which will favor the development of effective prevention and treatment methods for COPD in nonsmokers.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81001428), the Jiangsu Province Scientific Research Innovation Project of University Graduate Students (KYLX15_0979), the Jiangsu Province Special Program of Medical Science (BL2012012), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, JX10231802).
Disclosure
The authors declare no conflicts of interest in this work.
References
- RabeKFHurdSAnzuetoABarnesPJBuistSACalverleyPGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2007176653255517507545
- PauwelsRARabeKFBurden and clinical features of chronic obstructive pulmonary disease (COPD)Lancet2004364943461362015313363
- FangXWangXBaiCCOPD in China: the burden and importance of proper managementChest2011139492092921467059
- LokkeALangePScharlingHFabriciusPVestboJDeveloping COPD: a 25 year follow up study of the general populationThorax2006611193593917071833
- BascomRDifferential susceptibility to tobacco smoke: possible mechanismsPharmacogenetics1991121021061844866
- SilvermanEKChapmanHADrazenJMGenetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitisAm J Respir Crit Care Med1998157177017789620904
- SandfordAJJoosLParePDGenetic risk factors for chronic obstructive pulmonary diseaseCurr Opin Pulm Med200282879411845002
- KeatingsVMCollinsPDScottDMBarnesPJDifferences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthmaAm J Respir Crit Care Med199615325305348564092
- MuellerRChanezPCampbellAMBousquetJHeusserCDifferent cytokine patterns in bronchial biopsies in asthma and chronic bronchitisRespir Med199690279858730325
- SunGStaceyMAVittoriEMariniMBelliniAKleimbergJCellular and molecular characteristics of inflammation in chronic bronchitisEur J Clin Invest19982853643729650009
- BroggerJSteenVMEikenHGGulsvikABakkePGenetic association between COPD and polymorphisms in TNF, ADRB2 and EPHX1Eur Respir J200627468268816585076
- PapatheodorouALatsiPVrettouCDimakouAChroneouAMakrythanasisPDevelopment of a novel microarray methodology for the study of SNPs in the promoter region of the TNF-alpha gene: their association with obstructive pulmonary disease in Greek patientsClin Biochem2007401284385017509552
- TrajkovDMirkovska-StojkovikjJPetlichkovskiAStrezovaAEfinska-MladenovskaOSandevskaEAssociation of cytokine gene polymorphisms with chronic obstructive pulmonary disease in MacedoniansIran J Allergy Asthma Immunol200981314219279357
- Cordoba-LanusEBaz-DavilaRde-TorresJPRodriguez-PerezMCMaca-MeyerNVaroNTNFA-863 polymorphism is associated with a reduced risk of chronic obstructive pulmonary disease: a replication studyBMC Med Genet20111213221985478
- WilsonAGdi GiovineFSBlakemoreAIDuffGWSingle base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR productHum Mol Genet1992153531363876
- HuangSLSuCHChangSCTumor necrosis factor-alpha gene polymorphism in chronic bronchitisAm J Respir Crit Care Med19971565143614399372657
- SakaoSTatsumiKIgariHShinoYShirasawaHKuriyamaTAssociation of tumor necrosis factor alpha gene promoter polymorphism with the presence of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2001163242042211179116
- GingoMRSilveiraLJMillerYEFriedlanderALCosgroveSPChanEDTumour necrosis factor gene polymorphisms are associated with COPDEur Respir J20083151005101218256059
- ChierakulNWongwisutikulPVejbaesyaSChotvilaiwanKTumor necrosis factor-alpha gene promoter polymorphism is not associated with smoking-related COPD in ThailandRespirology2005101363915691236
- EzzeldinNShalabyASaad-HusseinAEzzeldinHEI LebedyDFaroukHAssociation of TNF-alpha −308G/A, SP-B 1580 C/T, IL-13-1055 C/T gene polymorphisms and latent adenoviral infection with chronic obstructive pulmonary disease in an Egyptian populationArch Med Sci20128228629522662002
- TeramotoSIshiiTIshiiMYamamotoHYamaguchiYHibiSVariation in the tumour necrosis factor-alpha gene is not associated with susceptibility to Asian COPDEur Respir J200831368268318310403
- HighamMAPrideNBAlikhanAMorrellNWTumour necrosis factor-alpha gene promoter polymorphism in chronic obstructive pulmonary diseaseEur Respir J200015228128410706492
- ChappellSDalyLMorganKBaranesTGRocaJRabinovichRVariation in the tumour necrosis factor gene is not associated with susceptibility to COPDEur Respir J200730481081217906092
- SeifartCDempfleAPlagensASeifartUClostermannUMullerBTNF-alpha-, TNF-beta-, IL-6-, and IL-10-promoter polymorphisms in patients with chronic obstructive pulmonary diseaseTissue Antigens20056519310015663746
- TanakaGSandfordAJBurkettKConnettJEAnthonisenNRParePDTumour necrosis factor and lymphotoxin A polymorphisms and lung function in smokersEur Respir J2007291344116971410
- CastaldiPJChoMHCohnMLangermanFMoranSTarragonaNThe COPD genetic association compendium: a comprehensive online database of COPD genetic associationsHum Mol Genet201019352653419933216
- SmolonskaJWijmengaCPostmaDSBoezenHMMeta-analyses on suspected chronic obstructive pulmonary disease genes: a summary of 20 years’ researchAm J Respir Crit Care Med2009180761863119608716
- ZhanPWangJWeiSZQianQQiuXYuLKTNF-308 gene polymorphism is associated with COPD risk among Asians: meta-analysis of data for 6,118 subjectsMol Biol Rep201038121922720364405
- ZhangSWangCXiBLiXAssociation between the tumour necrosis factor-alpha-308G/A polymorphism and chronic obstructive pulmonary disease: an updateRespirology201116110711520946339
- LiYGuoBZhangLHanJWuBXiongHAssociation between C-589T polymorphisms of interleukin-4 gene promoter and asthma: a metaanalysisRespir Med2008102798499218396027
- SagooGSJulianLHigginsJPTSystematic reviews of genetic association studiesPLoS Med200963e1000028
- StangACritical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol201025960360520652370
- ThakkinstianAMcElduffPD’EsteCDuffyDAttiaJA method for meta-analysis of molecular association studiesStat Med20052491291130615568190
- IshiiTMatsuseTTeramotoSNeither IL-1beta, IL-1 receptor antagonist, nor TNF-alpha polymorphisms are associated with susceptibility to COPDRespir Med200094984785111001075
- KeatingsVMCaveSJHenryMJMorganKO’ConnorCMFitzGeraldMXA polymorphism in the tumor necrosis factor-alpha gene promoter region may predispose to a poor prognosis in COPDChest2000118497197511035665
- ShiYZWangXJSunHMDetection of TNF-a gene polymorphism in COPD patientsBasic Med Sci Clin20002095
- KucukaycanMVan KrugtenMPenningsHJTumor necrosis factor-alpha +489G/A gene polymorphism is associated with chronic obstructive pulmonary diseaseRespir Res200232912537602
- FerrarottiIZorzettoMBeccariaMTumour necrosis factor family genes in a phenotype of COPD associated with emphysemaEur Respir J200321344444912661999
- HeBJiangLNingLTumor necrosis factor-a gene 308 A allele can predispose to chronic obstructive pulmonary disease in smokersChin J Respir Crit Care Med200312226229
- MaZZhangZHanZA study on relationship between tumor necrosis factor alpha gene promoter polymorphism and susceptibility to development of COPDPract Prev Med2004115417419
- BroekhuizenRGrimbleRFHowellWMPulmonary cachexia, systemic inflammatory profile, and the interleukin 1beta -511 single nucleotide polymorphismAm J Clin Nutr20058251059106416280439
- HegabAESakamotoTSaitohWPolymorphisms of TNFalpha, IL1beta, and IL1RN genes in chronic obstructive pulmonary diseaseBiochem Biophys Res Commun200532941246125215766560
- MaZZhangZHanZA study on the relationship between tumor necrosis factor a gene promoter polymorphism and the expressive levels of TNF-a proteinJ Clin Intern Med2005224230232
- JiangLHeBZhaoMWNingLDLiXYYaoWZAssociation of gene polymorphisms of tumour necrosis factor-alpha and interleukin-13 with chronic obstructive pulmonary disease in Han nationality in BeijingChin Med J2005118754154715820084
- LiYZhaiFZDuYRelationship of genetic polymorphisms of human betadefensin-1, tumor necrosis factor-alpha, mEH with chronic obstructive pulmonary diseaseJ Intern Med2006453225226
- JiangPLiYAssociation among genetic polymorphisms of HO-1, TNF-alpha and chronic obstructive pulmonary diseaseJ Shandong Univ (Health Sci)200644212531257
- ShiYZLiuBZhangWStudy of the relationship between COPD and TNF-alpha gene polymorphism in Heilongjiang regionChin J Tuberc Respir Dis200730233234
- ZhangYWangWXuSHAssociation between polymorphism in the gene coding for tumor necrosis factor-alpha and chronic obstructive pulmonary diseaseJ Taishan Med Coll200728106109
- DuYSunHMLiYThe relationship between genetic polymorphism of tumor necrosis factor-alpha, human beta-defensin-1 and the development of chronic obstructive pulmonary diseaseClin Med J China200815329331
- GongYJinMLRenTAssociation of tumor necrosis factor gene promoter polymorphism with chronic obstructive pulmonary diseaseClin Med J China200815166169
- HsiehMHChongIWHwangJJLeeCHHoCKYuMLLack of associations between several polymorphisms in cytokine genes and the risk of chronic obstructive pulmonary diseases in TaiwanKaohsiung J Med Sci200824312613718364273
- LiYDuYJiangPRelationship of genetic polymorphisms of heme oxygenase-1, tumor necrosis factor-alpha, human betadefensin-1 with chronic obstructive pulmonary diseaseChin J Geriatr200827333336
- TangMQMoHWChengYQThe relationship between the gene polymorphisms of TNF alpha and the genetic susceptibility to chronic obstructive pulmonary disease (COPD) with tuberculosisJ Qiqihar Med Coll20082929492951
- ZhangYQXiongJLCorrelation of genetic polymorphisms of TNF-alpha and chronic obstructive pulmonary disease in North Chinese Han peopleClin Med China200824129130
- StankovicMMNestorovicARTomovicAMTNF-alpha-308 promotor polymorphism in patients with chronic obstructive pulmonary disease and lung cancerNeoplasma200956434835219469656
- ChenYCLiuSFChinCHAssociation of tumor necrosis factor-alpha-863C/A gene polymorphism with chronic obstructive pulmonary diseaseLung2010188433934720352242
- YaoZGWangHYJiaNStudy on the relationship between TNF-α+308, TNF-α+489 gene polymorphism and chronic obstructive pulmonary diseaseMod J Integr Trad Chin Western Med2012212224002405
- ShuklaRKKantSBhattacharyaSMittalBAssociation of cytokine gene polymorphisms in patients with chronic obstructive pulmonary diseaseOman Med J201227428529023071879
- WangFELingMRelationship between tumor necrosis factor-α gene-308 promoter polymorphism and the susceptibility to chronic obstructive pulmonary disease in Xinjiang Uighur populationJ Pract Med2013292236863689
- YangLLiFYanMLiXAssociation of the CYP1A1 MspI and TNFα-308 polymorphisms with chronic obstructive pulmonary disease in Inner MongoliaGenet Mol Res20141323209321724841653
- OzdoganNTutarNDemirRSaatciCKanbayABuyukoglanHIs TNF-alpha gene polymorphism related to pulmonary functions and prognosis as determined by FEV1, BMI, COPD exacerbation and hospitalization in patients with smoking-related COPD in a Turkish population?Rev Port Pneumol2014206305310 Portuguese24818527
- ChiangCHChuangCHLiuSLTransforming growth factor-beta1 and tumor necrosis factor-alpha are associated with clinical severity and airflow limitation of COPD in an additive mannerLung201419219510224153451
- WuSMWangFELingMRelationship between tumor necrosis factor-α gene promoter polymorphism and susceptibility to development of COPD in Xinjiang Kazakh populationJ Xi’an Jiao Tong Univ2014356820823
- PauwelsRABuistASCalverleyPMJenkinsCRHurdSSGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summaryAm J Respir Crit Care Med200116351256127611316667
- COPD Branch in Chinese Thoracic SocietyThe guideline of diagnosis and management for COPD. COPD Branch in Chinese Thoracic SocietyChin J Tuberc Respir Dis200225453460
- American Thoracic SocietyStandards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic SocietyAm J Respir Crit Care Med19951525 Pt 2S77S1217582322
- The guideline of diagnosis and management for COPD. Chinese Thoracic SocietyChin J Tuberc Respir Dis199720199233
- Chinese Thoracic SocietyBTS guidelines for the management of chronic obstructive pulmonary disease. The COPD Guidelines Group of the Standards of Care Committee of the BTSThorax199752Suppl 5S1S28
- TanniSEPelegrinoNRAngeleliAYCorreaCGodoyISmoking status and tumor necrosis factor-alpha mediated systemic inflammation in COPD patientsJ Inflamm (Lond)201072920534161