Abstract
Background
During the treatment phase of active pulmonary tuberculosis (PTB), respiratory function impairment is usually restrictive. This may become obstructive, as a PTB-associated airflow obstruction (AFO) or as a later manifestation of underlying COPD.
Purpose
The aim of the study was to examine the potential causes and risks for AFO development in PTB by exploring the aspects of spirometry limitations and clinical implications for the underlying COPD detection, taking into account various confounding factors.
Patients and methods
Prospective, nest case–control study on 40 new cases of PTB with initial restrictive respiratory function impairment, diagnosed and treated according to the directly observed treatment short course (DOTS) strategy.
Results
From all observed patients, 37.5% of them developed AFO upon the completion of PTB treatment, with significantly increased average of forced vital capacity (%) (P<0.01). Their changes in forced expiratory volume in the first second (%) during the PTB treatment were strongly associated with the air pollution exposure in living (0.474%–20.971% for 95% confidence interval [CI]; P=0.041) and working environments (3.928%–20.379% for 95% CI; P=0.005), initial radiological extent of PTB lesions (0.018%–0.700% for 95% CI; P=0.047), leukocyte count (0.020%–1.328% for 95% CI; P=0.043), and C-reactive protein serum level (0.046%–0.205% for 95% CI; P=0.003) compared to the other patients. The multivariate logistic regression analysis model shows initial radiological extent of pulmonary tuberculosis lesions (OR 1.01–1.05 for 95% CI; P=0.02) and sputum conversion rate on culture (OR 1.02–1.68 for 95% CI; P=0.04) as the most significant predictors for the risk of AFO development.
Conclusion
AFO upon PTB treatment is a common manifestation of underlying COPD, which mostly occurs later, during the reparative processes in active PTB, even in the absence of major risk factors, such as cigarette smoking and biomass fuel dust exposure. Initial spirometry testing in patients with active PTB is not a sufficient and accurate approach in the detection of underlying COPD, which may lead to their further potential health deterioration.
Introduction
Severe (extensive) forms of active pulmonary tuberculosis (PTB), with its long-term evolution in cases of bronchogenic forms of the disease, contribute to serious destruction of lung parenchyma, including permanent scarring, bronchiectasis, and fibrosis, leading to significant respiratory function impairment.Citation1,Citation2 It is estimated that COPD affects 65 million people worldwide, most of them in low- and middle-income countries, where the prevalence of PTB remains high.Citation3,Citation4
During the treatment phase of active PTB, respiratory ventilation impairment is usually restrictive. This may persist, resolve, or become obstructive in nature, as a PTB-associated airflow obstruction (AFO) or as a late manifestation of already existing (underlying) COPD itself.Citation4,Citation5 Causes and development of AFO in patients with active PTB, in addition to the factors of infection, involve contributing factors of the patient (genetic factors, systemic inflammation response, and initial extent of PTB lesions), as well as from the environment (tobacco-smoking habits, air pollution, and economic income), while clinically manifested bronchial obstruction occurs as their mutual interplay.Citation6–Citation8 Recent studies indicate the strong association of matrix-metalloproteinase system in remodeling mechanisms of pulmonary extracellular matrix in the pathogenesis of AFO and also in extensive PTB, as in COPD.Citation9–Citation11 A relationship between active PTB and COPD developments has been suggested in several reports, but a serious limitation in these studies was the lack of diagnostic precision in distinguishing COPD from the other forms of structural lung disease (eg, bronchiectasis, bullous parenchymal destruction, and fibrosis), which are common in PTB.Citation12,Citation13 Based on the results of the few available longitudinal studies, the association of PTB and AFO appears to be causal.Citation4 Several mechanisms may account for the development of AFO and even clinically manifested COPD, after the active infection in PTB, including the structural damage of airways, bronchiolar narrowing, and bronchiolitis obliterans, as well as accelerated emphysematous change, caused by residual, chronic, or recurrent inflammation.Citation13 Previous studies of the origin of AFO in active PTB in individuals with or without underlying COPD have not shown the reliable solutions for adequate detection and differentiation of the major risks for the development of these pulmonary ventilation disorders.Citation4–Citation8
The aim of this study was to examine the potential causes and risks for AFO development in PTB, exploring the aspects of spirometry limitations and clinical implications for the presence of underlying COPD and its detection in patients with extensive PTB and initial restrictive respiratory function impairment, which are treated only with antituberculosis drugs over the standard 6-month regimen, taking into account the hereditary burden of COPD, air pollution exposure in living and working environments, smoking habits, radiological extent of PTB lesions, sputum conversion rate, and nonspecific systemic inflammatory response.
Patients and methods
Patients – the subjects of the study
The research was performed as a prospective, nest case–control study in the Clinic for Lung Diseases, Faculty of Medicine, University of Nis, Nis, Serbia, on 40 carefully selected patients defined as new cases of extensive (severe) PTB (new cases of PTB with cavities and pericavital parenchymal fibrocaseous infiltrates on standard chest radiograph) and initial (according to the first lung function test results) restrictive respiratory function (pulmonary ventilation) impairment, who were diagnosed and treated according to the directly observed treatment short course (DOTS) strategy and National Tuberculosis Programme of the Republic of Serbia, in the period from January 2005 to December 2012.Citation14 Among the 541 tuberculosis (TB) patients diagnosed in our clinic during the period, 83 patients were identified as new cases of extensive PTB with initial restrictive respiratory function impairment. After reviewing the obtained data by applying the exclusion criteria, 43 patients were excluded from the study, and 40 patients were enrolled for the final analysis. The criteria for initial restrictive respiratory function impairment according to the Official Statement of the European Respiratory Society were as follows: forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) ×100%≥70%, level of postbronchodilation FVC ≤80%, and level of total lung capacity (TLC) <5% of the reference values.Citation15
The study protocol was reviewed and approved by the Academic Council of Faculty of Medicine, University of Nis (No 04-642/04, December 13, 2005). The study procedures were conducted according to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all study patients after an explanation of the nature of the study and confirmed by the Academic Council of Faculty of Medicine, University of Nis. All study patients were Serbian Caucasians recruited from the Clinic for Lung Diseases.
The inclusion criteria for the selection of patients in this study were as follows: 1) typical symptoms for PTB (cough, sputum production, fever, night sweat, and weight loss); 2) negative personal history of TB and/or TB treatment; 3) typical fibrocavitary pulmonary infiltrates on standard chest radiographs; 4) at least one smear positive sputum, with the subsequent positive culture of Mycobacterium tuberculosis; and 5) all study patients, at the time of inclusion in the study, were ≥20 years of age and could already have been on the antituberculosis treatment (processed with all necessary radiological, microbiological, and laboratory and spirometric examinations, before starting the antituberculosis treatment) but not for >2 weeks.
The exclusion criteria for the patients in this study were as follows: 1) detection of mono- or multidrug-resistant TB bacilli in the first positive sputum culture; 2) coexisting lung disease, defined as reliable confirmation of lung pathology other than TB; and 3) patients with chronic heart, liver, and kidney failures, metabolic disorders, and pregnancy.
Risk factors for COPD, such as familial burden, air pollution exposure in living and working environments for >15 years, and smoking habits, were determined from medical history data, while the level of nicotine dependence in smoking patients was determined by filling the specific questionnaire of Fagerstörm’s test.Citation16
Determining the radiological extent of PTB lesions
Chest radiographs were collected at the beginning of PTB treatment and after 6 months, ie, at the end of PTB treatment. Interpretation of the extent of PTB lesions was performed by two clinicians who made an assessment and came to an agreement without prior access to the lung function test results, scoring the lesions, whereby each lung was divided into equal thirds, each of them scored on four-level scale from 0 to 3 points, with a maximal radiographic score of 18.Citation12
Bacteriological sputum analysis
Initially, at the beginning of anti tuberculosis treatment, sputum samples were taken in a series of three consecutive morning samples and analyzed by direct smear microscopy (Ziehl–Neelsen stained) and plating the same samples to Lowenstain–Jensen culture, as well as the resistance test on the first-line anti tuberculosis drugs, from the first positive culture. Furthermore, during the intensive phase of treatment, sputum conversion rate was checked in a series of two consecutive morning samples on a weekly level, and during the continuation phase of treatment, the rate was checked in a series of two consecutive morning samples on a monthly level.Citation14
Measurements of inflammatory serum markers
The following parameters were determined: erythrocyte sedimentation rate (ESR) in the first hour (mm/1 h), leukocyte (Le) count expressed in 109/L, which was obtained by peripheral blood smear, serum levels of C-reactive protein (CRP) (mg/L), and fibrinogen (g/L). The parameters of hematological and biochemical analyses mentioned earlier were determined at the beginning and after 6 months of TB treatment.
Lung function measurements
Respiratory function measurements were carried out in the morning, by spirometry testing, determined by the standard spirometric parameters, as follows: FVC (%), FEV1 (%), and their percentage ratio (FEV1/FVC ×100%) (%) and TLC (%), by body plethysmography testing (Version 4.54 gb; Master Lab system, E. Jaeger, Würzburg, Germany). For a final value of each of the examined lung function parameters, the best score of the respondents from three consecutive measurements was taken. A pharmacodynamic bronchodilation test of reversibility was performed, with 400 µg of salbutamol in metered dose inhalation spray on all patients, before entering the study. Remeasurement of FEV1 was performed 30 minutes after the inhalation, and positive result was considered as an increase of 200 mL of FEV1 or ≥12% from the baseline level. Criteria for restrictive respiratory function impairment were as follows: FEV1/FVC ×100%≥70%, level of postbronhodilation FVC ≤80%, and level of TLC <5% of the reference values.Citation15,Citation17,Citation18 Pulmonary function test was performed on the following three occasions: 1) at the beginning of the intensive phase of TB treatment, 2) at the end of the intensive phase of TB treatment (ie, after 2 months), and 3) at the end of the continuation phase of TB treatment (after 6 months).
Statistical analysis
Statistical calculations were performed using the SPSS software Version 10.0. Comparison of mean values of numerical characteristics between the three separate occasions of lung function measurements was done by single factor analysis of variance with Tukey’s post hoc test. The values of inflammatory markers at the beginning and at the end of the PTB treatment of observed patients were compared by Wilcoxon signed rank test. Analysis of the relationship between changes in FEV1 (%) values and factors of interest (major risk factors for COPD, such as familial burden for COPD, air pollution exposure in living and working environments for >15 years, and smoking habits, such as smoker, nonsmoker, pack/years index, Fagerstörm’s score, and nicotine dependence level), factor of PTB activity (radiographic score at the beginning and at the end of PTB treatment and sputum conversion rate by microscopy and on culture), and factors of systemic inflammatory response (values of inflammatory serum markers, such as ESR, CRP, and fibrinogen, at the beginning and at the end of PTB treatment), adjusted for age, sex, and body mass index (BMI) values during the PTB treatment was performed by univariate linear regression analysis (calculated the linear regression coefficient [β] and its 95% CI). The significance of the regression coefficient was checked by linear regression t-test. To assess the impact of the same major risk factors for COPD, systemic inflammatory response, and PTB activity on the development and characterization of residual AFO upon the TB treatment, the logistic regression analysis was applied, adjusted for age, sex, and BMI values. Assessing the value of statistical significance was performed by calculating the odds ratio (OR) Wald values. Factors for which the univariate logistic regression showed significant influence on the occurrence of AFO were included in multivariate regression models.Citation19
Results
Based on defined criteria, our study identified 83 out of 541 TB patients diagnosed as new cases of extensive PTB with restrictive respiratory function impairment from the territory of Nisava District, Republic of Serbia, with the population of 371,003 inhabitants, overall TB incidence of 25–14/105, from 2005 to 2012, with 88%–85% of new cases, 5%–13.5% relapses, and successful treatment outcome of 34%–62.8% annually.
The obtained data were reviewed, and 43 patients were excluded for the following reasons: 1) seven patients were relapses of PTB (medical history data), 2) five patients also had TB pleurisy (high-resolution CT scan and thoracocentesis), 3) 14 patients had coexisting lung diseases (five patients with massive bacterial pneumonia, three patients with lung cancer, four patients with chronic respiratory failure, and two patients with sarcoidosis) (medical history data, high-resolution CT scan, fiberoptic bronchoscopy with biopsy and bronchial lavage histopathology, cytology and microbiological confirmation, and arterial blood gas analysis), 4) eleven patients had chronic disease (four patients with heart failure, two patients with liver cirrhosis, three patients with renal failure, one patient with rheumatoid arthritis, and one patient with Crohn’s disease), and 5) there was loss of data or all the measurements were not made for six patients. Thus, 40 patients with definitive diagnosis of new cases of extensive PTB with restrictive respiratory function impairment were enrolled for the final analysis.
Demographic, clinical, and laboratory profiles
Demographic and clinical aspects of examined patients are shown in . The majority of patients were current smokers, with an average pack/year index of 1.36±0.67/29.61±12.83 and a high average nicotine dependence level of 6.26±2.14 Fargestörm score points (). Generally, ten (22.5%) patients did not have any risk factors for COPD ().
Table 1 General demographic profile of observed patients
Table 2 The major familial and environmental risks for COPD development among observed patients
Table 3 Clinical signs for COPD among the observed patients
The average radiological extent of PTB lesions during TB treatment was significantly reduced from 10.23±4.04 radiographic score points at the beginning to 3.93±2.44 points at the end (P<0.001), with an average reduction of 6.30±2.65 points. The average sputum smear conversion rate was 2.90±2.07 weeks, while on culture it was 4.12±2.24 weeks. The average values of inflammation serum marker levels (ESR, Le count, CRP, and fibrinogen), were significantly reduced during the PTB treatment (P<0.001) ().
Table 4 Comparing the values of serum markers of systemic inflammation
Pulmonary function test
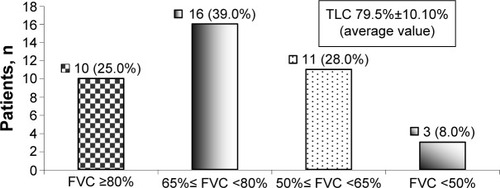
Initially, 16 (39.0%) patients had mild restrictive pulmonary ventilation disorder, eleven (27.5%) patients had moderate restrictive pulmonary ventilation disorder, and three (8.0%) patients had severe restrictive pulmonary ventilation disorder ().
Figure 1 Distribution of patients according to the severity of the initially registered restrictive pulmonary ventilation disorder.
Abbreviations: FVC, forced vital capacity; TLC, total lung capacity.
A positive response to bronchodilation test was verified in 7.7% of patients, while the average baseline level of TLC (%) increased until the end of PTB treatment, with no significant difference. The average baseline level of FVC (%) was lower and significantly increased during the PTB treatment (P<0.01), as opposed to FEV1 (%) ().
Table 5 Comparing the values of pulmonary ventilation parameters between the measurements made at the beginning, during, and at the end of the PTB treatment with the differences between the same
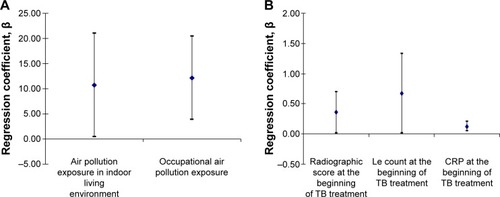
Linear regression analysis confirmed significant association between changes in the values of FEV1 (%), resulting in TB treatment completion, and the value of some risk factors for COPD, PTB activity, and factors of systemic inflammation, adjusted for age, sex, and BMI. Patients exposed to air pollution in indoor living and working environments for >15 years had significant higher changes in FEV1 (%) of 10.722% (0.474%–20.971% for 95% CI; P=0.041) and 12.153% (3.928%–20.379% for 95% CI; P=0.005), respectively, during the TB treatment when compared to the other patients. On the other hand, any increase in radiographic score, Le count, and CRP at the beginning of TB treatment for just one measurement unit was strongly associated with significantly increased changes in FEV1 (%) after the TB treatment completion: radiographic score of 0.359% (0.018%–0.700% for 95% CI; P=0.047), Le count of 0.674% (0.020%–1.328% for 95% CI; P=0.043), and CRP of 0.126% (0.046%–0.205% for 95% CI; P=0.003) ().
Figure 2 Significant factors of impact on the FEV1 (%) values upon the TB treatment completion.
Abbreviations: CRP, C-reactive protein; FEV1, forced expiratory volume in the first second; Le, leukocyte; TB, tuberculosis.
Outcome
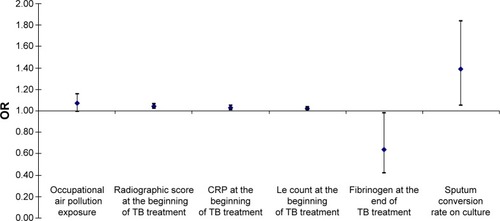
From the initial findings of restrictive pulmonary ventilation disorder, upon the completion of PTB treatment, 15 (37.5%) of all observed patients developed the AFO. In those patients, a univariate logistic regression analysis, adjusted for age, sex, and BMI, confirmed that occupational air pollution exposure for >15 years increases the risk of AFO development for 7%, but the level of error estimates of such claim in the sample is at a level of <10% (OR 0.99–1.16 for 95% CI; P=0.07). On the other hand, any increase in initial value of radiographic score of one measurement unit increases the risk of AFO development for 4% (OR 1.02–1.06 for 95% CI; P=0.01), as well as initial CRP serum level for 3% (OR 1.01–1.05 for 95% CI; P=0.02), initial Le count for 2% (OR 1.01–1.03 for 95% CI; P=0.03), and sputum conversion rate on culture for 39% (OR 1.05–1.84 for 95% CI; P=0.04), while any increase in fibrinogen serum level upon the TB treatment completion decreases the risk of AFO development for 36% (OR 0.42–0.98 for 95% CI; P=0.04) ().
Figure 3 Predictors for the AFO occurrence and development upon the TB treatment completion.
Abbreviations: AFO, airflow obstruction; CRP, C-reactive protein; Le, leukocyte; OR, odds ratio; TB, tuberculosis.
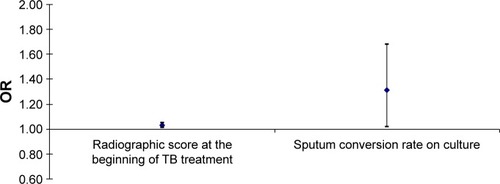
In multivariate logistic regression analysis model, adjusted for age, sex, and BMI, the most significant predictors for the development of AFO in the observed patients upon TB treatment completion were radiographic score at the beginning of TB treatment and sputum conversion rate on culture. Any increase in those predictors of one measurement unit significantly increases the risk of AFO development, as follows: radiographic score at the beginning of the TB treatment for 3% (OR 1.01–1.05 for 95% CI; P=0.02) and sputum conversion rate on culture for 31% (OR 1.02–1.68 for 95% CI; P=0.04) ().
Figure 4 The impact of initial radiological extent of pulmonary TB lesions (Snider’s radiological score) at the beginning of TB treatment and sputum conversion rate on culture as major risk factors for the AFO development upon the TB treatment completion.
Abbreviations: AFO, airflow obstruction; OR, odds ratio; TB, tuberculosis.
Discussion
According to the current GOLD criteria, there is increasing awareness of the heterogeneity of COPD. These criteria require the presence of chronic airflow limitation (CAO) that persists after use of a bronchodilator and a history of exposure to recognized risk factors for the development of AFO. Among these risks, the most common are cigarette smoking, biomass fuel dust exposure, childhood lung infections, and occupational exposures, including exposure to dust, gases, and fumes.Citation4,Citation20 The absence of tobacco-smoking history occurs in >20% of patients fulfilling the criteria for COPD by spirometry and has been reported in many population-based studies.Citation21,Citation22 Whether TB should be added to the list of recognized exposures for the future development of COPD is unclear.
A number of studies have shown that the treatment of pulmonary TB only with antituberculosis drugs may lead to the emergence or worsening of existing AFO in some patients.Citation6,Citation23 Some prospective studies with a longer follow-up period demonstrated a significant number of cases affected and/or treated for PTB, which had the same result in CAO, or the restrictive respiratory syndrome.Citation22,Citation24 In patients with active PTB, CAO occurs in 52.7% infiltrative, 56.6% fibrous and cavitary, and even 88.2% miliary forms.Citation23 It is debatable whether PTB-associated CAO should be considered as a part of the COPD spectrum, as a distinct phenotype of COPD, or as an unrelated disease. However, based on the results of the few available longitudinal studies, the association of PTB with CAO appears to be causal.Citation4 The strongest body of evidence comes from three large population-based, cross-sectional studies that showed a statistically significant association between previous PTB and COPD. These cross-sectional studies, such as PLATINO (N=5,571), PREPOCOL (N=5,539), and the study by Lam et al (N=8,066), provide an adjusted OR of between 1.37 and 2.94 for this association between previous PTB and COPD, an association greater than that with either cigarette smoking or wood-smoke exposure in the PREPOCOL study.Citation7,Citation24,Citation25 In addition, in the PLATINO study, a history of PTB was associated with more severe grades of bronchial obstruction.Citation7 Our earlier case–control study on PTB patients with initial normal respiratory ventilation confirmed the influence of positive familial history on COPD together with initial radiological extent of PTB lesions and sputum conversion rate on culture as significant predictive factors for the residual AFO upon TB treatment completion, but the lack of further follow-up information could not confirm a subsequent diagnosis of COPD with certainty.Citation26 These findings are also supported by the smaller cross-sectional, cohort, and case-control studies.Citation6,Citation22,Citation27
Cigarette smoking is the only external agent, which is cause-related to the occurrence and development of CAO in COPD. Reviewing the smoking habits of patients in our study, our results corresponded to available literature data.Citation28 Den Boon et alCitation29 confirmed the increase in COPD morbidity and mortality as a consequence of various respiratory tract infections, including PTB. In our investigation, 22.5% of patients were without any risk factors for COPD, which is in accordance with similar results in the available literature.Citation4,Citation5,Citation21
Although the extensive destruction of lung parenchyma in PTB with consequent restriction of airflow through the bronchial tree is clinically relatively common, the severity of AFO and bronchodilator response have still not been evaluated objectively.Citation6,Citation8,Citation22,Citation23 Most of all symptoms and limitations, including AFO, of these patients in everyday practice consider as a consequence of active or cured pulmonary TB and in most cases ignored the emergence of severe clinical manifestations and complications. All the mechanisms of systemic inflammatory response in the pathogenesis of AFO in active PTB remained still unclear, but it is certain that some inflammatory conditions act as exclusively linked. In our study, the observed patients had initially pathologically elevated average values of all observed systemic proinflammatory indices, which were significantly decreased to the normal ones at the end of the treatment, or subclinical values, which can be explained by the high intensity of TB infection in active extensive disease, as well as their decline at the end of TB treatment, due to the effect of DOTS therapy regimen.Citation27,Citation30
In our study, a positive response to bronchodilation test was verified in only 7.7% of patients, while some studies refer a positive test in overall 44%–88% of PTB patients, depending on the initial registered pulmonary ventilation disorder.Citation23,Citation31 From the initial restrictive pulmonary ventilation disorder upon the completion of PTB treatment, 37.5% of all observed patients in our study developed AFO and have a strong association between changes in FEV1 (%) values, resulting in PTB treatment completion, and air pollution exposure in indoor living and working environments, the initial value of radiographic score, Le count, and CRP at baseline level, as well as the sputum conversion rate on culture, representing the most significant risk factors for AFO development. Many authors confirmed the study of strong association between the past history of PTB and the actual presence of AFO.Citation4,Citation13,Citation22,Citation26 Estimates of association are further influenced by the presence of powerful confounding factors, such as cigarette smoking, which predisposes to the development of both COPD and TB infections.Citation20,Citation31 However, in some of the reported studies, the influence of smoking was avoided by separate analyses in nonsmokers or by excluding smokers.Citation13,Citation23,Citation25 In PLATINO and PREPOCOL studies, the association remained after adjusting for smoking and the use of biomass fuel.Citation7,Citation24 In our study, the influence of initial radiological extent of pulmonary TB lesions and sputum conversion rate on culture were significant predictive factors for the development of AFO upon TB treatment.Citation4,Citation6,Citation27
The strength of this study is that it was carried out in Nisava District, which is the capital city of Nis (Serbia’s third largest city), with similar incidence of TB as on national level. The study included all eligible patients within the district catchment area, minimizing the effect of selection bias.
There were several limitations of this study: first, TB patients who were not detected by the National Health System could not be included, and although this group is a small one, it is also likely to include marginalized groups, such as homeless, foreign refugees, and migrants. Second, a considerable number of enrolled patients after the TB treatment completion were transferred out in the next 3 years, with further longer follow-up information made unavailable, emphasizing on study design and delays in the final analysis of obtained data. Finally, the living conditions, air pollution exposure, and socioeconomic factors considerably vary according to the different district area, especially within the urban, industrial, and rural regions, which could not be evaluated in this study.
Significant findings in this study include a high degree of suspicion of the existence of underlying COPD in severe PTB patients even in the presence of initial restrictive pulmonary ventilation disorder, with the identification of patient significant initial clinical characteristics associated with “adverse pulmonary ventilation outcomes” as an AFO, CAO, or COPD upon the TB treatment completion. These findings suggest several recommendations for improving further medical care and the general welfare among TB patients. Patients with active PTB with clinical characteristics of initial spirometric restrictive respiratory ventilation impairment, male sex, long-term exposure to occupational air pollution, prevalence of extensive radiological extent of pulmonary TB lesions, and slower sputum culture conversion rate should be considered as a high risk for “adverse pulmonary ventilation outcome”, in the form of CAO or COPD, which justifies an increased awareness and care. In these patients, initial spirometry testing has serious limitations and lack of diagnostic accuracy for underlying CAO or COPD. However, for a more accurate evaluation of coexisting COPD, besides the characteristic clinical signs and risk factors of the disease, demographic details of the PTB patient’s specific district, together with occupational and living conditions and environment characteristics, within the regional category, also need to be taken into account.
Conclusion
The clinical syndrome of AFO or CAO among PTB-treated patients is often a common initial manifestation of underlying COPD, which mostly occurs later and appears in the results of our investigation as a consequence and significant functional impairment of the respiratory system, during the reparative processes in active PTB, even in the absence of major risk factors for COPD, such as cigarette smoking and biomass fuel dust exposure. Given the small number of longitudinal studies on this clinical problem, the results of our investigation point to the fact that initial spirometry testing in patients with active PTB is not a sufficient and accurate approach in the detection of underlying COPD, which may lead to their further potential health deterioration.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- DhedaKBarryCE3rdMaartensGTuberculosisLancet2016387100241211122626377143
- ChakrabartiBCalverleyPMDaviesPDTuberculosis and its incidence, special nature and relationship with chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis20072326327218229564
- LozanoRNaghaviMForemanKGlobal and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet201238098592095212823245604
- AlwoodBWMyerLBatemanEDA systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adultsRespiration2013861768523652030
- BaigIMSaeedWKhalilKFPost-tuberculous chronic obstructive pulmonary diseaseJ Coll Physicians Surg Pak201020854254420688021
- PasipanodyaJGMillerTLVecinoMPulmonary impairment after tuberculosisChest200713161817182417400690
- MenezesAMHallalPCPerez-PadillaRLatin American Project for the Investigation of Obstructive Lung Disease (PLATINO) TeamTuberculosis and airflow obstruction: evidence from the PLATINO study in Latin AmericaEur Respir J20073061180118517804445
- HnizdoESinghTChurchyardGChronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatmentThorax2000551323810607799
- EllkingtonPTFriedlandJSMatrix metalloproteinases in destructive pulmonary pathologyThorax200661325926616227332
- OngCWElkingtonPTFriedlandJSTuberculosis, pulmonary cavitation, and matrix metalloproteinasesAm J Respir Crit Care Med2014190191824713029
- WHOAn International Roadmap for Tuberculosis ResearchGenevaWorld Health Organization2011
- PlitMLAndersonRRensburgCEInfluence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosisEur Respir J19981223513569727784
- JordanTSSpencerEMDaviesPTuberculosis, bronchiectasis and chronic airflow obstructionRespirology201015462362820409028
- WHOTuberculosis-Treatment Guidelines for National ProgramsGenevaWHO Regional Office for Europe20101120
- Official Statement of the European Respiratory SocietyStandardized lung function testingEur Respir J1993161100
- DiFranzaJRWellmanRJSavageauJABecciaAUrsprungWWMcMillenRWhat aspect of dependence does the Fagerström test for nicotine dependence measure?ISRN Addict2012201390627625969829
- PrideNBTests of forced expiration and inspirationHughesJMBPrideNMLung Function TestsLondonW.B. Saunders2000326
- GoldmanMDSmithHJUlmerWTWhole-body plethysmographyGosselinkRStamHLung Function TestingWakefieldEuropean Respiratory Society Journals Ltd20051543
- PeacockJKerrySSingle group studiesPeacockJKerrySPresenting Medical Statistics from Proposal to PublicationLondonOxford University Press20074550
- VestboJHurdSSAgustíAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAmerican Journal of Respiratory and Critical Care Medicine2013187434736522878278
- LamprechtBMcburnieMAVollmerWMBOLD Collaborative Research GroupCOPD in never smokers: results from the population-based burden of obstructive lung disease studyChest2011139475276320884729
- LeeSWKimYSKimDOhYLeeSThe risk of obstructive lung disease by previous pulmonary tuberculosis in a country with intermediate burden of tuberculosisJ Korean Med Sci201126226827321286020
- SmelevEIKuklinaGMKalininaEETreatment of bronchial obstruction in patients with pulmonary tuberculosisProbl Tuberk Bolezn Legk20048576115478563
- CaballeroATorres-DuqueCAJaramilloCPrevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL Study)Chest2008133234334917951621
- LamKBJiangCQJordanREPrior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank cohort studyChest2010137359360019820078
- RadovicMRisticLStankovicIChronic airflow obstruction syndrome due to pulmonary tuberculosis treated with directly observed therapy – serious changes in lung functionMed Arh201165526526922073848
- KoYLeeYMLeeHYChanges in lung function according to disease extent before and after pulmonary tuberculosisInt J Tuberc Lung Dis201519558959525868029
- KolappanCGopiPGTobacco smoking and pulmonary tuberculosisThorax2002571196496612403879
- Den BoonSVan LillSWBorgdorffMWAssociation between smoking and tuberculosis infection: a population survey in a high tuberculosis incidence areaThorax200560755555715994262
- LeeJHChangJHLung function in patients with chronic airflow obstruction due to tuberculosis destroyed lungRespir Med200397111237124214635980
- Van Zyl SmithRNPaiMYewWWGlobal lung health: the colliding epidemics of tuberculosis, tobacco smoking, HIV and COPDEur Respir J2010351273320044459
