Abstract
Background
Cough and sputum production are frequent in chronic obstructive pulmonary disease (COPD). The objective of this study was to examine the relationship between cough and sputum production and health-related quality of life in COPD.
Methods
A cross-sectional study was conducted in the French Initiatives COPD cohort and assessed cough and sputum production within the past 7 days using the cough and sputum assessment questionnaire (CASA-Q), health-related quality of life, spirometry, smoking status, dyspnea, exacerbations, anxiety and depression, and comorbidities.
Results
One hundred and seventy-eight stable COPD patients were included (age, 62 [56–69] years, 128 male, forced expiratory volume in 1 second [FEV1]: 57 [37–72] % predicted) (median [Q1–Q3]). In univariate analyses, health-related quality of life (Saint George’s respiratory questionnaire total score) was associated with each CASA-Q domain and with chronic bronchitis, exacerbations, dyspnea, FEV1, depression, and anxiety. All four domains introduced separately were independently associated with health-related quality of life. When introduced together in multivariate analyses, only the cough impact domain remained independently associated with health-related quality of life (R2=0.60). With chronic bronchitis (standard definition) instead of the CASA-Q, the R2 was lower (R2=0.54).
Conclusion
This study provides evidence that current cough in the previous 7 days is an important determinant of health-related quality of life impairment in stable COPD patients.
Introduction
Cough and sputum production are frequent symptoms in chronic obstructive pulmonary disease (COPD) patients. The prevalence of chronic cough and sputum production varies from 14% to 74% in COPD patients, depending on the definitions used for chronic bronchitis, the case report forms, and the characteristics of the patients.Citation1–Citation6 Chronic cough and sputum production, usually defined using the classic definition of “chronic bronchitis” (cough and sputum production for at least 3 months of two consecutive years), are associated with a higher risk of exacerbation and hospitalization,Citation1,Citation3,Citation4,Citation7,Citation8 faster lung function decline,Citation9,Citation10 mortality,Citation8,Citation11–Citation14 and health-related quality of life (HRQoL) impairment.Citation2,Citation4 Current smoking is the main risk factor for chronic bronchitis in COPD.Citation15 Occupational and domestic exposures to gases, fumes, and dusts are also associated with a higher prevalence of chronic bronchitis in COPD patients.Citation16–Citation18
Until recently, despite the high prevalence of cough and sputum production in COPD and their association with the key features of COPD, these symptoms have been overlooked in clinical research on COPD, which likely relates to the lack of effective dedicated therapeutic agent. The natural history of chronic bronchitis in COPD is not clearly understood, and there is a lack of dedicated and objective tools for assessing the symptoms of cough and sputum production in COPD. Dedicated questionnaires have been designed, but their utility and relationship with other COPD outcomes have not been investigated intensively.
The present cross-sectional study was conducted in a subsample of the French Initiatives COPD cohort, a cohort of stable COPD patients. Cough and sputum production within the past 7 days were assessed using the cough and sputum assessment questionnaire (CASA-Q), which contains four domain scores, assessing the symptoms and impact of cough and sputum. This dedicated questionnaire allows a better characterization and quantification of the symptoms of cough and sputum than the classic definition of “chronic bronchitis”. HRQoL was assessed using the Saint George’s Respiratory Questionnaire (SGRQ). The objective of this study was to test the hypothesis that current cough and sputum production in the previous 7 days assessed by a dedicated questionnaire, the CASA-Q, are associated with HRQoL impairment in COPD.
Methods
Study design
This cross-sectional multicenter study was conducted in a subsample of the observational French Initiatives bronchopneumopathie chronique obstructive cohort including 18 French university hospitals.Citation1 The relationships between cough and sputum assessed by the CASA-Q and the HRQoL were assessed using univariate and multivariate analyses.
Patient selection
The inclusion criteria were as follows: a diagnosis of COPD defined as a postbronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity <0.7 measured in stable condition with no history of exacerbation within the four previous weeks. The exclusion criterion was a predominant diagnosis of asthma, bronchiectasis, or any other significant respiratory disease.
The study was approved by the ethics committee of Versailles, France (number: 04-479) for the protection of human beings involved in biomedical research. An informed written consent was obtained from each subject.
Data collection
Data collection has been extensively described previously.Citation1 A standardized questionnaire was used to collect demographic data (age, sex, body mass index [BMI]), smoking history (current status and cumulative smoking), comorbidities, symptoms of dyspnea using the modified Medical Research Council (mMRC) scale, symptoms of chronic bronchitis defined as daily cough and sputum production for at least 3 months per year during the past 2 years, hospital anxiety and depression scale, and the number of exacerbations in the previous year. HRQoL was assessed using the SGRQ. Pulmonary function tests were performed according to American Thoracic Society/European Respiratory Society standards.Citation19,Citation20
Cough and sputum assessment questionnaire
CASA-Q was added to the standardized questionnaires in the Initiatives BPCO cohort for the purpose of this study. This questionnaire has been described in detail elsewhere.Citation21,Citation22 Briefly, the CASA-Q is a self-administered questionnaire that assesses cough and sputum based on their frequency, severity, and impact on daily activities in the previous 7 days. The CASA-Q contains four domains: cough symptoms, cough impact, sputum symptoms, and sputum impact. Each domain contains three to eight items, each of which is answered in five categories from “never” to “always” for frequency and from “not at all” to “a lot/extremely” for intensity. For each domain, the items are summed and rescaled to obtain a score ranging from 0 to 100, with higher scores associated with fewer symptoms or less impact. The validated French translation of the CASA-Q was used in this study and is presented in the US English version in Table S1.Citation21,Citation22
Statistical analysis
Quantitative variables are expressed as median and quartiles and/or number and percentages. Univariate analyses were performed to determine the relationship between SGRQ total score and the following variables: age, sex, smoking status, cumulative smoking, BMI, FEV1% predicted, dyspnea mMRC grade, number of exacerbations in the previous year, hospital anxiety and depression scale (cutoff ≥10), cardiovascular comorbidities, chronic bronchitis, and CASA-Q domains. Multivariate models were built to assess the contribution of CASA-Q domains to quality of life impairment. Patients with complete data on the CASA-Q and the variables identified as being associated with the total SGRQ score in univariate analyses were included in the multivariate analyses. The first model was built with SGRQ total score as the explained variable and only the four CASA-Q domains as the possible explanatory variables (ie, no other variable was introduced in the model) (model 1). All other models also included all the variables tested with the SGRQ total score in univariate analysis as covariates (P<0.35). In the first of these models, all CASA-Q domains were introduced (model 2). In a following series of four models, each domain was introduced separately (one model for each domain) (models 3–6). Finally, a model was built without any CASA-Q domain, but with chronic bronchitis following its standard definition (model 7).
Results
Patient characteristics and CASA-Q results
Overall, 178 patients recruited in the Initiatives BPCO cohort were assessed for cough and sputum production using the CASA-Q. Clinical characteristics of the patients are presented in .
Table 1 Clinical characteristics of the population (N=178)
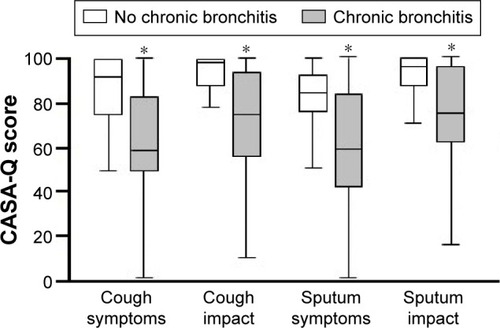
The median values for the four domains of the CASA-Q assessing cough and sputum in the previous 7 days were 70.8 (50.0–91.7) for cough symptoms, 84.4 (59.4–100.0) for cough impact, 66.7 (41.7–88.1) for sputum symptoms, and 87.5 (66.7–100.0) for sputum impact. Chronic bronchitis defined as daily cough and sputum production for at least 3 months each year during the past 2 years was found in 69% of the patients. Each score of the four domains of the CASA-Q was significantly lower with the presence of chronic bronchitis (P<0.0001), indicating more cough and sputum symptoms or impact when chronic bronchitis, based on the usual definition, was present ().
Figure 1 CASA-Q scores for each domain and chronic bronchitis.
Abbreviation: CASA-Q, cough and sputum assessment questionnaire.
Variables associated with the SGRQ total score in univariate analyses
Univariate analyses demonstrated that each CASA-Q domain score was associated with the total SGRQ score: cough symptoms (R=−0.391, P<0.0001), cough impact (R=−0.586, P<0.0001), sputum symptoms (R=−0.263, P<0.0005), and sputum impact (R=−0.481, P<0.0001). No significant association was found between the total SGRQ score and age, sex, BMI, smoking status, cumulative smoking, and cardiovascular comorbidities. Conversely, the total SGRQ score was associated with chronic bronchitis, number of exacerbations in the previous year, mMRC dyspnea grade, FEV1% predicted, and the hospital anxiety and depression subscales ().
Table 2 Associations between the total SGRQ score and other characteristics of patients in univariate analyses
Independent contribution of cough and sputum production to quality of life impairment
One hundred and forty-eight COPD patients with insightful data on the CASA-Q and the variables identified as associated with the total SGRQ score in univariate analyses were included in the multivariate analyses (). There were no statistically significant differences between the clinical characteristics of the 148 patients included in the multivariate analyses and the 30 patients not included because of incomplete data. When all the CASA-Q domains were considered without other variables, both the cough symptoms and cough impact scores were statistically associated with the total SGRQ score (model 1). Multivariate analyses including all CASA-Q domains, and FEV1% predicted, mMRC dyspnea grade, hospital anxiety and depression scale, cardiovascular comorbidities, and the number of exacerbations in the previous year as covariates retained the cough impact domain as the sole CASA-Q domain independently associated with SGRQ total score in the final model (model 2). When all the CASA-Q domains were introduced separately, they were all retained as independently associated with SGRQ total score in their specific models (models 3–6). When chronic bronchitis was introduced instead of CASA-Q domains, it was also independently associated with SGRQ total score, but the variance explained was lower than in the model with the CASA-Q cough impact domain (model 7).
Table 3 Relationships between the SGRQ total score, and CASA-Q domain scores and clinical variables in multivariate analyses
Discussion
This multicenter cross-sectional study demonstrates that current cough, assessed by the dedicated CASA-Q questionnaire in the 7 previous days, is independently associated with HRQoL impairment in COPD. Importantly, the cough impact domain of the CASA-Q was a stronger determinant of HRQoL impairment than chronic bronchitis, indicating that current cough, rather than current sputum production or chronic cough and sputum production, is responsible for reduced HRQoL in COPD patients.
We used the dedicated CASA-Q questionnaire to examine the contribution of current cough and sputum production to HRQoL impairment in COPD patients. A recent study in a large population of 4,513 subjects from the COPDGene cohort demonstrated that adding answers to the questions about cough and sputum from the SGRQ to the usual definition of chronic bronchitis allowed identifying more patients with similar phenotypes associated with poorer outcomes.Citation23 Our study concurs with the study by Kim et al in establishing new ways of assessing cough and sputum production and their contribution to important outcomes in COPD patients. Dedicated questionnaires (eg, the CASA-Q questionnaire) and/or cough monitorsCitation15 could represent useful tools for assessing these symptoms and their contribution to patient-reported outcomes in clinical trials and real life.
In the present study, the CASA-Q scores of each domain demonstrated significant association with HRQoL impairment. The correlation coefficients were relatively weak in univariate analyses, except for the cough impact domain which exhibited a stronger correlation coefficient. Interestingly, there was a large variability of the CASA-Q scores of each domain, even when chronic bronchitis was present. Chronic cough is often assessed by simple questions on daily or usual cough and sputum production, eventually completed by data related to chronology parameters such as date of onset and duration (at least 3 months each year during the past 2 years). In the present study, each domain score of the CASA-Q was strongly lower when chronic bronchitis was present, but multivariate analyses demonstrated that the association of HRQoL impairment was greater with the cough impact domain score than with the usual definition of chronic bronchitis. These results demonstrate that a dedicated questionnaire assessing cough in the previous 7 days is more accurate to identify cough-associated HRQoL impairment in COPD than the usual definition of chronic bronchitis assessing cough and sputum production during the past 2 years.
The CASA-Q targets cough and sputum by an original approach assessing both the symptoms and impacts in daily activities within the last 7 days.Citation21 A study assessing the evolution of the CASA-Q domains’ scores during and after COPD exacerbation demonstrated the responsiveness of the CASA-Q to clinical recovery after COPD exacerbation,Citation22 suggesting its usefulness for monitoring cough and sputum production symptoms and their impacts in longitudinal studies. Interestingly, the feasibility of using the CASA-Q for assessing cough and sputum was also demonstrated in idiopathic pulmonary fibrosis.Citation24 Our study confirmed this feasibility in a large group of COPD subjects included in a multicenter cohort. It should be pointed out that the diagnosis of COPD in the Initiative BPCO cohort is secured on international criteria using a standard definition of COPD and standardized questionnaires, allowing the collection of prospective data on demographics, symptoms, smoking history, and comorbidities.Citation1
One strength of this study is the strategy of multivariate analyses including data on all domains of the CASA-Q together with the major factors associated with poor outcomes in COPD, allowing to thoroughly characterize the contribution of cough to HRQoL impairment. When all variables were considered in the multivariate analyses, the CASA-Q cough impact score was found to be significantly associated with HRQoL impairment, compared to dyspnea assessed by the mMRC scale. The other variables significantly associated in the multivariate analyses were FEV1, exacerbations, depression, anxiety, and cardiovascular comorbidities. It should be noted that the R2 values of the different models of the multivariate analyses were not much different (0.54–0.62), indicating that each CASA-Q domain score remained pertinent for its association with HRQoL impairment. These results suggest that assessing cough is as important as assessing other key elements associated with poor outcomes and used to guide therapeutic choices in COPD, such as dyspnea, FEV1, and exacerbations. Accordingly, the CASA-Q could be of great interest to efficiently assess cough and sputum in COPD for better characterization of COPD phenotypes and to assess the response to innovative treatments in clinical trials.
Limitations
One limitation is the cross-sectional nature of the study, which prevents from linking CASA-Q data with longitudinally collected outcomes. The sample size is also a potential limitation; indeed, the generalizability of these results should be tested in larger populations. The 7-day period assessed with the CASA-Q does not provide information regarding the impact of cough and sputum symptoms on HRQoL in a shorter or longer than 7-day period. It would be interesting to investigate the relationships between the CASA-Q scores and the COPD assessment test questionnaire that includes eight items with two items assessing cough and sputum production and which is used in routine clinical practice. It could be argued that cough and sputum production are likely to relate to HRQoL as measured by the SGRQ, since this questionnaire actually comprises questions on cough and sputum production. However, there are only two questions on these symptoms in the SGRQ; so, their contribution to the total score is minimal. In addition, a sensitivity analysis was performed to assess the relationship between the CASA-Q and the total score of the SGRQ calculated after removing the two questions on cough and sputum production; identical results were found (data not shown).
Conclusion
This study provides evidence that current cough in the previous 7 days is an important determinant of HRQoL impairment in stable COPD patients and suggests that the dedicated questionnaire CASA-Q is an interesting tool to monitor cough and sputum production in COPD clinical trials.
Author contributions
GD, P-RB, J-LP, and NR have made substantial contributions to the conception or design of the work. GD, P-RB, RE, PC, IC-F, PN-M, GB-R, TP, GJ, DC, J-LP, and NR contributed to acquisition, analysis, and interpretation of data for the work. GD, P-RB, J-LP, and NR drafted the work. GD, P-RB, RE, PC, IC-F, PN-M, GB-R, TP, GJ, DC, J-LP, and NR revised the article critically for important intellectual content. GD, P-RB, RE, PC, IC-F, PN-M, GB-R, TP, GJ, DC, J-LP, and NR gave their final approval of the version to be published. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The French Initiative BPCO cohort is supported by an unrestricted grant from Boehringer Ingelheim and Pfizer, which had no role in the study design.
Supplementary material
Table S1 Cough and sputum assessment questionnaire (CASA-Q)
Disclosure
GD reports collecting personal fees from Boehringer Ingelheim, AstraZeneca, Novartis, Chiesi, and BTG/PneumRx; P-RB reports personal fees from Boehringer Ingelheim, Astra Zeneca, Novartis, GSK, and Vertex; RE reports personal fees from Boehringer Ingelheim, Novartis, AstraZeneca, Mundipharma, ALK, and Chiesi; PC reports personal fees from Almirall, Boehringer Ingelheim, Centocor, GSK, MSD, AstraZeneca, Novartis, Teva, Chiesi, and Schering Plough; IC-F reports personal fees from Boehringer Ingelheim, Novartis, and Pfizer; PN-M reports personal fees from Boehringer Ingelheim; GB-R reports personal fees from Boehringer Ingelheim, Novartis, GSK, Chiesi, and Pfizer; TP reports personal fees from Boehringer Ingelheim, Novartis, GSK, Chiesi, and Pierre Fabre; GJ reports personal fees from Boehringer Ingelheim, Pfizer, Novartis, and GSK; DC reports personal fees from Boehringer Ingelheim and Roche; J-LP reports no conflict of interest; NR reports personal fees from Boehringer Ingelheim, Novartis, Teva, GSK, AstraZeneca, Chiesi, Mundipharma, Cipla, and Pfizer. The authors report no other conflicts of interest in this work.
References
- BurgelPRNesme-MeyerPChanezPCough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjectsChest2009135497598219017866
- AgustiACalverleyPMCelliBEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigatorsCharacterisation of COPD heterogeneity in the ECLIPSE cohortRespir Res20101112220831787
- de OcaMMHalbertRJLopezMVThe chronic bronchitis phenotype in subjects with and without COPD: the PLATINO studyEur Respir J2012401283622282547
- KimVDaveyAComellasAPCOPDGene® InvestigatorsClinical and computed tomographic predictors of chronic bronchitis in COPD: a cross sectional analysis of the COPDGene studyRespir Res2014155224766722
- MiravitllesMGuerreroTMayordomoCSánchez-AgudoLNicolauFSegúJLFactors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study GroupRespiration200067549550111070451
- KimVCrinerGJThe chronic bronchitis phenotype in chronic obstructive pulmonary disease: features and implicationsCurr Opin Pulm Med201521213314125575367
- CorhayJLVinckenWSchlesserMBossuytPImschootJChronic bronchitis in COPD patients is associated with increased risk of exacerbations: a cross-sectional multicentre studyInt J Clin Pract201367121294130124246208
- LindbergASawalhaSHedmanLLarssonLGLundbäckBRönmarkESubjects with COPD and productive cough have an increased risk for exacerbations and deathRespir Med20151091889525528948
- VestboJPrescottELangePAssociation of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study GroupAm J Respir Crit Care Med19961535153015358630597
- CamiciottoliGBigazziFPaolettiMCestelliLLavoriniFPistolesiMPulmonary function and sputum characteristics predict computed tomography phenotype and severity of COPDEur Respir J201342362663523258785
- AnnesiIKauffmannFIs respiratory mucus hypersecretion really an innocent disorder? A 22-year mortality survey of 1,061 working menAm Rev Respir Dis198613446886933767125
- GuerraSSherrillDLVenkerCCeccatoCMHalonenMMartinezFDChronic bronchitis before age 50 years predicts incident airflow limitation and mortality riskThorax2009641089490019581277
- PelkonenMNotkolaILNissinenATukiainenHKoskelaHThirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural menChest200613041129113717035447
- LangePNyboeJAppleyardMJensenGSchnohrPRelation of ventilatory impairment and of chronic mucus hypersecretion to mortality from obstructive lung disease and from all causesThorax19904585795852402719
- SumnerHWoodcockAKolsumUPredictors of objective cough frequency in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187994394923471467
- RodríguezEFerrerJZockJPPAC-COPD Study GroupLifetime occupational exposure to dusts, gases and fumes is associated with bronchitis symptoms and higher diffusion capacity in COPD patientsPLoS One201492e8842624516659
- DijkstraAEde JongKBoezenHMRisk factors for chronic mucus hypersecretion in individuals with and without COPD: influence of smoking and job exposure on CMHOccup Environ Med201471534635224642640
- MarchettiNGarshickEKinneyGLCOPDGene InvestigatorsAssociation between occupational exposure and lung function, respiratory symptoms, and high-resolution computed tomography imaging in COPDGeneAm J Respir Crit Care Med2014190775676225133327
- QuanjerPHTammelingGJCotesJEPedersenOFPeslinRYernaultJCLung volumes and forced ventilatory flowsEur Respir J19936Suppl 1654024576915
- MillerMRHankinsonJBrusascoVATS/ERS Task ForceStandardisation of spirometryEur Respir J200526231933816055882
- CrawfordBMonzBHohlfeldJDevelopment and validation of a cough and sputum assessment questionnaireRespir Med2008102111545155518662868
- MonzBUSachsPMcDonaldJCrawfordBNivensMCTetzlaffKResponsiveness of the cough and sputum assessment questionnaire in exacerbations of COPD and chronic bronchitisRespir Med2010104453454119917525
- KimVCrapoJZhaoHCOPDGene InvestigatorsComparison between an alternative and the classic definition of chronic bronchitis in COPDGeneAnn Am Thorac Soc201512333233925575351
- GriesKSEsserDWiklundIContent validity of CASA-Q cough domains and UCSD-SOBQ for use in patients with idiopathic pulmonary fibrosisGlob J Health Sci2013165613114124171881
