Abstract
Objectives
Cellular senescence is a state of irreversible growth arrest induced either by telomere shortening (replicative senescence) or stress. The bronchial epithelial cell is often injured by inhaled toxic substances, such as cigarette smoke. In the present study, we investigated whether exposure to cigarette smoke extract (CSE) induces senescence of bronchial epithelial cells; and Cordyceps sinensis mechanism of inhibition of CSE-induced cellular senescence.
Methods
Human bronchial epithelial cells (16HBE cells) cultured in vitro were treated with CSE and/or C. sinensis. p16, p21, and senescence-associated-galactosidase activity were used to detect cellular senescence with immunofluorescence, quantitative polymerase chain reaction, and Western blotting. Reactive oxygen species (ROS), PI3K/AKT/mTOR and their phosphorylated proteins were examined to testify the activation of signaling pathway by ROS fluorescent staining and Western blotting. Then, inhibitors of ROS and PI3K were used to further confirm the function of this pathway.
Results
Cellular senescence was upregulated by CSE treatment, and C. sinensis can decrease CSE-induced cellular senescence. Activation of ROS/PI3K/AKT/mTOR signaling pathway was enhanced by CSE treatment, and decreased when C. sinensis was added. Blocking ROS/PI3K/AKT/mTOR signaling pathway can attenuate CSE-induced cellular senescence.
Conclusion
CSE can induce cellular senescence in human bronchial epithelial cells, and ROS/PI3K/AKT/mTOR signaling pathway may play an important role in this process. C. sinensis can inhibit the CSE-induced senescence.
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive disabling illness associated with an abnormal inflammatory response of the airways and the lungs to noxious stimuli. It is characterized by persistent airflow limitation that is not fully reversible and airway inflammation.Citation1 Recently, it has been shown that cellular senescence might play a role in the pathogenesis of COPD.Citation2
Cellular senescence is a process that results from a variety of stresses, leading to a state of irreversible growth arrest. Senescent cells accumulate during aging and have been implicated in promoting a variety of age-related diseases, such as ischemic heart disease, Alzheimer’s disease, and COPD.Citation3
Telomere shortening, activation of PI3K-AKT-mTOR signaling pathway, impaired autophagy, mitochondrial dysfunction, stem cell exhaustion, and low grade chronic inflammation are the main mechanisms of cell senescence.Citation4 Many of these pathways are driven by chronic oxidative stress. Increased oxidative stress is well documented in the lungs of patients with COPD. This increase in oxidative stress is due to oxidants in cigarette smoke and activated inflammatory cells. Reactive oxygen species (ROS) are generated in oxidative stress, and activate PI3K, then, via activation of the kinase AKT, activates mTOR, the PI3K–AKT–mTOR signaling pathway is activated. Then, the activated pathway inhibits autophagy via unc-51-like autophagy activating kinase-1 and reduces sirtuin-1 activity, both of which accelerate the aging process.Citation5 The pathway of mTOR is a key modulator of aging and age-related disease.Citation6 Activation of the mTOR pathway may play an important role in multimorbidity, and so inhibition of this pathway may offer a good future therapeutic opportunity.Citation7
In the People’s Republic of China, many traditional Chinese medicines modalities are regularly used in the treatment of patients with COPD. Several clinical trialsCitation8,Citation9 have shown that traditional Chinese medicines might have a therapeutic effect on patients with COPD, including improvement of symptoms, quantity of life, and lung function. Cordyceps sinensis, or Dong Chong Xia Cao in Chinese, is a special type of medicinal mushroom, which forms on an insect larva infected by the C. sinensis fungus; it is rich in nucleoside, polysaccharide, sterol, protein, amino acid, and polypeptide.Citation10 Now research on C. sinensis is mainly focused on chemical constituents and pharmacological actions.Citation11 C. sinensis has the functions of antiaging, anti-inflammatory, antioxidant, antitumor, antiapoptosis, and can regulate endocrine, respiratory, immune, and nervous systems.Citation12 But the specific inhibitory mechanism of C. sinensis is not clear. Singh et alCitation13 found C. sinensis could decrease oxidative stress in human lung epithelial cells. Antioxidation plays an important role in the functions of C. sinensis. The oxidative stress in the respiratory system is mainly caused by smoke, which has a close association with cell senescence.Citation14
Based on earlier research mentioned in the previous paragraph, the functions of C. sinensis are wide, but there is no definite research about its effect on cell senescence and specific cellular mechanisms. Here, we investigate the inhibitory effect of C. sinensis on the senescence of human bronchial epithelial cells induced by cigarette smoke extract (CSE) and its mechanism.
Materials and methods
Cells and regents
Ethical approval was not required by the institutional review board of Qilu Hospital, Shandong University, because the cells mentioned in the experiment were derived from cell lines. The human bronchial epithelial cell line, 16HBE, was purchased from a cell bank (ATCC, Manassas, VA, USA) and cultured in high glucose Dulbecco’s Modified Eagle’s Medium (H-DMEM) complete medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/mL of penicillin, and 100 μg/mL of streptomycin at 37°C under conditions of 5% CO2. After 2 days in culture, the adherent cells were consistently 50% of epithelial morphology. The cells were treated with CSE and/or C. sinensis (2 hours before adding CSE).
CSE was prepared by a modification of the method of Carp and Janoff; briefly, three cigarettes without filters were combusted in a modified gas collecting pipe.Citation15 The smoke was bubbled through 3 mL of phosphate-buffered saline. The resulting suspension was adjusted to pH 7.4 with concentrated NaOH and then filtered through a 0.22 μm pore filter (MILLEX®GP) to remove bacteria and large particles. CSE was applied to 16HBE cultures within 30 minutes of preparation. To make sure the concentration of CSE was stable, the burning time and the pressure of gas collecting pipe were fixed. The initial absorbance value was determined in the range of CSE (270–280 nm) by using the spectrophotometer, and the absorbance value of CSE was the same as that for each preparation. CSE solution was diluted by adding H-DMEM containing 10% FBS to concentrations of 0.5%, 1%, 2%, and 5%.
Cultured C. sinensis extracts were provided by Hangzhou Zhongmei Huadong Pharmaceutical Co Ltd. (Hangzhou, People’s Republic of China) at a concentration of 0.99 g/mL; it was microfiltered to remove bacteria. C. sinensis was diluted by adding H-DMEM containing 10% FBS to a concentration of 100 mg/L.Citation16,Citation17
The PI3K signaling pathway inhibitor Ly294002 (#9901, Cell Signaling Technology, Danvers, MA, USA) 10 μMCitation18 and ROS inhibitor N-acetylcysteine (S0077, Beyotime, Haimen, People’s Republic of China) 10 mM were added.Citation19
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay
16HBE cells plated in 96-well plates were treated with CSE in different doses and time points. Cell viability was assayed using the Vibrant® MTT Cell Proliferation Assay Kit (Molecular Probes, Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s protocol.
Staining for senescence-associated-β-galactosidase
16HBE cells were fixed in 2% formaldehyde and 0.2% glutaraldehyde for 10 minutes at room temperature, washed in phosphate-buffered saline, and incubated for 24 hours at 37°C with senescence-associated β-galactosidase (SA-β-gal) (Sigma-Aldrich, St Louis, MO, USA) in staining solution (40 mM sodium citrate [pH 6], 150 mM NaCl, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, and 1 mg/mL bromo-4-chloro-3-indolyl-β-d-galactoside).
Western blotting
16HBE cells were grown to confluence in six-well plates. After treatment with CSE, C. sinensis, N-acetylcysteine, or LY294002, 16HBE cells were lysed with a cell extraction buffer (Thermo Fisher Scientific), and the total amount of protein was quantitated using the Pierce BCA Protein Assay Kit (P0010S, Beyotime). Equal amounts of total protein (30 μg) were separated on 10%–12% Bis–Tris gels in MOPS SDS Running Buffer (Thermo Fisher Scientific), transferred to a polyvinylidene difluoride membrane (Immobilon, Millipore Corp, Bedford, MA, USA).
Then the polyvinylidene difluoride membrane was blocked with 5% skimmed milk in Tris-buffered saline (50 mM Tris–Cl, pH 7.5, 150 mM NaCl) for 60 minutes at room temperature and probed with anti-p16 antibody (1:2,000; Abcam, Cambridge, MA, USA), anti-p21 antibody (1:500; Abcam), anti-PI3K antibody (1:500; Cell Signaling Technology), anti-AKT antibody (1:2,000; Cell Sig-naling Technology), anti-p-AKT antibody (1:1,000; Cell Signaling Technology), anti-mTOR antibody (1:1,000; Cell Signaling Technology), and anti-p-mTOR antibody (1:1,000; Cell Signaling Technology) followed by overnight incubation with primary antibodies. The membrane was washed three times with 1× TBS-T for 5 minutes and then incubated for 1 hour with secondary antibodies conjugated to horseradish peroxidase at room temperature. The membrane was exposed to a multicolor fluorescence gel imaging analysis system (FluorChem®Q, Cell Biosciences, Santa Clara, CA, USA) and visualized using the ECL system (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The integrated density value of band intensities from the films was analyzed by the ImageJ software (National Institutes of Health, Bethesda, MA, USA).
Real-time quantitative polymerase chain reaction analysisCitation20
16HBE cells were grown to confluence in six-well plates. After transfection with CSE and/or C. sinensis extract, RNA was isolated using TRIzol (Thermo Fisher Scientific), and quantified using a NanoDrop (Thermo Fisher Scientific). RNA was analyzed by real-time polymerase chain reaction (PCR) amplification. Briefly, 1 μg of total RNA per sample was denaturated at 70°C for 10 minutes and laid on ice for 10 minutes, PCR reactions were performed in a volume of 20 μL containing 4 μL 5× reverse transcriptase (RT) buffer (Toyobo, Osaka, Japan), 1 μL RT Enzyme Mix (Toyobo), 1.0 μL (5 pmol) of each primer (sense and antisense) in the presence of PCR buffer (Toyobo). The complementary DNAs (cDNAs) were predenaturated for 2 minutes at 95°C followed by 35 cycles of 30 seconds denaturation at 95°C, 30 seconds annealing at 60°C, and 1 minute elongation at 68°C.
p16 was amplified by using the following primers (157 bp): forward primer (5′-3′): CTACTCTCCTCCGCTGGGAA and reverse primer (5′-3′): GGCCTAACTTAGCGCTGCTT. p21 was amplified by using the following primers (74 bp): forward primer (5′-3′): 5′-CAGGCTCAGGAGTTAGCAAGG and reverse primer (5′-3′): TCAACACCCTGTCTTGTCTTCG. Glyceraldehyde 3-phosphate dehydrogenase was amplified by using the following primers (89 bp): forward primer (5′-3′): ATGATTCATCCCACGGCAAG and reverse primer (5′-3′): CTGGAAGATGGTGATGGGTT.
Real-time PCR reactions were performed in a volume of 20 μL containing 2 μL of cDNA, 8 μL of each primer (10 pmol/μL,10 μM) and 10 μL of QuantiTect™ SYBRs Green PCR containing DNA polymerase, dNTP mix, buffer, MgCl2, and fluorescent dyes (Qiagen, Mississauga, Ontario, Canada). The PCR protocol consisted of three programs: denaturation, amplification, and melting curve analysis for product identification. The denaturation and amplification conditions were 95°C for 20 minutes followed by 40 cycles of PCR. Each cycle included denaturation at 95°C for 30 seconds, annealing of 10 seconds at 60°C, and extension of 15 seconds at 72°C. The primers of p16, p21, and glyceraldehyde 3-phosphate dehydrogenase were stated as above. The temperature transition rate was 20°C/s, except when heating at 72°C, when it was 5°C/s. Fluorescence was measured at the end of every cycle to allow quantification of cDNA. Data were acquired and analyzed with the computer software Opticon Monitor (version 2.02.24; BioRad Laboratories, Hercules, CA, USA). Normalized expressions were calculated by using the calculated efficiency of each PCR reaction with a cDNA standard curve.
Immunofluorescence microscopyCitation21
16HBE cells cultured on glass slides were fixed with 4% paraformaldehyde for 10 minutes, incubated with 0.5% Triton X-100 for 30 minutes, and then blocked with 1% bovine serum albumin for 30 minutes at room temperature. The slides were incubated with anti-p16 (1:50) and anti-p21 (1:200) overnight at 4°C and subsequently with goat anti-rabbit immunoglobulin G-fluorescein isothiocyanate mAb (1:200) for 1 hour. 4′,6-Diamidino-2-phenylindole (500 ng/mL in 95% ethanol) was used to stain the nuclei for 20 seconds. Cover slips were mounted with 80% glycerol (Zsbio, Beijing, People’s Republic of China). The slides were examined under fluorescence microscopy (Nikon, Gotemba, Japan).
ROS
ROS assay kit (E004, Nanjing Jiangcheng Bioengineering Institute, Nanjing, People’s Republic of China) was used to detect intracellular ROS level following the manufacturer’s protocol. 4′,6-Diamidino-2-phenylindole (500 ng/mL in 95% ethanol) was used to stain the nuclei for 20 seconds. Stained cells were observed with fluorescence microscope (Nikon).
Statistical analysis
All data were from at least three separate experiments for Western blotting and ten separate experiments for MTT and staining for SA-β-gal. Statistical analysis was performed using the SPSS 12.0 software package (SPSS Inc., Chicago, IL, USA). Data were analyzed using one-way analysis of variance between the groups studied. Significance was defined for P<0.05.
Results
CSE-induced cellular senescence in human bronchial epithelial cells in vitro
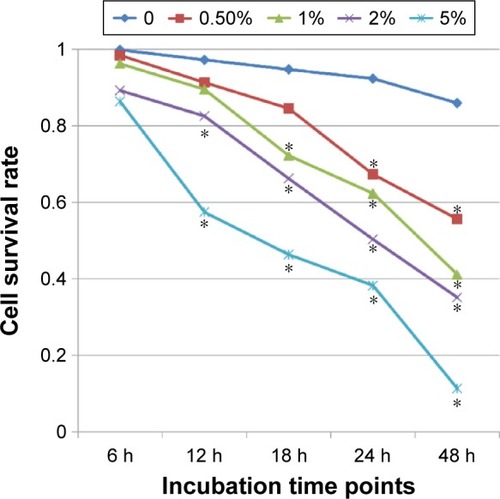
To detect the appropriate dose and action time of CSE, the cell survival rate was observed by MTT assays. 16HBE cells were treated with CSE at different doses and time points. The relative cell number was detected to evaluate cell growth. Cell survival rate was inhibited by CSE in a time- and dose-dependent manner. According to the survival curve (), considering IC50 and the obvious downward trend, stimulation by 2% CSE for 24 hours could be used in the follow-up experiments.
Figure 1 16HBE cells were incubated with CSE at different doses and time points.
Abbreviations: CSE, cigarette smoke extract; HBE, human bronchial epithelial; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide; SE, standard error.
C. sinensis can inhibit the CSE-induced cellular senescence
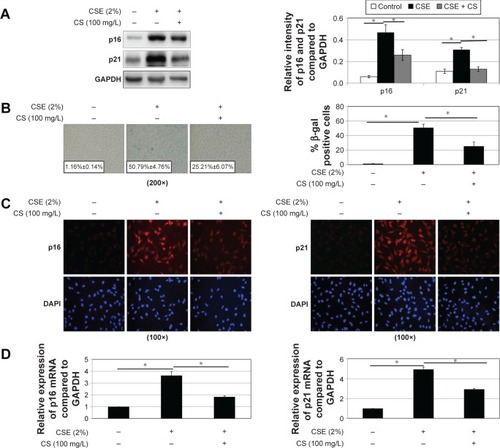
Previous reports have demonstrated that cigarette smoke induces bronchial epithelial cellular senescence,Citation22 and senescent cells are readily found in airway epithelia of patients with COPD.Citation23 Two major signaling pathways are involved in senescence: the p19ARF/p53/p21 and the p16INK4/CDK/pRb pathways.Citation24 We examined p21 and p16 protein levels to evaluate cell senescence, and higher p16 and p21 protein levels were found in epithelial cells in a time- and dose-dependent manner after CSE treatment (). However, higher p16 and p21 expressions induced by CSE could be reduced when treated with C. sinensis (). Immunofluorescence cytochemistry and messenger RNA levels of p16 and p21 were also analyzed to verify the result. They were significantly increased in 16HBE cells after CSE stimulation, but decreased when the cells were treated with C. sinensis before CSE stimulation (). SA-β-gal staining was performed to examine senescent cells. β-Galactosidase, the lysosomal hydrolase, is active at pH 4, but SA-β-gal is active at pH 6 and is only present in senescent cells; allowing the two activities to be readily distinguished.Citation25 We found SA-β-gal positive cells ratio was obviously increased by CSE stimulation. After adding C. sinensis, the ratio was able to be decreased compared to CSE group (). These data indicated that CSE stimulation could induce cellular senescence in human bronchial epithelial cells, and C. sinensis can inhibit the senescence induced by CSE.
Figure 2 Cellular senescence induced by CSE stimulation.
Notes: (A) 16HBE cells were stimulated with different doses of CSE for 24 hours, protein expressions of p16 and p21 were detected by Western blotting (*P<0.05, **P>0.05). (B) 16HBE cells were stimulated by 2% CSE for different time durations and protein expressions of p16 and p21 were detected by Western blotting (*P<0.05). Data are expressed as mean ± SE. Results represent at least three independent experiments.
Abbreviations: CSE, cigarette smoke extract; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HBE, human bronchial epithelial; SE, standard error.
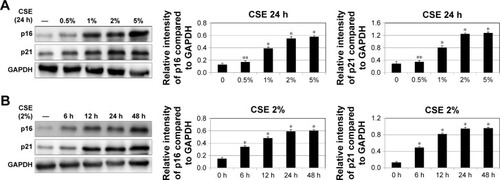
Figure 3 Cellular senescence affected by CSE and Cordyceps sinensis. 16HBE cells were stimulated with 2% CSE and/or C. sinensis (100 mg/L) (2 hours before adding CSE) for 24 hours.
Abbreviations: CS, Cordyceps sinensis; CSE, cigarette smoke extract; DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HBE, human bronchial epithelial; mRNA, messenger RNA; qPCR, quantitative polymerase chain reaction; SA-β-gal, senescence-associated β-galactosidase; SE, standard error.
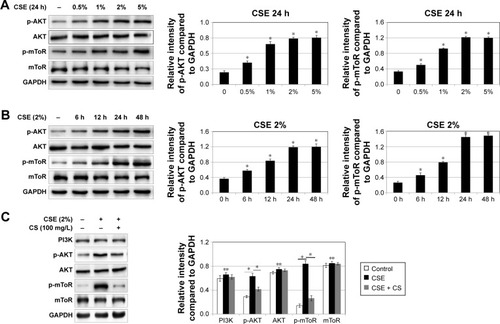
The role of ROS/mTOR signaling pathway and C. sinensis in CSE-induced senescence
ROS and PI3K/AKT/mTOR signaling pathway are involved in a variety of physiological processes, including cellular senescence.Citation26 According to previous studies,Citation26 we intend to determine the importance of ROS/mTOR signaling pathway in cellular senescence and the correlation between each other in this process. ROS can be caused by smoke, we used CSE to stimulate 16HBE cells and detect the change of ROS and the activation of PI3K/AKT/mTOR signaling pathway. We found ROS fluorescence was enhanced by CSE, and C. sinensis can weaken the ROS change (). The expressions of p-AKT and p-mTOR were promoted by CSE in a time- and dose-dependent manner (). Activation of mTOR signaling pathway was increased in the CSE group, and decreased when C. sinensis was added ().
Figure 4 ROS fluorescence in 16HBE cells.
Notes: 16HBE cells were stimulated with 2% CSE and/or Cordyceps sinensis (100 mg/L) for 24 hours, and stained with ROS fluorescence. Results represent three independent experiments.
Abbreviations: CSE, cigarette smoke extract; DAPI, 4′,6-diamidino-2-phenylindole; HBE, human bronchial epithelial; ROS, reactive oxygen species.
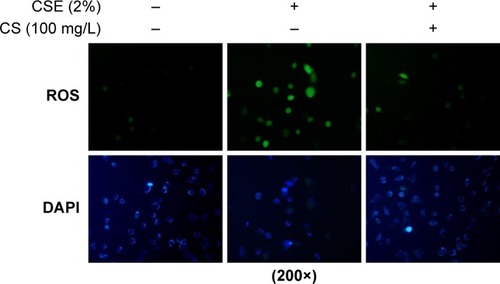
Figure 5 Expressions of PI3K, AKT, p-AKT, mTOR, p-mTOR in 16HBE cells.
Abbreviations: CS, Cordyceps sinensis; CSE, cigarette smoke extract; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HBE, human bronchial epithelial; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide-3-kinase; SE, standard error.
As we discussed earlier, CSE can induce cellular senescence, and C. sinensis was able to block the progress. ROS generation and ROS/mTOR signaling pathway play important roles in CSE-induced cellular senescence.
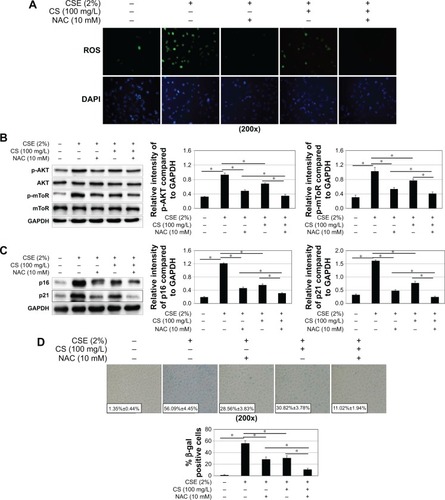
Blocking ROS/mTOR signaling pathway can alleviate CSE-induced cellular senescence in human bronchial epithelial cell
To confirm these findings, p16 and p21 expression analysis and SA-β-gal staining were also performed. As shown in , we inhibited ROS with N-acetylcysteine, and ROS generation and cellular senescence induced by CSE was weakened. Activation of mTOR signaling pathway was decreased ().
Figure 6 The effect of mTOR pathway activation and cellular senescence by blocking ROS. 16HBE cells were stimulated with 2% CSE, 100 mg/L Cordyceps sinensis or/and 10 mM NAC.
Abbreviations: CS, Cordyceps sinensis; CSE, cigarette smoke extract; DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HBE, human bronchial epithelial; mTOR, mammalian target of rapamycin; NAC, N-acetylcysteine; SA-β-gal, senescence-associated β-galactosidase; ROS, reactive oxygen species; SE, standard error.
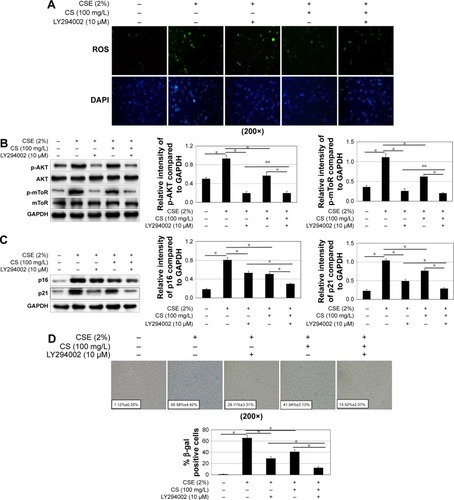
Next, we inhibited PI3K with LY294002. The cellular senescence induced by CSE was decreased, same as earlier (). The activation of mTOR signaling pathway was decreased (). However, there was no change in ROS generation ().
Figure 7 The effect of mTOR pathway activation and cellular senescence by blocking PI3K. 16HBE cells were stimulated with 2% CSE, 100 mg/L Cordyceps sinensis or/and 10 μM Ly294002.
Abbreviations: CS, Cordyceps sinensis; CSE, cigarette smoke extract; DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HBE, human bronchial epithelial; mTOR, mammalian target of rapamycin; ROS, reactive oxygen species; SE, standard error.
Our study suggested that CSE can induce human bronchial epithelial cell senescence via ROS and mTOR signaling pathway. In this process, ROS generated from CSE, activates mTOR signaling pathway, which leads to senescence. C. sinensis can block the process induced by CSE.
Discussion
Recently, it has been shown that cellular senescence might play a role in the pathogenesis of COPD.Citation2 Cellular senescence is a process that results from a variety of stresses, leading to a state of irreversible growth arrest.Citation27 Cellular senescence may be the same mechanism in many chronic diseases,Citation28 including COPD.
The aim of current routine pharmacotherapy for stable COPD is to control disease progression by using bronchodilators and anti-inflammatory drugs; however, the treatment outcomes remain less than satisfactory.Citation29 Treatment with Chinese herbal medicine is currently considered a complementary or alternative treatment and is being increasingly accepted worldwide.Citation30,Citation31 C. sinensis is one of the effective Chinese herbal medicines.
C. sinensis is a rare and precious medicinal mushroom having great medicinal value. Treatment with C. sinensis appreciably attenuated hypoxia-induced ROS generation, oxidation of lipids and proteins, and maintained antioxidant status.Citation12 Current evidence suggested that C. sinensis was effective in treating COPD.Citation32
Cellular senescence in COPD has a close association with smoking. Cigarette smoke exposure, the known main cause of COPD,Citation33 has been widely demonstrated to accelerate cellular senescence.Citation34,Citation35 We proved that CSE could upregulate the level of senescence in human bronchial epithelial cells, the same as in previous studies.
Purported effects of the C. sinensis suggested a wide range of biological functions, such as use as an aphrodisiac, analgesic, and immune modulator and free radical scavenger.Citation36 In recent years, C. sinensis has been investigated in animals and in vitro studies for antiaging effects.Citation37 Similarly, we proved that C. sinensis could weaken the CSE-induced senescence. C. sinensis may be a useful method to resist senescence in patients with COPD.
The correlation between CSE and C. sinensis is not clear. There was research about genetic damage induced by cigarette smoke and the protective effects of the C. sinensis.Citation38 Through our research, it was proven that CSE and C. sinensis are associated with cellular senescence.
Although the precise mechanisms of cellular senescence are still obscure, “the free radical theory of aging” has thus far been widely accepted.Citation39 Cellular senescence has close association with chronic oxidative stress which originated from smoke.Citation40 Under oxidative stress, the ROS increase.Citation26 ROS is a series of derivatives that are generated during the metabolic process by aerobic cells, and participate in and influence a range of cellular signaling pathways, including activating PI3K/AKT/mTOR signaling pathway.Citation41
There is increasing evidence that PI3K/AKT/mTOR signaling pathway is of critical importance in cellular senescence and aging, and inhibition of this pathway may extend the lifespan of many species.Citation42,Citation43 Xu et alCitation42 reported that active AKT inhibits the transcriptional activity of FOXO3a and thereby downregulates MnSOD, leading to an increased level of ROS that induced activation of the p53/p21 pathway and senescence. Senescent cells secrete cytokines and chemokines, known as the senescence-activated secretory phenotype, which amplifies and spreads cellular senescence.Citation44 This suggests that activation of the mTOR pathway may play an important role in multimorbidity and inhibition of this pathway offers a future therapeutic opportunity.Citation7
We detected ROS and the expressions of phospho-AKT and phospho-mTOR to evaluate the activation of ROS and PI3K/AKT/mTOR signaling pathway. Meanwhile, we found there was the same trend between the activation of ROS/PI3K/AKT/mTOR signaling pathway and the increased senescence. Then, we blocked ROS and PI3K, and found the same weakening on cellular senescence. So we confirmed that ROS and PI3K/AKT/mTOR pathway was one of the important mechanisms of cellular senescence. We also found that there were still significant differences between CSE group and CSE + C. sinensis group in activation of mTOR signaling pathway and senescence after blocking ROS; there were significant differences between CSE group and CSE + C. sinensis group in senescence after blocking PIK3.
The mechanism of cigarette smoke exposure resulting in cellular senescence mostly focused on excessive ROS production,Citation45 mitochondrial damage, telomere shortening, p53, and p16-retinoblastoma protein pathways.Citation46 We confirmed that CSE can affect ROS generation, as reported earlier. At the same time, we investigated the connection between CSE and PI3K/AKT/mTOR signaling pathway. We found that CSE could affect the activation of mTOR signaling pathway, by testing the phosphorylation AKT and mTOR; blocking PI3K had influence on the senescence induced by CSE. We believe that the PI3K/AKT/mTOR signaling pathway is involved in the mechanism of CSE-induced senescence.
Our study had confirmed the effect of C. sinensis on senescence. But the specific mechanism is not clear. Zou et alCitation12 found the inhibition effect of C. sinensis on oxidative stress. Park et alCitation47 reported Cordyceps militaris extract might have protective effects against oxidative stress-induced premature senescence via scavenging ROS. In our study, we found that C. sinensis could affect ROS, which was consistent with previous studies. We also explored the influence of C. sinensis on the activation of mTOR signaling pathway, blocking ROS and PI3K could affect the function of C. sinensis. Antisenescence of C. sinensis may have close association with ROS and PI3K/AKT/mTOR signaling pathway.
Although ROS and PI3K/AKT/mTOR signaling pathway play important roles in CSE-induced senescence, there were no specific quantified standards for us to find the particular points CSE and C. sinensis worked on. So, future studies need to be performed.
This study demonstrated that exposure to CSE-induced cellular senescence, which can be reduced by C. sinensis. The CSE induces senescence via the ROS and PI3K/AKT/mTOR signaling pathway, and C. sinensis can weaken the pathway, which then reduces the CSE-induced cellular senescence. Our research provided a novel strategy for slowing the human aging process and aging-related diseases.
Acknowledgments
This research is supported by the National Nature Science Foundation of China (NSFC) (NSFC for LD, Grant number: 81270072) and the Natural Science Funding committee of Shandong province (SDNSF) (Grant number: ZR2015PH022). The funders had no roles in study design, data collection and analysis, decision to publish, and preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease Updated 2014. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2014.pdfAccessed April 16, 2014
- KumarMSeegerWVoswinckelRSenescence-associated secretory phenotype and its possible role in chronic obstructive pulmonary diseaseAm J Respir Cell Mol Biol201451332333325171460
- ZieglerDVWileyCDVelardeMCMitochondrial effectors of cellular senescence: beyond the free radical theory of agingAging Cell20151411725399755
- ManuelCBlascoMAManuelSCellular senescence in cancer and agingCell2007130222323317662938
- BarnesPJMechanisms of development of multimorbidity in the elderlyEur Respir J201545379080625614163
- JohnsonSCRabinovitchPSKaeberleinMmTOR is a key modulator of ageing and age-related diseaseNature2013493743233834523325216
- LammingDWYeLSabatiniDMBaurJARapalogs and mTOR inhibitors as anti-aging therapeuticsJ Clin Invest2013123398098923454761
- LiJSLiSYYuXQBu-Fei Yi-Shen granule combined with acupoint sticking therapy in patients with stable chronic obstructive pulmonary disease: a randomized, double-blind, double-dummy, active-controlled, 4-center studyJ Ethnopharmacol2012141258459121911051
- LiSYLiJSWangMHEffects of comprehensive therapy based on traditional Chinese medicine patterns in stable chronic obstructive pulmonary disease: a four-center, open-label, randomized, controlled studyBMC Complement Altern Med20121219723107470
- LiuYWangJWangWZhangHZhangXHanCThe chemical constituents and pharmacological actions of Cordyceps sinensisEvid Based Complement Alternat Med2015201557506325960753
- DongCHYaoYJIn vitro evaluation of antioxidant activities of aqueous extracts from natural and cultured mycelia of Cordyceps sinensisFood Sci Technol2008414669677
- ZouYLiuYRuanMCordyceps sinensis oral liquid prolongs the lifespan of the fruit fly, Drosophila melanogaster, by inhibiting oxidative stressInt J Mol Med201536493994626239097
- SinghMTulsawaniRKogantiPChauhanAManickamMMisraKCordyceps sinensis increases hypoxia tolerance by inducing heme oxygenase-1 and metallothionein via Nrf2 activation in human lung epithelial cellsBiomed Res Int2013201356920624063008
- KirkhamPABarnesPJOxidative stress in COPDChest2013144126627323880677
- CarpHJanoffAPossible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidantsAm Rev Respir Dis19781183617621101105
- CuiMGuoXHLiHCordyceps sinensis extractant protect against cyclosporine induced injury of tubular epithelium cellJ Nephrol Daily Transplant20041314448
- MaLLYangXYGaoJZExperimental study on effect of Bailing Capsule on dendritic cells in miceZhong Guo Zhong Xi Yi Jie He Za Zhi20072710905908
- BadinlooMEsmaeili-MahaniSPhosphatidylinositol 3-kinases inhibitor LY294002 potentiates the cytotoxic effects of doxorubicin, vincristine, and etoposide in a panel of cancer cell linesFundam Clin Pharmacol201428441442223837575
- ChenSRenQZhangJN-acetyl-L-cysteine protects against cadmium-induced neuronal apoptosis by inhibiting ROS-dependent activation of Akt/mTOR pathway in mouse brainNeuropathol Appl Neurobiol201440675977724299490
- WuJDongFWangRACentral role of cellular senescence in TSLP-induced airway remodeling in asthmaPLoS One2013810e7779524167583
- ZhaoJPJiaoXAWuJXFIZZ1 promotes airway remodeling in asthma through the PTEN signaling pathwayInflammation20153841464147225655389
- KuilmanTMichaloglouCVredeveldLCOncogene-induced senescence relayed by an interleukin-dependent inflammatory networkCell200813361019103118555778
- TuderRMYoshidaTStress responses affecting homeostasis of the alveolar capillary unitProc Am Thorac Soc20118648549122052924
- CampisiJd’Adda di FagagnaFCellular senescence: when bad things happen to good cellsNat Rev Mol Cell Biol20078972974017667954
- DimriGPLeeXBasileGA biomarker that identifies senescent human cells in culture and in aging skin in vivoProc Natl Acad Sci U S A19959220936393677568133
- SchieberMChandelNSROS function in redox signaling and oxidative stressCurr Biol20142410R453R46224845678
- NyunoyaTMonickMMKlingelhutzAYarovinskyTOCagleyJRHunninghakeGWCigarette smoke induces cellular senescenceAm J Respir Cell Mol Biol200635668168816840774
- FabbriEZoliMGonzalez-FreireMSaliveMEStudenskiSAFerrucciLAging and multimorbidity: New tasks, priorities, and frontiers for integrated gerontological and clinical researchJ Am Med Dir Assoc201516864064725958334
- QaseemAWiltTJWeinbergerSEDiagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory SocietyAnn Intern Med2011155317919121810710
- ChenXMayBDiYMOral Chinese herbal medicine combined with pharmacotherapy for stable COPD: A systematic review of effect on BODE index and six minute walk testPLoS One201493e9183024622390
- HuJZhangJZhaoWZhangYZhangLShangHCochrane systematic reviews of Chinese herbal medicines: an overviewPLoS One2011612e2869622174870
- WeiMSongYLZhangSZhangLMinFUCordyceps sinensis for chronic obstructive pulmonary diseases: a systematic reviewChin J Evidence-Based Med2013131113731381
- EisnerMDAnthonisenNCoultasDAn official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2010182569371820802169
- HaraHArayaJTakasakaNInvolvement of creatine kinase B in cigarette smoke-induced bronchial epithelial cell senescenceAm J Respir Cell Mol Biol201246330631221980054
- FujiiSHaraHArayaJInsufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary diseaseOncoimmunology20121563064122934255
- YamaguchiYKagotaSNakamuraKShinozukaKKunitomoMAntioxidant activity of the extracts from fruiting bodies of cultured Cordyceps sinensisPhytother Res200014864764911114006
- JiDBYeJLiCLWangYHZhaoJCaiSQAntiaging effect of Cordyceps sinensis extractPhytother Res200923111612218803231
- MaJBiSProtective effects of Cordyceps sinensis (Berk) Sacc, artificial pypha on genetic damage induced by cigarette smokeBeijing Da Xue Xue Bao. Zi ran ke xue bao1996324521526
- HarmanDFree radical theory of aging: an update: increasing the functional life spanAnn NY Acad Sci20061067102116803965
- BonoRTassinariRBellisarioVUrban air and tobacco smoke as conditions that increase the risk of oxidative stress and respiratory response in youthEnviron Res201513714114625531819
- JinSYLeeHSKimEKHaJMKimYWBaeSReactive oxygen species and PI3K/Akt signaling in cancerFree Radic Biol Med201475Suppl 1S34S3526461347
- XuYLiNXiangRSunPEmerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescenceTrends Biochem Sci201439626827624818748
- HarrisonDEStrongRSharpZDRapamycin fed late in life extends lifespan in genetically heterogeneous miceNature2009460725339239519587680
- SalamaRSadaieMHoareMNaritaMCellular senescence and its effector programsGenes Dev20142829911424449267
- ItoSArayaJKuritaYPARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesisAutophagy201511354755925714760
- HaraHArayaJItoSMitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescenceAm J Physiol Lung Cell Mol Physiol201330510L737L74624056969
- ParkJMLeeJSLeeKRHaSJHongEKCordyceps militaris extract protects human dermal fibroblasts against oxidative stress-induced apoptosis and premature senescenceNutrients2014693711372625230212
