Saito T, Takeda A, Hashimoto K, et al. Int J Chron Obstruct Pulmon Dis. 2015;10:2393–2404.
On page 2395, right column, line 3, “Philadelphia, PA, USA” should have read “Hoechberg, Germany”.
On page 2396, was incorrect. The corrected figure is shown below.
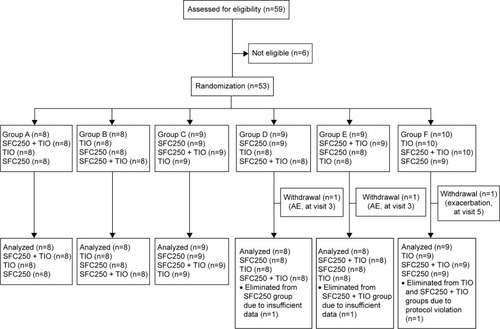
Figure 2 CONSORT flow diagram.
On page 2396, right column, line 1, “although FEV1 and FVC were not log transformed prior to analysis” should have read “although sRaw and sGaw were log transformed prior to analysis”.
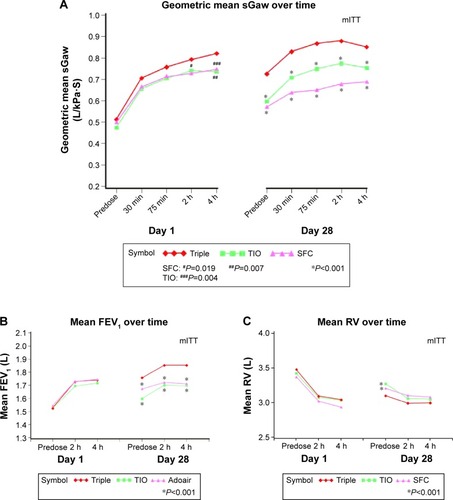
On page 2397, right column, Lung volumes section, line 5, “Statistical significance was observed for postdose RV immediately after dosing (time =0) on day 28 for SFC250 + TIO compared to TIO and SFC250 (Tables S3, S4). Improvements on day 28 were also seen in postdose TGV, IC, and TLC following treatment with SFC250 + TIO compared to each component, but none were significant (Table S4). Improvements were seen for all parameters for their adjusted mean values on day 1, but none were significant (Table S3).” Should have read “Statistical significance was observed for trough RV (time =0) on day 28 for SFC250 + TIO compared to TIO and SFC250 (Table S3). Improvements on day 28 were also seen in postdose TGV, IC, and TLC following treatment with SFC250 + TIO compared to each component, but statistical treatment differences were not observed, except for after dosing (240 min) IC at day 28 (Table S3). Improvements were seen for all parameters for their adjusted mean values on day 1, but statistical treatment differences were not observed, except for SFC250 after dosing (240 min) TGV at day 1 (Table S3).”
On page 2398, was incorrect. The corrected figure is shown below.
Figure 3 (A) sGaw, (B) FEV1, and (C) RV on days 1 and 28 of treatment.
On page 2398, Table 3, TGV (L) row, SFC250 + TIO vs TIO (n=50): 97.5% CI column, “−0.029” should have read “0.029”.
On page 2398, Table 3, TGV (L) row, SFC250 + TIO vs SFC250 (n=50): 97.5% CI column, “−0.028” should have read “0.028”.
On page 2398, Table 3, Note section, “Difference” should have read “Adjusted mean difference”.
On page 2402, Table S2, figure caption, “Postdose raw mean (standard deviation log) values of sGaw and sRaw (mITT population)” should have read “Postdose raw geometric mean (standard deviation log) values of sGaw and sRaw (mITT population)”.
On page 2402, Table S2, Time (minutes) column, sGaw, third line down, “SFC250 + TIO” should have read “SFC250”.
On page 2402, Table S2, Time (minutes) column, sRaw, third line down, “SFC250 + TIO” should have read “SFC250”.
