Abstract
Objectives
The objectives of this study were to estimate the impact of recruitment source and outcome definition on the incidence of acute exacerbations of COPD (AECOPD) and explore possible predictors of AECOPD.
Patients and methods
During a 1-year follow-up, we performed a baseline visit and four telephone interviews of 81 COPD patients and 132 controls recruited from a population-based survey and 205 hospital-recruited COPD patients. Both a definition based on health care utilization and a symptom-based definition of AECOPD were applied. For multivariate analyses, we chose a negative binomial regression model.
Results
COPD patients from the population- and hospital-based samples experienced on average 0.4 utilization-defined and 2.9 symptom-defined versus 1.0 and 5.9 annual exacerbations, respectively. The incidence rate ratios for utilization-defined AECOPD were 2.45 (95% CI 1.22–4.95), 3.43 (95% CI 1.59–7.38), and 5.67 (95% CI 2.58–12.48) with Global Initiative on Obstructive Lung Disease spirometric stages II, III, and IV, respectively. The corresponding incidence rate ratios for the symptom-based definition were 3.08 (95% CI 1.96–4.84), 3.45 (95% CI 1.92–6.18), and 4.00 (95% CI 2.09–7.66). Maintenance therapy (regular long-acting muscarinic antagonists, long-acting beta-2 agonists, inhaled corticosteroids, or theophylline) also increased the risk of AECOPD with both exacerbation definitions (incidence rate ratios 1.65 and 1.73, respectively). The risk of AECOPD was 59%–78% higher in the hospital sample than in the population sample.
Conclusion
If externally valid conclusions are to be made regarding incidence and predictors of AECOPD, studies should be based on general population samples or adjustments should be made on account of a likely higher incidence in other samples. Likewise, the effect of different AECOPD definitions should be taken into consideration.
Introduction
Acute exacerbations of COPD (AECOPD) are associated with mortality and poorer quality of life, leading to higher consumption of health resources, and a more rapid decline in lung function compared to COPD patients without frequent exacerbations.Citation1–Citation3 AECOPD pose a great burden to both patients and society.Citation4
There is scarce knowledge on the incidence and predictors of COPD exacerbations in COPD patients from the general population. Estimates from previous studies have shown an AECOPD rate per person per year from 0.65 to 1.40.Citation5–Citation7 These estimates vary by exacerbation definitions. A study comparing health care utilization and symptom-defined AECOPD observed higher incidence using the symptom-based definition.Citation8 The symptoms defining AECOPD are common, and even healthy individuals experience them at times.Citation5,Citation9 Hence, if the symptom-based definition is to be used, inclusion of a control group is necessary for adjustment of the baseline burden of these symptoms in the healthy population.
Most previous studies are done on selected populations without a plausible control group.Citation1,Citation3–Citation6,Citation8,Citation10–Citation13 Nevertheless, studies have indicated that exacerbation risk increases with higher age,Citation6,Citation7,Citation10–Citation13 a history of previous exacerbations,Citation5–Citation7,Citation10,Citation13,Citation14 increasing airflow obstruction,Citation5,Citation6,Citation10–Citation16 inflammatory biomarkers,Citation13,Citation17–Citation20 gastroesophageal reflux,Citation16,Citation21,Citation22 depression,Citation23,Citation24 reduced quality of life,Citation5,Citation8,Citation13 low body mass index (BMI), or weight loss,Citation6,Citation8,Citation25 in addition to chronic respiratory symptoms.Citation7,Citation11,Citation15,Citation26
Only two previous studies have genuine population-based study samples. The PLATINO study used a symptom-based definition of AECOPD in a general population,Citation15 but it was retrospective, did not define a control group, and did not report utilization-based exacerbations. Based on the COPDGene sample, Bowler et alCitation5 reported utilization-defined exacerbations gathered by six-monthly telephone interviews, but they did not include a control group without airflow obstruction. Thus, to our knowledge, there is no study where two exacerbation definitions were applied to the same study population and where a control group was included.
Important treatment-related decisions are currently made based on studies using different definitions of AECOPD and based on samples that are not population based;Citation3,Citation6,Citation14 thus, the effect of these choices needs to be estimated and the impact on predictors of exacerbations needs to be examined. The aims of this study were to estimate the incidence of AECOPD in the general population with two different exacerbation definitions, compare the results to a hospital-based COPD study sample, and explore predictors of AECOPD in both COPD study samples. We hypothesized that the population sample exacerbated less often than the hospital sample and that the symptom-based exacerbation definition resulted in a higher exacerbation rate compared to the health care utilization-based definition.
Methods
Our data were from the EconCOPD study, a 1-year prospective observational study conducted between March 2005 and August 2006 at Haukeland University Hospital, Bergen, Norway. The study was approved by the Regional Committee for Medical and Health Research Ethics in Western Norway (REK Vest case number 252.04), and all participants provided written informed consent. Details on sampling procedures and data collection have previously been published.Citation27
Study population and design
EconCOPD recruited three groups of participants who went through the same study during the same time frame: COPD patients from Haukeland University Hospital’s COPD register and COPD cases and control subjects from a general population cohort. The population-based cases and controls were recruited from a follow-up examination of the Hordaland County Respiratory Health Survey in 2003–2004, a random and representative sample of the population in Hordaland County in 1985.Citation28 COPD patients from the general population sample who had received treatment at the University Hospital were only registered as participants in the population-based sample.
Participants were all current or former smokers of ≥ 2.5 pack-years and were at least 40 years old. The choice of using 2.5 pack-years as the lower limit for smoking exposure was made to exclude nontobacco-associated COPD cases.Citation29 COPD was defined as a post-bronchodilator ratio of the forced expiratory volume in 1 second (FEV1) to the forced vital capacity <0.70 and FEV1 <80% predicted according to age, sex, and height.Citation30 Postbronchodilator spirometry was performed according to American Thoracic Society standards.Citation31 The control subjects had an FEV1/forced vital capacity ratio >0.70 and FEV1 > 80% predicted. The latter group was included to be able to adjust for a baseline risk of having exacerbation-like symptoms or events in a general population without respiratory disease.
All included participants were interviewed at baseline concerning smoking habits, education and employment status, and comorbidities. They were all clinically assessed by the project physician (RG). At 12 weeks, 24 weeks, 36 weeks, and 52 weeks, a follow-up interview was conducted by telephone, gathering information on productivity losses, health care utilization, and exacerbations of respiratory symptoms. Follow-up by telephone was considered satisfactory as no biological measurements were needed, the interval between interviews was short, and telephone coverage was reliable in the area.Citation32,Citation33 Information on comorbidities was gathered by asking for conditions listed in the Charlson Comorbidity Index.Citation34
Exacerbation definition
We defined a symptom-based AECOPD as an increase in two major symptoms (dyspnea, sputum volume, or sputum color) or one major and one minor symptom (cough, sore throat, nasal secretion, wheezing, or asthenia) for at least two consecutive days (modified Anthonisen criteria).Citation35,Citation36 A health care utilization-defined exacerbation was defined by use of antibiotics or corticosteroids due to respiratory disease or by hospitalization due to respiratory disease.
Statistical analyses
To test the distribution of characteristics across participant groups, we used parametric (t-test, analysis of variance) or nonparametric (χ2, Kruskal–Wallis) tests.
The frequency of exacerbations was skewed. Thus, we chose Kruskal–Wallis tests with ties and negative binomial regression for bivariate and multivariate analyses, respectively.Citation37 For the latter, we first performed bivariate analyses of each possible predictor and included those that were significant with a P-value of <0.10 in the final multivariate model. To estimate the effect of sampled population, we pooled the population-based and hospital-recruited participants and adjusted for participant group as well as COPD severity (Global Initiative on Obstructive Lung Disease [GOLD]-defined FEV1 categories). We estimated regression models both for symptom-defined exacerbations and exacerbations identified by health care utilization. The models included age, sex, smoking status, pack-years, educational level, FEV1% predicted, number of comorbid conditions, maintenance therapy (defined as regular use of long-acting muscarinic antagonists, long-acting beta-2 agonists, inhaled corticosteroids, or theophylline), influenza vaccination, pneumococcal vaccination, oxygen therapy, and BMI. The incidence rate ratio for each predictor gives their associated relative risk of exacerbation, adjusting for the other predictors.
All analyses were performed using Stata SE 13.1 (Stata-Corp LP, College Station, TX, USA).
Results
Characteristics
The characteristics and unadjusted exacerbation rates of each study group are shown in . Sex and smoking status did not vary between the participant groups, but differences were found for age, education, lung function, comorbidities, and being underweight (P<0.001). Nearly all hospital-recruited COPD patients received maintenance therapy for their COPD (80%), whereas a significantly lower percentage used maintenance therapy in both the population-based COPD sample and the control group (P<0.001). A similar pattern applied to vaccination status.
Table 1 Characteristics of hospital- and population-recruited COPD cases and population-recruited control subjects in the EconCOPD study
A population-recruited COPD patient had an average of 0.4 utilization-defined exacerbations per year and 2.9 symptom-defined exacerbations per year. The respective numbers for COPD patients from the hospital register were 1.0 utilization-defined exacerbations per year and 5.9 symptom-defined exacerbations per year, while the participants in the control group had 0.1 and 0.7 exacerbations per year with the respective exacerbation definitions (). For all three groups, the resulting exacerbation rates were skewed, with a total of 349 (83%) and 264 (63%) participants having zero or one exacerbation per year with the utilization-based and the symptom-based definitions, respectively.
Bivariate analyses
In bivariate analyses, we found that receiving maintenance therapy was associated with a higher exacerbation rate. With the utilization-based definition, increased GOLD stage was related to increased exacerbation rate. We found no consistent pattern for age, sex, education, smoking status, BMI, or vaccination (Tables S1 and S2).
Multivariate analyses
Tables S3 and S4 show the results from the bivariate and multivariate negative binomial regression analyses, including all COPD patients defined by either of the two study samples, and the results are illustrated in .
Figure 1 IRRs for COPD exacerbations when using a utilization-based and symptom-based definition.
Abbreviations: IRR, incidence rate ratio; gen pop, general population; GOLD, Global Initiative on Obstructive Lung Disease; yrs, years.
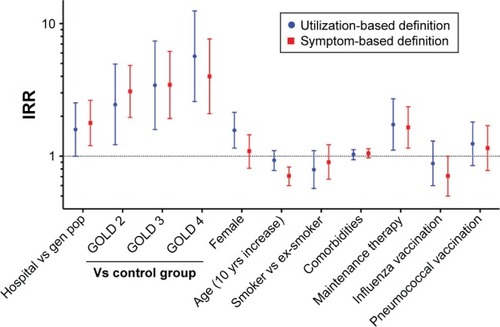
Applying the utilization-based exacerbation definition (Table S3), we found that the incidence rate ratio for COPD exacerbations was significantly higher in the hospital sample compared to the population sample, even after extensive adjustment for potential confounders. There were increasingly higher exacerbation risks with increasing severity of COPD. Female sex and receiving maintenance therapy were also significantly associated with higher risk of exacerbation in the multivariate model. Applying the symptom-based exacerbation definition (Table S4), we found, with three exceptions, the same main predictors as with the utilization-based definition. The exceptions were female sex that was not significantly associated with exacerbation risk, increasing age that was significantly associated with a lower exacerbation risk, and having undergone influenza vaccination that was significantly associated with a lower risk of exacerbation.
Discussion
We have shown that on average, a community-dwelling COPD patient had 0.4 utilization-based exacerbations per year, while COPD patients selected from a hospital register had one exacerbation per year (2.5 times more). The results for the symptom-based definition were 2.9 and 5.9 exacerbations per year (2.0 times more), respectively, for the two groups. In multivariate regression analysis, belonging to the hospital sample corresponded with a 59%–78% increased relative risk of experiencing an exacerbation compared to the population sample. In both groups, there were increasingly higher exacerbation rates with increasing COPD severity. By including control subjects, we have adjusted for the incidence of exacerbation-like events in subjects without respiratory disease.
This confirmed our hypothesis that COPD patients from a hospital register have more exacerbations than COPD patients found in the general population and, furthermore, that using a symptom-based definition results in a higher exacerbation rate than with a utilization-based definition.
The only population-based study that provides comparable data is the PLATINO study. It estimated an exacerbation-rate of 0.58/person/yr with a symptom-based exacerbation definition.Citation15 The study was retrospective with a longer recollection period, and the subjects might have been more prone to recall bias. Bowler et alCitation5 found an exacerbation rate of 0.65/person/yr for COPD patients using a utilization-based definition. They included all GOLD stages, including stage I. The results from the TORCH study, where the sample was from outpatient clinics and a utilization-based definition was applied, showed an annual exacerbation rate between 0.85 and 1.13.Citation6 In the study performed by Husebø et al,Citation7 they used a utilization-based definition and found an annual exacerbation rate per person to be 1.40 for their outpatient sample of COPD patients. Our results are in line with the results from the PLATINO study, but extend the previous knowledge on exacerbation rates and give enhanced understanding of the implications of how the selection of study samples and exacerbation definitions affect the results.
The independent predictors of increased exacerbation rates were belonging to the hospital sample, decreasing FEV1/increasing airflow limitation, female sex, and receiving maintenance therapy for the utilization-based definition. For the symptom definition, the same predictors were significantly associated with higher exacerbation risk apart from female sex, which did not prove significant, and adding increasing age and influenza vaccination, which were associated with a lower risk if present.
It is a novel, but not surprising, finding that hospital samples gave higher exacerbation rates than population-based samples. The participants recruited from the hospital register can be expected to have more severe disease as seen in the newly published study by Müllerova et alCitation13 and therefore to exacerbate more often. Müllerova et al found a hazard ratio for hospitalization due to AECOPD at 1.12 per 5% drop of FEV1% predicted, and those who did not exacerbate had a significantly lower BODE (Body mass index, airflow Obstruction, Dyspnea and Exercise capacity) index. We think our analyses demonstrated that studies on AECOPD recruiting from outpatient or hospital samples are biased toward higher exacerbation rates as compared with a general population. This might be not only due to differences in airflow limitation but also due to unidentified factors associated with the so-called frequent exacerbator phenotype.
The observed exacerbation risk associated with decreasing FEV1 has previously been seen by various authors.Citation1,Citation3,Citation7,Citation10,Citation11,Citation15,Citation38 As the airflow limitation increases, even minor influences from exacerbation-causing agents may lead to a worsening where a change in medication or even hospitalization is needed.
Whether female sex is truly associated with a greater risk of COPD exacerbations is an ongoing debate. It is not known if females perceive their symptoms differently, seek medical aid more frequently, or are genuinely more prone to exacerbations than men.Citation39,Citation40 Studies have shown both biological and cultural associations between sex and respiratory disease.Citation41 We found that female sex was only significantly associated with exacerbation risk with the utilization-based exacerbation definition. One explanation for this finding might be that women have more severe COPD exacerbations requiring medical care, as recently found by Kilic et al.Citation42 The alternative would be that men seek medical advice less frequently than women (a cultural explanation). In addition, the doctor’s response to their patients’ symptoms might differ by the patient sex.
In the multivariate analysis, receiving maintenance therapy was independently and significantly associated with elevated risk of exacerbation. We interpret this association as an expression of disease severity. The patients with the most impairing disease are also probably those advised to use medication and hence more prone to exacerbation due to their grade of disease and not due to the medication itself. This view is strengthened by the fact that 80% of the hospital samples used maintenance therapy (the group with the lowest FEV1 and most comorbidities in our dataset) and as few as 38% of the population-based COPD cases used such medication.
Having undergone influenza vaccination was significantly associated with a lower exacerbation risk. This confirms previous results from large datasets where prophylactic vaccination is proven to reduce acute exacerbations and does not provoke exacerbations when administered.Citation43 We could not find an association between pneumococcal vaccination and exacerbation risk. We believe this is due to the fact that as few as 47% of the hospital-based COPD patients and 5% of the population-based COPD cases had taken this vaccine, and hence, we had no power to evaluate this effect.Citation43
The main strength of our study is that we included both a population-based and a hospital-based group of COPD patients and that the analyses were performed on both groups highlighting the importance of source origin. We included two much-used definitions of an exacerbation and performed our analyses for these definitions separately on the same dataset. To our best knowledge, this has not been done before including a population-based sample. The Hokkaido cohort included several exacerbation definitions, but no population sample nor a control group.Citation8 Additionally, our project was prospective and had trained health personnel doing telephone interviews at intervals minimizing recall bias.Citation44 The overall response rate was high (79%), which enabled us to generalize our results to the Norwegian population.
Some possible weaknesses deserve mentioning. First, never-smoking subjects and subjects younger than 40 years were excluded. This was to avoid confounding with asthma patients and to ensure that potential differences between COPD cases and controls could not be explained by distinct smoking history. Second, the number of population-recruited COPD cases was lower than the number of participants from the other two groups and there were fewer with severe and very severe airflow limitation in this group. Yet, even in this group, there was a significantly increasing exacerbation risk with worsening grade of COPD, suggesting sufficient power. Third, participants of the current study were recruited from the city of Bergen, Western Norway, and eleven surrounding municipalities. However, a comparison between national Norwegian survey data for individuals older than 40 years with patients from the original cohort study that EconCOPD recruited from showed no discrepancy.Citation45 Finally, with our given sample size, the current analyses might have been prone to type II errors. Nevertheless, we were able to demonstrate a clear effect of the main explanatory variable (population) on the outcome.
Our results can help clinicians in identifying groups of patients at high risk of exacerbation who can benefit from better prevention of modifiable predictors and early onset of treatment, which may reduce morbidity and mortality.
We have found that COPD patients from a population sample exacerbate 2.0–2.5 times less frequently than hospital-based COPD patients, depending on the definition used. Apart from belonging to the hospital sample, increasing COPD severity gives significantly higher risk of exacerbation.
Conclusion
Our results, combined with previous findings,Citation46,Citation47 demonstrate that several studies on exacerbation rate use selected populationsCitation6,Citation14,Citation38 and hence suggest exaggerated rates of exacerbation, which in turn overstate the effect of medication. Thus, our finding implies that any study with AECOPD as the primary outcome should recruit from population-based samples and, if not possible, explicitly state from which population patients are recruited. Furthermore, implications of the chosen definition of AECOPD should be discussed.
Author contributions
Design, planning, and data collection were carried out by RG, AG, and AJ. Data management and quality control was performed by ME and RG. Statistical analyses were carried out by ME, RG, AJ, and TME. Analysis planning was done by RG, AJ, and ME. Drafting was carried out by ME. Revision and approval of drafts were performed by RG, TME, AJ, AG, PB, and ME. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors are indebted to Margrete Klemmetsby, Hege Marie Schnelle, Idunn Riisnes, Jan Egil Romestrand, Erik Helgeland, Jan Schille, Lene Svendsen, Tonje Lauvaasvaag, Heike Wiegmann, and Lene Kvamsdal for their contribution in collecting the data for EconCOPD. ME has recently received a research grant from AstraZeneca. Within the last 3 years, TME has received travel grants from InterMune for the AIR conferences, a grant for the MicroILD study from Boehringer Ingelheim, and speaker fees from AstraZeneca and Boehringer Ingelheim. RG reports grants from the Norwegian Association of Heart and Lung Patients and EXTRA funds from the Norwegian Foundation for Health and Rehabilitation as well as YaraPraxair during the conduct of the study, grants and personal fees from Boehringer Ingelheim, personal fees from AstraZeneca, and personal fees from GlaxoSmithKline outside the submitted work. AG has during the last 3 years participated in the advisory boards of Chesi Pharma AS, Sverige, Novartis Norge AS, Takeda Nycomed Norge, AstraZeneca Norge, and Boehringer Ingelheim, Norway. The Norway GenKOLS study, where he was the principal investigator, was supported by GlaxoSmithKline Research & Development Limited, UK.
Supplementary materials
Table S1 Number of events of exacerbations of respiratory symptoms in population-recruited cases by sex, age, smoking status, education, and FEV1% predicted
Table S2 Number of events of exacerbations of respiratory symptoms in hospital-recruited cases by sex, age, smoking status, education, and FEV1% predicted
Table S3 IRRs (95% CI) for AECOPD with a resource-based exacerbation definition
Table S4 IRRs (95% CI) for AECOPD with a symptom-based exacerbation definition
Disclosure
The authors report no conflicts of interest in this work.
References
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- SullivanSDRamseySDLeeTAThe economic burden of COPDChest20001172 suppl5S9S10673466
- HalpinDMDecramerMCelliBKestenSLiuDTashkinDPExacerbation frequency and course of COPDInt J Chron Obstruct Pulmon Dis2012765366123055714
- SeemungalTADonaldsonGCPaulEABestallJCJeffriesDJWedzichaJAEffect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19981575 pt 1141814229603117
- BowlerRPKimVReganECOPDGene InvestigatorsPrediction of acute respiratory disease in current and former smokers with and without COPDChest2014146494195024945159
- JenkinsCRCelliBAndersonJASeasonality and determinants of moderate and severe COPD exacerbations in the TORCH studyEur Respir J2012391384521737561
- HusebøGRBakkePSAanerudMPredictors of exacerbations in chronic obstructive pulmonary disease – results from the Bergen COPD cohort studyPLoS One2014910e10972125279458
- SuzukiMMakitaHItoYMHokkaido COPD Cohort Study InvestigatorsClinical features and determinants of COPD exacerbation in the Hokkaido COPD cohort studyEur Respir J20144351289129724232696
- TanWCBourbeauJHernandezPCanCOLD Collaborative Research GroupExacerbation-like respiratory symptoms in individuals without chronic obstructive pulmonary disease: results from a population-based studyThorax201469870971724706040
- NiewoehnerDELokhnyginaYRiceKRisk indexes for exacerbations and hospitalizations due to COPDChest20071311202817218552
- MiravitllesMGuerreroTMayordomoCFactors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study GroupRespiration200067549550111070451
- BeehKMGlaabTStowasserSCharacterisation of exacerbation risk and exacerbator phenotypes in the POET-COPD trialRespir Res20131411624168767
- MüllerovaHMaselliDJLocantoreNECLIPSE InvestigatorsHospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohortChest20151474999100725356881
- HurstJRVestboJAnzuetoAEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) InvestigatorsSusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- de OcaMMTálamoCHalbertRJFrequency of self-reported COPD exacerbation and airflow obstruction in five Latin American cities: the Proyecto Latinoamericano de Investigacion en Obstruccion Pulmonar (PLATINO) studyChest20091361717819349388
- OzyilmazEKokturkNTeksutGTatliciogluTUnsuspected risk factors of frequent exacerbations requiring hospital admission in chronic obstructive pulmonary diseaseInt J Clin Pract201367769169723758448
- GroenewegenKHPostmaDSHopWCCOSMIC Study GroupIncreased systemic inflammation is a risk factor for COPD exacerbationsChest2008133235035718198263
- EaganTMUelandTWagnerPDSystemic inflammatory markers in COPD: results from the Bergen COPD Cohort StudyEur Respir J201035354054819643942
- ThomsenMIngebrigtsenTSMarottJLInflammatory biomarkers and exacerbations in chronic obstructive pulmonary diseaseJAMA2013309222353236123757083
- TufvessonEEkbergMBjermerLInflammatory biomarkers in sputum predict COPD exacerbationsLung2013191441341623689877
- SakaeTMPizzichiniMMTeixeiraPJSilvaRMTrevisolDJPizzichiniEExacerbations of COPD and symptoms of gastroesophageal reflux: a systematic review and meta-analysisJ Bras Pneumol201339325927123857694
- TeradaKMuroSSatoSImpact of gastro-oesophageal reflux disease symptoms on COPD exacerbationThorax2008631195195518535116
- QuintJKBaghai-RavaryRDonaldsonGCWedzichaJARelationship between depression and exacerbations in COPDEur Respir J2008321536018321938
- LaurinCMoullecGBaconSLLavoieKLImpact of anxiety and depression on chronic obstructive pulmonary disease exacerbation riskAm J Respir Crit Care Med2012185991892322246177
- HallinRKoivisto-HurstiUKLindbergEJansonCNutritional status, dietary energy intake and the risk of exacerbations in patients with chronic obstructive pulmonary disease (COPD)Respir Med2006100356156716019198
- CorhayJLVinckenWSchlesserMBossuytPImschootJChronic bronchitis in COPD patients is associated with increased risk of exacerbations: a cross-sectional multicentre studyInt J Clin Pract201367121294130124246208
- NielsenRKlemmetsbyMGulsvikAEconomics of COPD: literature review and experiences from field workClin Respir J20082suppl 110411020298358
- JohannessenAOmenaasERBakkePSGulsvikAImplications of reversibility testing on prevalence and risk factors for chronic obstructive pulmonary disease: a community studyThorax2005601084284716085729
- LamprechtBMcBurnieMAVollmerWMBOLD Collaborative Research GroupCOPD in never smokers: results from the population-based burden of obstructive lung disease studyChest2011139475276320884729
- GulsvikATostesonTBakkePHumerfeltSWeissSTSpeizerFEExpiratory and inspiratory forced vital capacity and one-second forced volume in asymptomatic never-smokers in NorwayClin Physiol200121664866011722472
- ATSStandardization of spirometryAm J Respir Crit Care Med1995199515211071136
- LandisSHMuellerovaHManninoDMContinuing to Confront COPD International Patient Survey: methods, COPD prevalence, and disease burden in 2012–2013Int J Chron Obstruct Pulmon Dis2014959761124944511
- JanssonSABackmanHStenlingALindbergARönmarkELundbäckBHealth economic costs of COPD in Sweden by disease severity – has it changed during a ten years period?Respir Med2013107121931193823910072
- CharlsonMEPompeiPAlesKLMacKenzieCRA new method of classifying prognostic comorbidity in longitudinal studies: development and validationJ Chronic Dis19874053733833558716
- AnthonisenNRManfredaJWarrenCPHershfieldESHardingGKNelsonNAAntibiotic therapy in exacerbations of chronic obstructive pulmonary diseaseAnn Intern Med198710621962043492164
- DonaldsonGCWedzichaJACOPD exacerbations. 1: epidemiologyThorax200661216416816443707
- KeeneONCalverleyPMJonesPWVestboJAndersonJAStatistical analysis of exacerbation rates in COPD: TRISTAN and ISOLDE revisitedEur Respir J2008321172418591336
- SpencerSCalverleyPMBurgePSJonesPWImpact of preventing exacerbations on deterioration of health status in COPDEur Respir J200423569870215176682
- CampPGGoringSMGender and the diagnosis, management, and surveillance of chronic obstructive pulmonary diseaseProc Am Thorac Soc20074868669118073404
- AryalSDiaz-GuzmanEManninoDMCOPD and gender differences: an updateTransl Res2013162420821823684710
- BecklakeMRKauffmannFGender differences in airway behaviour over the human life spanThorax199954121119113810567633
- KilicHKokturkNSariGCakırMDo females behave differently in COPD exacerbation?Int J Chron Obstruct Pulmon Dis20151082383025977604
- VarkeyJBVarkeyABVarkeyBProphylactic vaccinations in chronic obstructive pulmonary disease: current statusCurr Opin Pulm Med2009152909919532022
- EvansCCrawfordBPatient self-reports in pharmacoeconomic studies. Their use and impact on study validityPharmacoeconomics199915324125610537432
- NielsenRCosts of Chronic Obstructive Pulmonary Disease in a General PopulationMethodological Aspects and Longitudinal Perspectives (dissertation)BergenUniversity of Bergen2011
- NielsenRJohannessenAOmenaasERBakkePSAskildsenJEGulsvikAExcessive costs of COPD in ever-smokers. A longitudinal community studyRespir Med2011105348549321030232
- ErdalMJohannessenAAskildsenJEEaganTGulsvikAGrønsethRProductivity losses in chronic obstructive pulmonary disease: a population-based surveyBMJ Open Respir Res201411e000049
