Abstract
Background
In acute exacerbation of COPD, increased plasma levels of cardiac troponin are frequent and associated with increased mortality. Thus, we aimed at prospectively determining the diagnostic value of coronary angiography in patients with exacerbated COPD and concomitantly elevated cardiac troponin.
Patients and methods
A total of 88 patients (mean age 72.9±9.2 years, 56.8% male) hospitalized for acute exacerbation of COPD with elevated plasma troponin were included. All patients underwent coronary angiography within 72 hours after hospitalization. Complementary 12-lead electrocardiogram, transthoracic echocardiography, pulmonary function, and angiological testing were performed.
Results
Coronary angiography objectified the presence of ischemic heart disease (IHD) in 59 patients (67.0%), of whom 34 patients (38.6% of total study population) underwent percutaneous coronary intervention. Among these 34 intervened patients, the vast majority (n=26, 76.5%) had no previously known IHD, whereas only eight out of 34 patients (23.5%) presented an IHD history. Patients requiring coronary intervention showed significantly reduced left ventricular ejection fraction (45.8%±13.1% vs 55.1%±13.3%, P=0.01) and a significantly more frequent electrocardiographic ST-segment depression (20.6% vs 7.4%, P=0.01). Neither additional laboratory parameters for inflammation and myocardial injury nor lung functional measurements differed significantly between the groups.
Conclusion
Angiographically confirmed IHD that required revascularization occurred in 38.6% of exacerbated COPD patients with elevated cardiac troponin. In this considerable portion of patients, coronary angiography emerged to be of diagnostic and therapeutic value.
Introduction
COPD constitutes a leading cause of disability and mortality worldwide, affecting >10% of the adult population.Citation1 As a complex respiratory disorder, its course is frequently complicated by its systemic manifestations.Citation2 Among these, cardiovascular disease – in particular ischemic heart disease (IHD) – shows major affliction.Citation3 Previous studies irrefutably indicate that recent respiratory infections are strongly associated with cardiovascular events, notably with myocardial infarction (MI).Citation4 This contradicts the assumption that COPD and IHD are only linked to each other by their common risk profile, foremost smoking.
Exacerbations of COPD sustainably influence the natural history of the disease. Apart from accelerating the rate of lung function decline,Citation5 periexacerbational inflammation is not restricted to the airways but constitutes a systemic inflammatory process.Citation6 In its course, an increase in inflammatory markers is detectable, predisposing to cardiovascular events such as MI.Citation7 As such, elevation of cardiac troponin in the context of acute exacerbation of COPD (AECOPD) has frequently been observed.Citation8,Citation9 Though its increase has been associated with an increased risk of all-cause mortality,Citation10 its predictive value for IHD that requires revascularization remains undetermined.
Therefore, the aims of this prospective cohort study were 1) to determine the diagnostic yield of coronary angiography in patients hospitalized for AECOPD with concomitant troponinemia and 2) to correlate the angiographic results with the findings obtained by noninvasive cardiological testing.
Methods
Between July 2014 and December 2015, patient recruitment took place at three hospitals of Bonn (Germany): the University Hospital, the Johanniter Hospital, and the Malteser Hospital. Patients aged ≥18 years with a history of spirometrically and clinically confirmed COPD and presenting with AECOPD at one of the hospitals’ emergency departments were screened for eligibility for this prospectively conducted clinical cohort trial. In conformity with the Global Initiative for Chronic Obstructive Lung Disease (GOLD),Citation1 acute exacerbation was defined by an acute, sustainable worsening of the patient’s respiratory symptoms, leading to a change in medication. In all patients, cardiac troponin I (cTnI) was measured in the context of routine emergency department laboratory assessment. In case of an increase in cTnI, defined by a serum level exceeding 0.05 ng/mL (high-sensitivity troponin I assay; Siemens Healthcare GmbH, Erlangen, Germany), patients were confirmed eligible and asked for study participation. If patients initially presented at the Johanniter or Malteser Hospital’s emergency units, they were transferred to the University Hospital’s cardiological inpatient department to receive local medical care. Patients primarily recruited from the University Hospital’s emergency department continued on-site medical treatment. Questionnaire-based recording of preexisting cardiac comorbidities and established risk factors for cardiovascular disorders was conducted. COPD staging was performed on the basis of pulmonary function testing results obtained in the last exacerbation-free period prior to hospitalization, as assessed by collection from the patient’s medical reports. Exclusion criteria for study participation comprised principally ischemic pectoral symptomatology, severe renal insufficiency with an estimated glomerular filtration rate <30 mL/min/1.73 m2, or acute kidney injury. Written informed consent was obtained from each patient at the time of confirmed troponin increase. The study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Medical Ethics Committee of Bonn (Germany).
Coronary angiography
Within a timeframe of 72 hours after initial hospital presentation, all patients underwent coronary angiography (Allura Xper FD10/10; Philips Medical Systems, Koninklijke Philips N.V., Hamburg, Germany) with a transfemoral or transradial approach, as appropriate. In accordance with the European Society of Cardiology guidelines,Citation11 IHD was defined as a degree of coronary stenosis ≥50%. The concrete coronary interventional approach was left to the discretion of the treating operator and standard practice.Citation11
Electrocardiogram and transthoracic echocardiography
At the time of initial diagnosis of cTnI elevation, 12-lead electrocardiogram was obtained, allowing for assessment of arrhythmias, complexes, and intervals, with special focus on signs of current ischemia or prior MI.
Transthoracic echocardiography was conducted at the University Hospital’s cardiological unit prior to coronary angiography and was performed by experienced cardiac sonographers and cardiologists with multiannual technical expertise in echocardiographic circulatory imaging. Echocardiographic recordings were obtained by conventional equipment (Vivid 7; GE Healthcare Systems, Little Chalfont, United Kingdom; Philips Medical Systems, Koninklijke Philips N.V., Hamburg, Germany), in accordance with the recommendations of the American Society of Echocardiography.Citation12
Laboratory testing
Immediately after patient’s transfer to the University Hospital, a fasting blood sample was taken, encompassing full blood count, complete lipid panel, plasma glucose concentration, and high sensitivity C-reactive protein. Moreover, cardiac biomarkers of myocardial injury – creatine kinase, creatine kinase-myocardial band, and sequential cTnI – were assessed. In case of patient’s initial evaluation at the University Hospital, the aforementioned complementary blood sample was taken in an ~4-hour interval to the first cTnI measurement.
Pulmonary function testing
Subsequent to coronary angiography and in conformity with the European Respiratory Society guidelines,Citation13 patients underwent postbronchodilator spirometry and body plethysmography. Parameters were recorded as absolute measures and percentages predicted for age, sex, and height indices. Concurrently, capillary blood gas analysis was performed for evaluation of oxygenation and ventilation status.
Angiological testing
With the objective of evaluating generalized atherosclerotic processes and arterial stiffness as an established independent predictor for cardiovascular risk,Citation14 we measured brachial-ankle central pulse wave velocity (cPWV) using AngE Pro8® (Sonotechnik Austria, Maria Rain, Austria), with values exceeding 12 m/s considered to be pathological. Moreover, angiological diagnostics for peripheral arterial disease comprised assessment of the ankle-brachial index (ABI), obtained by division of the posterior tibial artery’s systolic pressure by its brachial correlate. All angiological examinations were conducted in line with the current European guidelines.Citation15
Statistical analysis
Descriptive data are presented as absolute numbers and percentages, mean ± standard deviation – if normally distributed – or as median and range – if not normally distributed. In the case of continuous parameters, a Student’s t-test or a Mann–Whitney U-test – as appropriate – was employed for comparison between two groups. For comparison of more than two groups, nonparametric testing by Kruskal–Wallis one-way analysis of variance was used. Categorical variables were analyzed for association using Pearson’s χ2 test. Statistical significance was assumed when the null hypothesis could be rejected at P<0.05. All statistical analyses were performed using SPSS Statistics 23 software (IBM Corporation, Armonk, NY, USA).
Results
Demographic characteristics and clinical data of the 88 patients enrolled are summarized in . The study population exhibited a slight male predominance (56.8%, n=50) and a mean age of 72.9±9.2 years. The vast majority of study participants had a smoking history (60.2% and 37.5% were current and former smokers, respectively) and evidenced a median amount of 40.0 (0–100) pack-years. As to stable course staging prior to exacerbation, 14.8%, 32.9%, 21.6%, and 30.7% of patients pertained to COPD GOLD groups A–D, respectively, with symptom severity evaluation performed using the COPD assessment test. At the time of hospitalization for exacerbation, pulmonary function testing revealed a mean forced expiratory volume in one second of 1.10 L (±0.5 L) in absolute terms or 44.2%±16.9% of the predicted value. The residual volume averaged 4.06 L (±1.3 L) or 172.6%±63.4% of predicted value. In terms of capillary blood gas analysis, median resting oxygen tension accounted for 63.6 mmHg (42.3–79.2 mmHg), with 4.5% of patients undercutting a value of 55 mmHg. Median carbon dioxide tension averaged 37.8 mmHg (28.7–67.9 mmHg); hypercapnia was present in 4.5% of patients.
Table 1 Demographic, clinical, and echocardiographic characteristics of total study population at the time of hospitalization for acute exacerbated COPD
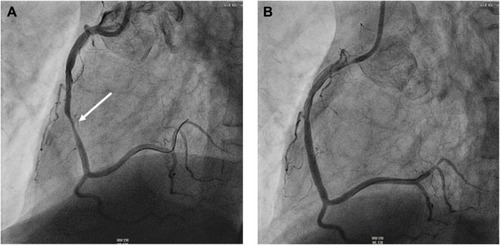
Coronary angiography identified IHD based on the presence of a coronary stenosis ≥50% in 67.0% (n=59) of the whole study population. Single-, two-, and three-vessel disease was present in 29.5% (n=26), 14.8% (n=13), and 22.7% (n=20) of patients, respectively. A total of 34 participants, ie, 38.6% of the total study cohort, showed necessity for and underwent percutaneous coronary intervention. Distribution analysis of intervened coronary arteries identified the right coronary artery to be predominately affected (44.1% of cases; ); left main coronary artery disease requiring revascularization was objectified in one patient who likewise received treatment by a percutaneous approach. Differentiation of the 34 intervened patients as a function of previously known IHD revealed 23.5% (8/34) of patients to have an IHD history, whereas in the vast majority of patients (26 out of 34 patients) no IHD was previously diagnosed. Neither the prevalence of IHD per se nor the presence of coronary lesions requiring revascularization significantly differed within COPD GOLD stages (P=0.66 and P=0.97, respectively).
Figure 1 Coronary artery angiography of a 71-year old male with acute exacerbated COPD GOLD D and elevated plasma troponin.
Laboratory data are given in . Mean cTnI level averaged 1.93±4.92 ng/mL and showed no significant distributive difference between patients with and without indication for percutaneous coronary intervention (2.66±6.49 ng/mL vs 1.48±3.60 ng/mL, respectively; P=0.28). Among those 34 patients undergoing coronary revascularization, 23 patients (67.6%) had a rising sequential cTnI pattern by 150.7%±50.6% of baseline value. Among those 54 patients without indication for percutaneous coronary intervention, a rising sequential cTnI pattern was exhibited by 38 patients (70.4%; 151.7%±48.9% rise from baseline value) and did not significantly differ from that in intervened patients (P=0.38). Likewise, the additionally assessed cardiac biomarkers of myocardial damage were balanced between groups. The sole laboratory parameters identified to correlate with the necessity for coronary revascularization were low-density lipoprotein cholesterol and fasting plasma glucose concentrations (P=0.04 and P<0.01, respectively).
Table 2 Comparison of laboratory and pulmonary parameters as a function of angiographically evidenced indication for percutaneous coronary intervention
The 12-lead electrocardiographic and transthoracic echocardiographic results are outlined in . The sole electrocardiographic finding that differed between groups was ischemic ST depression that was significantly more frequent among intervened patients (P=0.01). A trend toward a major burden of tachycardiac heart rate alterations was ascertained in the intervened patient subgroup (P=0.06). As to echocardiography, left ventricular ejection fraction was significantly lower in those patients undergoing coronary revascularization (45.8%±13.1% vs 55.1%±13.3%, P=0.01).
Table 3 Data comparison of noninvasive cardiological and angiological testing as a function of angiographically evidenced indication for percutaneous coronary intervention
In terms of angiological testing, mean calculated ABI values in patients requiring coronary intervention were <0.9 and thus implied lower extremity circulatory compromise. Pulse wave recording evidenced bilaterally concordant pulse wave indices. Neither pulse wave index nor cPWV differed between the groups ().
Discussion
In the present study, we aimed to determine the diagnostic value of invasive coronary angiography in patients with AECOPD and elevated cTnI levels. We observed that 1) coronary angiography detected IHD in 59 out of 88 examined patients (67.0%). 2) IHD that required revascularization was present in 38.6% of total study population. 3) When compared with the results obtained by coronary angiography, the diagnostic value of noninvasive cardiological testing emerged to be moderate.
To the best of our knowledge, this is the first prospective study to invasively examine coronary status in AECOPD patients with troponinemia. In a considerable portion of patients, we identified cTnI elevation to occur as a conse quence of MI that demanded coronary intervention.
Occurrence and frequency of acute exacerbation in COPD exert a sustainably negative impact on the disease’s course not only by negatively affecting the patient’s quality of life but also additionally and mainly by accelerating the rate of persistent lung function decline.Citation5 As to exacerbation-related mortality, autopsy results attribute the leading cause of early death in hospitalized AECOPD patients to cardiac failure.Citation16 The prevalence of increase in cardiac troponin during AECOPD has repeatedly been focused on and is reported to comprise percentages from 10%Citation17 up to 74%,Citation18 dependent on the employed assay’s sensitivity. Against this backdrop, numerous efforts to identify its diagnostic value have been undertaken. In a recently published meta-analysis, a predictive role has been ascribed to derangements in cardiac troponin in hospitalized patients for AECOPD, as it was associated with an increased risk for all-cause mortality.Citation10 Moreover, Campo et alCitation19 reported positive troponin in AECOPD to be an independent predictor of cardiac death alone and of nonfatal MI; surprisingly, its prognostic role was limited to patients without previous IHD history. The term “cardiopulmonary continuum” summarizes this pathophysiological correlation between AECOPD and coronary processes.Citation20 As described by Gan et al,Citation21 COPD is characterized by a systemic inflammatory process, whose dimension increases by acute exacerbatory events. Systemic inflammation markers comprising leukocytes, C-reactive protein, and diverse inflammatory cytokines have been identified to play a major role in COPD exacerbation frequency and severity,Citation6 with low-grade systemic inflammation occurring in stable COPD. Likewise, inflammation promotes endothelial damage, resulting in endothelial dysfunction that in turn precedes atherosclerosis. An abrupt alteration in systemic inflammatory activity can cause silent atherosclerotic lesions to rupture with ensuing acute myocardial ischemia.Citation22 In keeping with this and in order to estimate the systemic atherosclerotic effect induced by inflammation-enhanced endothelial dysfunction, we performed complementary angiological examination. In the setting of exacerbated and stable-state COPD, Patel et alCitation23 reported arterial stiffness to rise acutely during exacerbation and to not recover for several weeks, with frequent COPD exacerbators manifesting a higher arterial stiffness than infrequent exacerbators. We presently assessed cPWV as a marker for arterial stiffness and ascertained no distributive differences between patients with and without indication for coronary revascularization; mean cPWV values were within the normal range in both cohorts. Nonetheless, intervened patients manifested a significant higher prevalence of concomitant peripheral artery disease, as defined by a reduction in ABI values, suggesting a systemic affection of inflammation-driven atherosclerotic lesions.
As a consequence of the aforementioned inflammation-driven proatherosclerotic processes, manifest atherosclerotic conditions, such as coronary artery disease, may develop. In this context, troponin elevation can be ascribed to spontaneous MI (type 1) consequent to atherosclerotic plaque rupture, ulceration, or dissection that induces myocyte necrosis.Citation24 Alternative pathways responsible for troponin elevation during AECOPD comprehend: MI (type 2) due to an oxygen supply-demand imbalance,Citation18 acute right ventricular dysfunction consequent to pressure elevation in the pulmonary circulation, and exacerbating left ventricular failure that secondarily causes AECOPD. Despite these diverse approaches, their respective causative values remain to be ascertained, in order to enable clinicians to decide to what extent patients with elevated cardiac troponin should receive a different treatment from those with normal values.Citation25 In view of the abovementioned, we aimed at defining the role of type 1 and type 2 MI by prospectively performing coronary angiography. For this purpose, we consciously included all AECOPD patients irrespective of any prediagnosed IHD so as to prevent preselection and provide insights into the true risk for MI in this specific clinical setting. In line with the European Society of Cardiology guidelines,Citation11 coronary angiography revealed concomitant IHD in 67.0% of patients. The portion of patients evidencing high-grade coronary stenosis and undergoing revascularization accounted for 38.6%. Comparison to the data obtained by Stripe et alCitation26 in a non-AECOPD cohort with mild troponin elevation (11% and 8.6% of patients had IHD and underwent revascularization, respectively) implies that troponinemia in AECOPD, when compared with troponinemia in a non-AECOPD setting, is a stronger indicator for coronary processes. Data comparison based upon the patient’s history of IHD revealed no statistically significant intercohortal difference in the need for revascularization: 8/15 patients (53.3%) with and 26/73 patients (35.6%) without a prior history of IHD, respectively, received coronary intervention (P=0.20). Moreover, though ST-segment depression and left ventricular systolic dysfunction were significantly more frequent in those patients requiring coronary revascularization, in 23 out of 34 intervened patients, both electro- and echocardiographic ischemic changes were absent. Among these 23 individuals, 15 patients had an increasing sequential cTnI pattern. The remaining eight out of 34 intervened patients had decreasing sequential cTnI values and normal electro- and echocardiographic findings. Thus, MI type 1, characterized by electro-and echocardiographic ischemia and sequentially rising troponin values, was present in 26 patients (76.5% of the intervened cohort), whereas troponin release in the remaining eight patients (23.5% of the intervened cohort) was primarily attributable to an imbalance between myocardial oxygen supply and demand, designated as type 2 MI.Citation27 There are several limitations of this study that should be addressed. The study design was cross-sectional and not drafted to perform longitudinal follow-up that in turn would have permitted estimation of the impact exerted by angiographic results on long-term mortality. Additional performance of coronary angiography in AECOPD patients without concomitant troponin elevation would have been a valuable comparatory complement to our observations, whose realization, however, emerges to be questionable from an ethical point of view. Though within guideline-defined standard clinical practice, the concrete choice of the interventional strategy was based on the operator’s discretion and may therefore have affected our revascularization data. Finally and in view of the relative impreciseness of the official AECOPD definition, we cannot exclude with complete certainty that a portion of the patients studied may have had a primary coronary event that aggravated their dyspnea. Interestingly, among those patients who required percutaneous coronary intervention, the right coronary artery was preferentially affected and intervened (44.1% of cases). This raises the question if the resulting right ventricular dysfunction predisposes to be misinterpreted – in our study and in real-world settings – as a respiratory-driven event on the basis of a prediagnosed COPD.
Conclusion
Cardiac troponin elevation at the time of hospitalization for AECOPD is – in a considerable portion of patients – indicative of IHD requiring revascularization. In the acute medical treatment of this portion of patients, invasive coronary angiography emerges to be of substantial diagnostic and therapeutic value. The establishment of a standardized diagnostic strategy to identify this portion of patients who require an invasive coronary approach is mandatory.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The study was financially supported by Novartis Pharma GmbH (Nuremberg, Germany).
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016 [homepage on the Internet] Available from: http://www.goldcopd.org/Accessed April 15, 2016
- AgustiASobradilloPCelliBAddressing the complexity of COPDAm J Respir Crit Care Med201118391129113721169466
- ManninoDMThornDSwensenAHolguinFPrevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPDEur Respir J200832496296918579551
- ClaytonTCThompsonMMeadeTWRecent respiratory infection and risk of cardiovascular disease: case–control study through a general practice databaseEur Heart J20082919610318063596
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- HurstJRPereraWRWilkinsonTMDonaldsonGCWedzichaJASystemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20061731717816179639
- WedzichaJASeemungalTAMacCallumPKAcute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levelsThromb Haemost200084221021510959691
- BrekkePHOmlandTHolmedalSHSmithPSøysethVTroponin T elevation and long-term mortality after chronic obstructive pulmonary disease exacerbationEur Respir J200831356357018032444
- HarveyMGHancoxRJElevation of cardiac troponins in exacerbation of chronic obstructive pulmonary diseaseEmerg Med Australas200416321221515228464
- PavasiniRd’AscenzoFCampoGCardiac troponin elevation predicts all-cause mortality in patients with acute exacerbation of chronic obstructive pulmonary disease: systematic review and meta-analysisInt J Cardiol201519118719325965630
- RoffiMPatronoCColletJP2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC)Eur Heart J20163726731526320110
- DouglasPSGarciaMJHainesDEACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiographyJ Am Soc Echocardiogr201124322926721338862
- MillerMRHankinsonJBrusascoVStandardisation of spirometryEur Respir J201526231933816055882
- LaurentSCockcroftJVan BortelLEuropean Network for Noninvasive Investigation of Large ArteriesExpert consensus document on arterial stiffness: methodological issues and clinical applicationsEur Heart J200627212588260517000623
- TenderaMAboyansVBartelinkMLESC Committee for Practice Guidelines. ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the task force on the diagnosis and treatment of peripheral artery diseases of the European Society of Cardiology (ESC)Eur Heart J201132222851290621873417
- ZvezdinBMilutinovSKojicicMA postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbationChest2009136237638019318666
- McAllisterDAMaclayJDMillsNLDiagnosis of myocardial infarction following hospitalisation for exacerbation of COPDEur Respir J20123951097110322323574
- HøisethADNeukammAKarlssonBDOmlandTBrekkePHSøysethVElevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary diseaseThorax201166977578121653926
- CampoGPavasiniRMalagùMRelationship between troponin elevation, cardiovascular history and adverse events in patients with acute exacerbation of COPDCOPD201512556056725775224
- UkenaCMahfoudFKindermannMThe cardiopulmonary continuum systemic inflammation as ‘common soil’ of heart and lung diseaseInt J Cardiol2010145217217620570377
- GanWQManSFSenthilselvanASinDDAssociation between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysisThorax200459757458015223864
- HanssonGKInflammation, atherosclerosis, and coronary artery diseaseN Engl J Med2005352161685169515843671
- PatelARKowlessarBSDonaldsonGCCardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201318891091109924033321
- BrekkePHOmlandTSmithPSøysethVUnderdiagnosis of myocardial infarction in COPD – Cardiac Infarction Injury Score (CIIS) in patients hospitalised for COPD exacerbationRespir Med200810291243124718595681
- ChangCLRobinsonSCMillsGDBiochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPDThorax201166976476821474497
- StripeBRechenmacherSJurewitzDLeeCSchaeferSThe diagnostic yield of cardiac catheterization in low-risk troponinemiaJAMA Intern Med2013173222088209024100658
- ThygesenKAlpertJSJaffeASJoint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial InfarctionThird universal definition of myocardial infarctionCirculation2012126162020203522923432
