Abstract
Background
Low-dose opioids can relieve breathlessness but may be underused in late-stage COPD due to fear of complications, contributing to poor symptom control.
Objectives
We aimed to study the period prevalence and indications of opioids actually prescribed in people with end-stage COPD.
Methods
The study was a longitudinal, population-based study of patients starting long-term oxygen therapy (LTOT) for COPD between October 1, 2005 and June 30, 2009 in Sweden. A random sample (n=2,000) of their dispensed opioid prescriptions was obtained from the national Prescribed Drugs Register from 91 days before starting LTOT until the first of LTOT withdrawal, death, or study end (December 31, 2009). We analyzed medication type, dispensed quantity, date of dispensing, and indications categorized as pain, breathlessness, other, or unknown.
Results
In total, 2,249 COPD patients (59% women) were included. During a median follow-up of 1.1 (interquartile range 0.6–2.0) years, 1,034 patients (46%) were dispensed ≥1 opioid prescription (N=13,722 prescriptions). The most frequently prescribed opioids were tramadol (23%), oxycodone (23%), morphine (16%), and codeine (16%). Average dispensed quantity was 9.3 (interquartile range 3.7–16.7) defined daily doses per prescription. In the random sample, the most commonly stated indication was pain (97%), with only 2% for breathlessness and 1% for other reasons.
Conclusion
Despite evidence that supported the use of opioids for the relief of breathlessness predating this study, opioids are rarely prescribed to relieve breathlessness in oxygen-dependent COPD, potentially contributing to less-than-optimal symptom control. This study creates a baseline against which to compare future changes in morphine prescribing in this setting.
Introduction
COPD is a leading cause of mortality worldwide, and is associated with high burden of symptoms, often poorly controlled in advanced disease stages.Citation1–Citation4 The prevalence and severity of breathlessness are higher in end-stage COPD than in advanced lung cancer, both during the final year of life and in the terminal weeks and days near death.Citation5,Citation6 Despite long-term oxygen therapy (LTOT), most COPD patients suffer from breathlessness at rest or on minimal exertion that greatly limits even the basic activities of daily living.Citation7–Citation9 Breathlessness that persists at rest or on minimal exertion despite optimal treatment of the underlying disease(s) is termed “refractory breathlessness.”Citation10,Citation11
Opioids have a growing evidence base for decreasing refractory breathlessness in advanced COPD.Citation10,Citation12 Jennings et al concluded in a Cochrane meta-analysis that low doses of oral sustained-release morphine can reduce chronic refractory breathlessness,Citation12 which was confirmed by an adequately powered crossover trial in 2003.Citation10
Clinicians may, however, be reluctant to prescribe opioids due to a fear of adverse events including confusion, falls, and respiratory depression in patients with respiratory compromise.Citation13 Among stable outpatients with advanced COPD, 94% reported moderate to severe breathlessness but only 2% used opioids, such as morphine.Citation13 In palliative care, only one-fourth of COPD patients received opioids during their last 6 months of life, compared to half of the patients with lung cancer.Citation14
No study has evaluated the indications for actual prescribed opioids, and data are limited on the temporal trends and prescription as death approaches. A recent study reported that opioid therapy, mostly short-term, was commonly used in COPD but did not analyze the indications for opioid use.Citation15 Underuse of opioids for breathlessness in severe COPD could contribute to insufficient symptom control and unnecessary suffering.
The aim of this study was to evaluate the indications, medication types, and temporal patterns of opioid prescription in severe oxygen-dependent COPD.
Materials and methods
Design and participants
This was an observational, population-based study of patients aged 45 years or older who started LTOT for COPD in the Swedish Register for Respiratory Failure between October 1, 2005 and June 30, 2009. The database was used in a recent safety study of opioids.Citation16
Data sources
The Swedish Register for Respiratory Failure prospectively includes patients starting LTOT in Sweden with a nationwide coverage of approximately 85%.Citation17 It contains physiological data including arterial blood gas tensions when breathing air and oxygen, body mass index, World Health Organization (WHO) performance status,Citation18 and forced expiratory volume in 1 second registered at the start of LTOT. Details of the register have been published.Citation19
Data on all dispensed outpatient prescriptions were obtained from the Swedish Prescribed Drugs RegisterCitation20 during the study period, which was from 91 days before the start of LTOT until the first of LTOT withdrawal, death, or study end (December 31, 2009). Drugs were categorized according to the Anatomical Therapeutic Chemical Classification System (ATC codes) as previously described.Citation16 Vital status was obtained from the Swedish Causes of Death Register.
Opioid prescriptions
A random sample (n=2,000) of the cohort’s dispensed opioid prescriptions during the study period was derived from the database using the commando runiform in the statistical package Stata, version 13 (StataCorp LP; College Station, TX, USA). The random sample was analyzed in relation to medication type, dispensed quantity as WHO defined daily doses,Citation21 date of dispensing, and free-text indication. Each indication was reviewed by a respiratory physician and categorized as pain, breathlessness, other, or unknown (insufficient information to identify an indication). Each prescription could have multiple indications and could include both a regular and an “as-needed” dose. In Sweden, the stated free-text indications are decided entirely by the prescribing physician and do not undergo external review by the pharmacy or clinic. Analysis of primary interest was the period prevalence of opioid prescriptions during the follow-up period.
Ethics
Participants provided their verbal consent when registered in Swedevox, and the consent procedure and the study were approved by the Lund University Research Ethics Committee (157/2007 and 350/2008), the Swedish National Board of Health and Welfare, and the Data Inspection Board.
Statistical analyses
Baseline characteristics were summarized as counts and percentages for categorical variables, mean values with standard deviation or median with interquartile range (IQR) or range for normally and nonnormally distributed continuous variables, respectively. Differences were tested using a t-test for continuous variables and chi-square test for unpaired categorical data. All tests were two sided, and statistical significance was defined as P<0.05. Statistical analyses were conducted using Stata version 12.1 (StataCorp LP, College Station, TX, USA).
Results
Participants
A total of 2,249 COPD patients (59% women) were included prospectively and followed-up for a median 1.1 years (IQR 0.6–2.0). During the study period, 1,034 patients (46 %) were dispensed at least one prescription of an opioid (in total 13,722 dispensed opioid prescriptions). As shown in , patients who were prescribed opioids were aged 74±8 years, were mostly women (66%), and 70% were mostly ambulatory with WHO status 1–2 at LTOT start. The random sample of 2,000 (15%) of the opioid prescriptions was dispensed by 575 patients.
Table 1 Characteristics of oxygen-dependent COPD patients who were prescribed opioids
Types of opioids
The most frequently prescribed opioids were tramadol (23%), oxycodone (23%), morphine (16%), and codeine (16%), as shown in . The average dispensed quantity was 9.3 (IQR 3.7–16.7) defined daily doses per prescription. Of the opioid prescriptions, 417 (21%) included an as-needed dose (). No nebulized opioids were prescribed. The indication was present in 33% (n=662) of the opioid prescriptions and absent in 67% (n=1338) of the opioid prescriptions (). Characteristics were similar between the patients whose indications for opioids were known compared to those with unknown indications ().
Table 2 Opioid prescriptions in oxygen-dependent COPD
Table 3 Indications of 2,000 random opioid prescriptions among 575 patients with oxygen-dependent COPD
Temporal patterns of indications
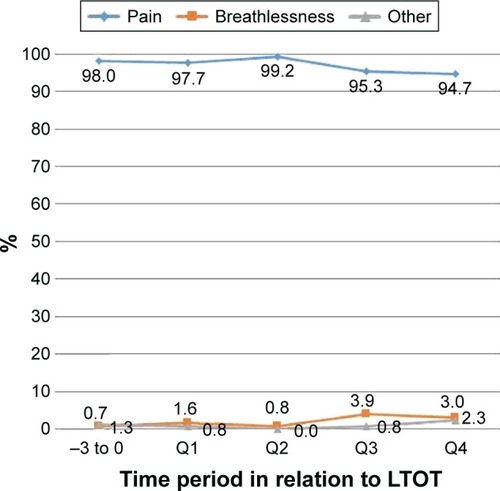
shows indications for the prescriptions of opioids before and after the start of LTOT. The vast majority of the stated indications were pain (97%), with only 2% for breathlessness and 1% for other reasons among the prescriptions with known indications. Prescriptions with dual indications (pain and breathlessness) occurred in only 0.7% (n=5) of the known indications. During the last 6 months of the patients’ lives, the period prevalence of opioid prescriptions for breathlessness was only 4% ().
Table 4 Prescribed opioids at LTOT start versus before death in oxygen-dependent COPD patients who died (n=278)
Opioids were prescribed predominantly among patients with WHO performance status 1–2 ().
Table 5 Indications of opioid prescriptions by WHO performance status in oxygen-dependent COPD
shows the temporal pattern of opioid prescriptions during follow-up by indication. Pain was the predominant indication throughout the study period. Breathlessness as an indication increased slightly during follow-up but the absolute numbers were low.
Figure 1 Trend for stated indications of opioid prescriptions in 575 patients with oxygen-dependent COPD.
Discussion
Key findings
In oxygen-dependent COPD, breathlessness was an indication in less than 2% of opioid prescriptions and only 4% in the last 6 months of life. Among patients, 46% took an opioid during follow-up. Of the stated indications, the vast majority was pain (97%) and as many as 67% of opioid prescriptions lacked any information on the indication.
Strengths and limitations
Strengths of the present study include its national population-based design with complete longitudinal data on all dispensed outpatient opioid prescriptions and vital status nationwide. National registry data enabled complete follow-up in these frail patients with advanced disease. The use of a randomized prescription sample enabled analysis of the free-text indications, and the timing of prescription was analyzed in relation to LTOT and death.
A possible limitation was that a high proportion of prescriptions lacked a stated indication. This would probably not lead to bias because the opioid indication is not externally reviewed and does not affect the price or the patient’s ability to dispense the opioid prescription in Sweden. It also reflects clinical reality and is an important finding in its own right by identifying an area of possible improvement in clinical practice. Clear information regarding the indication and aim of the treatment should be written on all prescriptions, especially regarding sick elderly patients and when informal caretakers are involved. Prescription data did not include opioids given in hospital, but this is unlikely to substantially change the relations between the treatment indications. Another possible limitation is that the study included data between 2005 and 2009. However, evidence that supported the use of opioids for the relief of breathlessness was already available from a Cochrane meta-analysis published in 2002Citation12 and an adequately powered trial published in 2003.Citation10 We did not have patient-reported data including breathlessness or quality of life scores to further characterize patients in this study.
Given the evidence of the efficacy of opioids for breathlessness,Citation10,Citation12,Citation16,Citation22 the study design, and the striking difference in prescribing for pain (97% of prescriptions) and breathlessness (2%), the finding that opioids are rarely used for refractory breathlessness in advanced and end-stage COPD likely has high validity.
Relation to current evidence
Patients with advanced and oxygen-dependent COPD are known to suffer from high levels of breathlessness despite LTOT, both in outpatients and at the end of life.Citation6–Citation9 Opioids have been reported to be less prescribed near death in advanced COPD than in lung cancer, despite the presence of more breathlessness in COPD and similar rates of pain.Citation6,Citation14
The current findings are consistent with a Dutch study that chest physicians rarely prescribed opioids for refractory breathlessness to outpatients with advanced COPD.Citation23 The most frequent barriers to opioid prescription were the physician’s fear of possible serious adverse events, including respiratory depression and resistance of some patients.Citation23 Although more research is needed, the evidence to date supports the safety of low-dose opioids for symptomatic treatment in advanced diseases including COPD. This evidence consists of a Cochrane meta-analysis,Citation12 a systematic review,Citation24 a randomized trial,Citation10 and several studies.Citation16,Citation22 Sustained-release morphine should be considered as a first line treatment and should be initiated at a low dose regularly and titrated upward over days and weeks, balancing beneficial and adverse effects.Citation25,Citation26 All treatments assume adequate follow-up of the patient’s clinical condition and symptoms, including proper prophylaxis and treatment for expected effects such as opioid-related constipation.Citation10,Citation25 Opioid side effects include initial nausea, worsened constipation, or dizziness, which have been reversible upon dose reduction or discontinuation.Citation16 There have been no reported serious adverse events related to titrated low-dose opioids, including no case of respiratory depression.Citation12,Citation16,Citation22,Citation24 A study using the present database found that low-dose opioids were not associated with increased rates of hospitalization or death in patients with severe oxygen-dependent COPD.Citation16 In a pharmacovigilance study of 83 patients who received 10–30 mg oral morphine per day, no episodes of respiratory depression or hospitalizations were reported up to 3 months.Citation22 A recent meta-analysis (16 studies; 271 patients) reported that opioids safely improved breathlessness in severe COPD.Citation24 The findings from this meta-analysis are consistent with those of Jennings et al.Citation12 Rocker et al reported that patients experienced beneficial effects from opioid therapy for breathlessness that sustained over months.Citation27 The present study suggests that, despite the high symptom burdenCitation6 and the available safety data,Citation12,Citation16,Citation22,Citation24 there is a widespread reluctance to prescribe opioids for breathlessness in advanced COPD.
What this study adds
This is, to the authors’ knowledge, the first study looking at the indications for prescribing opioids in severe COPD. This study shows that patients with oxygen-dependent COPD are treated with opioids; 46% of patients were dispensed at least one prescription during follow-up. Pain was the dominating opioid indication (97%), and breathlessness was the indication in only 2% of the prescriptions. Although breathlessness as an indication increased, slightly during follow-up, absolute numbers were low and during the last 6 months of life, only 4% of opioid prescriptions were for breathlessness. These findings further support undertreatment of breathlessness both for symptom relief and at the end of life.Citation6
Implications and the future
This study forms a crucial baseline against which to evaluate temporal trends in opioid prescribing for COPD as the evidence base continues to evolve. Research is needed to inform and implement evidence-based opioid treatment for the relief of refractory breathlessness in severe COPD. Large-scale longitudinal studies are needed to evaluate adverse effects and the net clinical benefit of opioids in clinical practice.Citation16,Citation28 Initiatives for implementing evidence-based treatment for symptom relief are warranted in severe respiratory diseases.
For clinicians, this study identifies potential improvement opportunities in the management of chronic refractory breathlessness. Changing the threshold at which people experience breathlessness is likely to have important implications for activities of daily living. It is likely that people exert themselves to the same level of breathlessness, and if this takes longer or more intense exertion to reach, then people are likely to be more active, therefore breaking the cycle of deconditioning. Structured measurement of the breathlessness severity in routine care is important in evidence-based symptomatic treatment.Citation29 Given their growing evidence of efficacy and safety, low-dose opioids, after careful initiation and titration, can be prescribed with better confidence for the relief of refractory breathlessness in advanced COPD.Citation30,Citation31 Another important finding is that more than half of the opioid prescriptions lacked any written indication. Clear oral and written information on why opioid is given and on the aim of the therapy is likely important for the safety of the therapy, compliance, and symptom control in these often elderly, frail, and often multi-morbid patients with end-stage respiratory diseases.
Conclusion
In severe oxygen-dependent COPD, almost half of patients were treated with opioids at some point, largely for pain (97%). Breathlessness was a rare indication for opioids (2%), even near death.
Author contributions
ME had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. DCC, ME, and ZA conceptualized and designed the study; EB and ME were responsible for the acquisition of the data; EB, ME, and ZA analyzed the data; DCC, ME, and ZA interpreted the data; ME and ZA drafted the article; and DCC, EB, ME, and ZA revised important intellectual content and approved the version to be published.
Acknowledgments
We thank all the physicians and nurses who collected the data and cared for the patients. This study was funded by unrestricted grants from the Scientific Committee of Blekinge County Council, Swedish Society of Medicine, Swedish Respiratory Society, Swedish Heart-Lung Foundation, and the Wera and Emil Cornell Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
- LozanoRNaghaviMForemanKGlobal and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet201238098592095212823245604
- WalkeLMByersALTinettiMEDubinJAMcCorkleRFriedTRRange and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failureArch Intern Med2007167222503250818071174
- JohnsonMJBowdenJAAbernethyAPCurrowDCTo what causes do people attribute their chronic breathlessness? A population surveyJ Palliat Med201215774475022687264
- MüllerováHLuCLiHTabbererMPrevalence and burden of breathlessness in patients with chronic obstructive pulmonary disease managed in primary carePLoS One201491e8554024427316
- MoensKHigginsonIJHardingREURO IMPACTAre There differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic reviewJ Pain Symptom Manage201448466067724801658
- AhmadiZLundströmSJansonCEnd-of-life care in oxygen-dependent COPD and cancer: a national population-based studyEur Respir J20154641190119326250490
- ElkingtonHWhitePAddington-HallJHiggsREdmondsPThe healthcare needs of chronic obstructive pulmonary disease patients in the last year of lifePalliat Med200519648549116218161
- GulbasGGunenHInEKilicTLong-term follow-up of chronic obstructive pulmonary disease patients on long-term oxygen treatmentInt J Clin Pract201266215215722188416
- LawSBoydSMacdonaldJRaesideDAndersonDPredictors of survival in patients with chronic obstructive pulmonary disease receiving long-term oxygen therapyBMJ Support Palliat Care201410.1136/bmjspcare-2012-000432
- AbernethyAPCurrowDCFrithPFazekasBSMcHughABuiCRandomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoeaBMJ2003327741452352812958109
- ParshallMBSchwartzsteinRMAdamsLAn official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspneaAm J Respir Crit Care Med2012185443545222336677
- JenningsALDaviesANHigginsJPGibbsJSBroadleyKEA systematic review of the use of opioids in the management of dyspnoeaThorax2002571193994412403875
- JanssenDJSpruitMAUszko-LencerNHScholsJMWoutersEFSymptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failureJ Palliat Med201114673574321510771
- AuDHUdrisEMFihnSDMcDonellMBCurtisJRDifferences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancerArch Intern Med2006166332633116476873
- VozorisNTWangXFischerHDIncident opioid drug use among older adults with chronic obstructive pulmonary disease: a population-based cohort studyBr J Clin Pharmacol201581116117026337922
- EkströmMPBornefalk-HermanssonAAbernethyAPCurrowDCSafety of benzodiazepines and opioids in very severe respiratory disease: national prospective studyBMJ2014348g44524482539
- Swedish National Register for Respiratory Failure (Swedevox)Annual Report2014 Available from: www.ucr.uu.se/swedevox/Accessed October 9, 2015
- OkenMMCreechRHTormeyDCToxicity and response criteria of the Eastern Cooperative Oncology GroupAm J Clin Oncol1982566496557165009
- EkströmMPHermanssonABStromKEEffects of cardiovascular drugs on mortality in severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187771572023328521
- WettermarkBHammarNForedCMThe new Swedish Prescribed Drug Register – opportunities for pharmacoepidemiological research and experience from the first six monthsPharmacoepidemiol Drug Saf200716772673516897791
- WHO Collaborating Centre for Drug Statistics MethodologyGuidelines for ATC Classification and DDD Assignment 2011WHO2010
- CurrowDCMcDonaldCOatenSOnce-daily opioids for chronic dyspnea: a dose increment and pharmacovigilance studyJ Pain Symptom Manage201142338839921458217
- JanssenDJde HossonSMbij de VaateEMoorenKJBaasAAAttitudes toward opioids for refractory dyspnea in COPD among Dutch chest physiciansChron Respir Dis2015122859225676931
- EkströmMNilssonFAbernethyAACurrowDCEffects of opioids on breathlessness and exercise capacity in chronic obstructive pulmonary disease: a systematic reviewAnn Am Thorac Soc20151271079109225803110
- RockerGHortonRCurrowDGoodridgeDYoungJBoothSPalliation of dyspnoea in advanced COPD: revisiting a role for opioidsThorax2009641091091519786716
- CurrowDCQuinnSGreeneABullJJohnsonMJAbernethyAPThe longitudinal pattern of response when morphine is used to treat chronic refractory dyspneaJ Palliat Med201316888188623746231
- RockerGMSimpsonACJoanne YoungBOpioid therapy for refractory dyspnea in patients with advanced chronic obstructive pulmonary disease: patients’ experiences and outcomesCMAJ Open201311E27E36
- JohnsonMJAbernethyAPCurrowDCGaps in the evidence base of opioids for refractory breathlessness. A future work plan?J Pain Symptom Manage201243361462422285285
- MularskiRACampbellMLAschSMA review of quality of care evaluation for the palliation of dyspneaAm J Respir Crit Care Med2010181653453820056904
- EkströmMBornefalk-HermanssonAAbernethyACurrowDLow-dose opioids should be considered for symptom relief also in advanced chronic obstructive pulmonary disease (COPD)Evid Based Med20152013925564658
- EkströmMPAbernethyAPCurrowDCThe management of chronic breathlessness in patients with advanced and terminal illnessBMJ2015349g761725556037
