Abstract
To investigate the association between inhaled corticosteroid (ICS) exposure patterns and the risk of pneumonia in chronic obstructive pulmonary disease (COPD) patients, we performed a nested case-control study. Between 1998 and 2010, 51,739 patients, including 19,838 cases of pneumonia, were matched to 74,849 control subjects selected from a cohort of COPD patients using ICSs via risk-set sampling of the database constructed by the National Health Research Institutes of Taiwan. After adjusting for covariates, the current use of ICSs was associated with a 25% increase in the risk of pneumonia (odds ratio [OR] =1.25, 95% confidence interval [CI] =1.20–1.30), and there was an increase in the OR with increase in the average daily dosage. Additionally, users of fluticasone/salmeterol, fluticasone, and either fluticasone/salmeterol or fluticasone were more likely to be at a higher risk of pneumonia (OR =1.35, 95% CI =1.28–1.41; OR =1.22, 95% CI =1.10–1.35; and OR =1.33, 95% CI =1.27–1.39, respectively). In contrast, there were no statistically significant associations between the risk of pneumonia and the use of budesonide/formoterol, budesonide, or either budesonide/formoterol or budesonide. In conclusion, ICSs are significantly associated with an increased risk of pneumonia in COPD patients. The effect is prominent for fluticasone-containing ICSs but not for budesonide-containing ICSs.
Introduction
The burden of COPD has been growing worldwide, and ~218 million persons are estimated to have been affected in 2010.Citation1–Citation3 Most importantly, COPD can cause significant morbidity and mortality.Citation4–Citation6 Exacerbations are important events for COPD patients and can elicit deleterious effects on health status and contribute to disease progression.Citation7 To prevent COPD exacerbations and reduce their associated hospital admissions, some pharmacologic therapies, such as ICSs; LABA; LAMA; macrolide; N-acetylcysteine; and nonpharmacologic interventions, including pulmonary rehabilitation, long-term oxygen therapy, and home noninvasive ventilator support, have been developed.Citation7–Citation12 Among these therapies, ICSs have been widely used and are recommended to reduce repeated exacerbations in COPD patients with risk factors.Citation8,Citation12
However, safety-related issues regarding ICS remain a serious concern,Citation13 and several adverse effects, including oral candidiasis, osteoporosis, hip fractures, glaucoma, cataracts, and hyperglycemia, have been reported.Citation13–Citation18 Additionally, several studies have also demonstrated a significant association between the use of ICSs and an increase in the risk of pneumonia in COPD patients.Citation19–Citation23 However, the magnitude of risk and the effects of various preparations and doses remain unclear.Citation24 Such inadequacy in information on pharmaceutical risk brings to clinicians more challenges in making appropriate decisions to balance positive and negative effects of ICSs for COPD patients. Moreover, the majority of the previous studiesCitation20–Citation23 were conducted in the Western countries. Therefore, the findings of these studies may not be generalizable to Asian populations, such as the Taiwanese. Additionally, previous studies have not evaluated the dose–response effects of the combined use of ICS and ICS/LABA or the intra-class differences between different ICSs and ICS/LABA; however, these factors may represent confounds.
The NHI program in Taiwan offers comprehensive medical care coverage to all residents. More than 99% of the residents in Taiwan have been involved in this program since 1996, and the loss of follow-up data in this database is extremely rare. By taking advantage of Taiwan’s nationwide, population-based database, we were able to conduct an investigation of a large population with long-term follow-up. Moreover, we used a nested case-control design to overcome the possible dose–response effects of the combined use of ICS or ICS/LABA and the intra-class differences between the ICSs and ICS/LABA. Overall, the aim of this study was to investigate the influence of ICS on the risk of pneumonia in COPD patients.
Methods
Data source
We used the database constructed by the NHRI of Taiwan. This database includes outpatient visits, hospital admissions, prescriptions, and disease and vital status data for 99% of the population (23 million people) in Taiwan. The patient records and information were anonymized and de-identified prior to analysis. Therefore, informed consent was not required and was specifically waived by the Institutional Review Board of NHRI. Ethics approval was obtained from the Institutional Review Board of NHRI.
The NHRI used original data from the NHI database to construct a longitudinal database of COPD patients from 1998 to 2010. This cohort included 2,200,000 patients representing 60.5% of all patients with heart or lung disease in the NHI database (n=3,635,539).
Study cohort
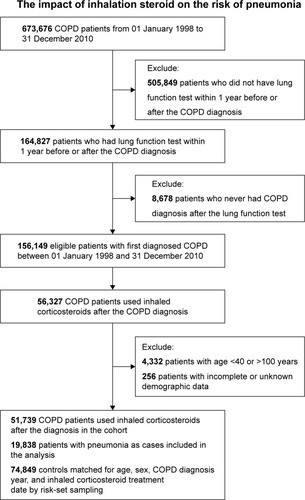
The study cohort comprised all patients who had experienced a hospital admission or at least three outpatient visits with a COPD diagnostic code (according to the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 491, 492, and 496) within 1 year between 1998 and 2010. Patients were excluded for the following reasons: 1) age <40 or >100 years, 2) unknown demographic data, 3) had not undergone a lung function test within 1 year before or after the COPD diagnosis, 4) had not received a COPD diagnosis after the lung function test, or 5) had not used ICSs after a COPD diagnosis. Consequently, the study cohort comprised 51,739 COPD patients who received treatment with ICSs. The index date was defined as the date of the first prescription for an ICS after a COPD diagnosis. The patients were followed from the index date until the first hospitalization for pneumonia, death, or 31 December 2010.
Pneumonia cases and controls
To investigate the associations between ICS drug exposure patterns and the risk of pneumonia, we performed a nested case-control study with the study cohort. All individuals in the study cohort with the serious pneumonia diagnosis codes (ICD-9-CM codes 480–486 and 507) were identified as cases. Serious pneumonia was defined as a hospitalization for or death from pneumonia. Risk-set sampling, matched for age (within 1 year), sex, COPD diagnosis year, and ICS treatment date, was used to find controls from the study cohort. Controls could not have pneumonia diagnosis within 1 year after matched case’s date of pneumonia diagnosis. Up to four controls were selected for each case.
Inhaled pharmacotherapy exposure and covariate assessment
All dispensed prescriptions for inhaled drugs used for COPD during the follow-up duration were identified. These drugs included ICSs (ATC code R03BA), LABAs (ATC codes R03AC12 and R03AC13), SABAs (ATC code R03AC), and fixed ICS/LABA combinations (ATC codes R03AK06 and R03AK07). All treatment periods on the same drug from initiation to termination (estimated based on the prescribed pack size and the prescribed number of daily inhalations) were summarized in terms of treatment length and events. All events were assigned to the treatment that the patient received at the time of the event. The amounts of oral corticosteroids and ICSs were converted to the number of DDDs, as defined by the ATC and DDD systems (http://www.whocc.no/atc_ddd_index/). The converted doses of the inhalation steroids were categorized as 0–500 μg, 500–1,000 μg, and >1,000 μg. The cumulative duration of inhalation steroid use was categorized as ≤1, 1–2, 2–3, and >3 years. Besides, we divided the person-time product into current use, recent use (from ICS discontinuation to pneumonia <6 months), and past use (from ICS discontinuation to pneumonia ≥6 months) by using nonuse as the reference.
The demographic and clinical characteristics of the patients at 1 year before the index date were recorded. These characteristic covariates included age, exacerbation of COPD (eg, hospitalization for COPD, number of visits to emergency departments due to COPD, the use of oral steroids or antibiotics), COPD drug use (SABA, LABA, or LAMA), and comorbidity (DM, cancer, asthma, chronic liver disease, renal failure, hypertension, heart failure, stroke, ischemic heart disease, chronic kidney disease, ESRD, and CCI). Additionally, the COPD-related health care utilization was categorized in terms of COPD hospitalization (no or yes), the number of days in hospital for COPD (0, 1, 2, or >2 weeks), and the number of outpatient visits for COPD (0, 1–5, or >5 times).
Data analysis
This study used SAS software version 9.1.3 (SAS Institute Inc., Cary, NC, USA) and R software version 2.14.1 (Free Software Foundation, Inc., Boston, MA, USA) to perform all statistical analyses. A significance level of 5% was used for all analyses.
The yearly rates of severe exacerbation events (hospitalizations for COPD, emergency department visits, and oral steroid or antibiotic use) were estimated using Poisson regressions. The crude and adjusted ORs for the associations of pneumonia with the independent variables (eg, oral and inhaled corticosteroid use, COPD-related health care utilization, and different types of inhalation steroids) with the 95% CIs were computed with conditional logistic regression. The confounding factors in the model were age, sex, days from COPD diagnosis to ICS, exacerbation of COPD, SABA, LABA, LAMA, DM, cancer, asthma, chronic liver disease, renal failure, hypertension, heart failure, stroke, ischemic heart disease, chronic kidney disease, ESRD, and CCI.
Results
Between 1998 and 2010, a total of 673,676 patients in the NHRI database were diagnosed with COPD. Among these patients, 164,827 had undergone lung function tests within 1 year prior to or after the COPD diagnosis. Seven thousand three hundred fifteen patients without diagnoses of COPD after the lung function test, 101,185 patients who did not use ICSs, 4,332 patients aged <40 or >100 years, and 256 patients without complete demographic data were excluded. Of the remaining 51,739 patients, 19,838 cases of pneumonia were matched to 74,849 control subjects selected from the COPD cohort using ICS by risk-set sampling ().
The demographic characteristics of the study subjects are shown in . The case group was older and had more underlying comorbidities, including DM, cancer, asthma, chronic liver disease, renal failure, hypertension, heart failure, stroke, ischemic heart disease, chronic kidney disease, and ESRD, compared with the control group. The pneumonia cases were more likely to be associated with frequent exacerbations, hospitalizations for COPD, and emergency department visits. The mean yearly rates of exacerbations, hospitalizations for COPD, and emergency department visits were 1.93, 0.63, and 0.46, respectively, in the pneumonia group and 1.03, 0.23, and 0.15 in the control group, respectively. Additionally, the case group had significantly higher rates and doses of oral steroids, antibiotics, SABA, LAMA, and LAMA than the control group.
Table 1 Characteristics of pneumonia cases and their matched controls selected from a cohort of patients with COPD
Compared with the control group, the pneumonia case group was associated with a significantly higher utilization rate of COPD-related health care (COPD hospitalization, days in hospital for COPD, and outpatient visits for COPD) within the year prior to the event (). After adjusting for age, sex, days from COPD diagnosis to ICS, oral steroid use, SABA, LABA, LAMA, and comorbidity, the conditional logistic regression analysis revealed that those with ≥1 COPD hospitalization were at a significantly higher risk for developing pneumonia than those without COPD hospitalization (OR =2.58, 95% CI =2.48–2.69). A dose–response relationship was found between the days in the hospital for COPD and the risk of pneumonia development. Among our study subjects, the adjusted ORs were 1.81 (95% CI =1.70–1.93), 2.38 (95% CI =2.23–2.54), and 3.73 (95% CI =3.53–3.95) for the patients who spent 1–7, 8–14, and >14 days in the hospital for COPD, respectively. Additionally, there was a slight trend toward an increased risk of pneumonia among those with >5 outpatient visits for COPD (OR =1.25, 95% CI =1.19–1.31).
Table 2 COPD-related health care utilization in the past year of the event
depicts the comparisons of the timing and average daily dose (DDD) of oral corticosteroids and ICSs between the cases and the control group. After adjusting for the covariates, the patients currently using (within 60 days) oral corticosteroids exhibited a 2.2-fold increase in the risk of pneumonia (OR =2.18, 95% CI =2.10–2.28). However, the current use of an ICS was associated with a 25% increase in the risk of pneumonia (OR =1.25, 95% CI =1.20–1.30). Additionally, the mean daily dosages ranged from 0.15 DDD/day for oral corticosteroids in the control group to 0.43 DDD/day for ICSs in the case group.
Table 3 Oral and inhaled corticosteroid use in pneumonia cases and control patients
reveals that the current use of ICS was associated with a 1.26-fold increase in the risk of pneumonia (OR =1.26, 95% CI =1.21–1.32). However, there was no significant association between the past use of ICS and the risk of pneumonia (OR =0.94, 95% CI =0.86–1.04). The OR increased with increase in the average daily dosage (DDD), which ranged from 10% for the >0 to 500 μg group (OR =1.10, 95% CI =1.04–1.16) to 63% for the >1,000 μg ICS group (OR =1.63, 95% CI =1.50–1.78). The significantly highest risk of pneumonia was observed in those with 2–3 years of exposure to ICSs (OR =1.38, 95% CI =1.21–1.59).
Table 4 Crude and adjusted odds ratios of pneumonia associated with inhalation steroid use
We performed conditional logistic regression to examine whether significant heterogeneity among the different types of ICSs contributed to the risk of pneumonia (). After adjusting for the covariates, the users of fluticasone/salmeterol, fluticasone, and the users of either fluticasone/salmeterol or fluticasone were more likely found to be at greater risks for pneumonia (OR =1.35, 95% CI =1.28–1.41; OR =1.22, 95% CI =1.10–1.35; and OR =1.33, 95% CI =1.27–1.39, respectively). Interestingly, there were no significant differences in the risks of pneumonia following the use of budesonide/formoterol (adjusted OR =1.02, 95% CI =0.96–1.08), budesonide (adjusted OR =1.06, 95% CI =0.99–1.13), or either budesonide/formoterol or budesonide (adjusted OR =1.03, 95% CI =0.99–1.08).
Table 5 Risk of overall pneumonia associated with the use of any individual inhalation steroid
Discussion
In this large, national population-based study of COPD patients who used ICSs, we identified several significant findings. Most importantly, we found that the use of ICSs was significantly associated with an increased risk of pneumonia among the COPD patients. Furthermore, we observed that the current users of ICSs exhibited a 1.25-fold increase in the risk of pneumonia (OR =1.25, 95% CI =1.20–1.30). In contrast, we did not identify any significant association between the past use of ICSs and the risk of pneumonia (OR =0.94, 95% CI =0.86–1.04). This finding is in line with the previous studyCitation22 that showed the ICS-associated risk of serious pneumonia would decline gradually after stopping ICS use and even disappear after 6 months. Therefore, these findings hint that the effect of ICS on the risk of pneumonia for COPD patients may not last for long, and the increasing risk was only evident for current users. We also found that the risk of pneumonia increased as the dose increased or the ICS exposure duration increased. These findings are consistent with those reported by previous studies.Citation22 Suissa et alCitation22 investigated a cohort of 163,514 patients and found that the current use of ICS was associated with a 69% increase in the rate of serious pneumonia (RR =1.69, 95% CI =1.63–1.75). In another recent review,Citation25 budesonide and fluticasone delivered alone or in combination with a LABA were found to be associated with an increased risk of serious adverse pneumonia events that required hospital admission. For fluticasone, the OR for the increase in nonfatal serious adverse pneumonia events was 1.78 (95% CI =1.50–2.12; ie, 18 more per 1,000 treated over 18 months), and for budesonide, the OR was 1.62 (95% CI =1.00–2.62; six more per 1,000 treated over 9 months).Citation25 In contrast, Suissa et al also reported that the discontinuation of ICSs was associated with a 37% decrease in the rate of serious pneumonia (RR =0.63, 95% CI =0.60–0.66) and that the risk reduction persisted for both fluticasone (RR =0.58, 95% CI =0.54–0.61) and budesonide (RR =0.87, 95% CI =0.78–0.97). All of these findings demonstrated that the use of ICSs by COPD patients increased the risk of pneumonia.
Additionally, we found that the risk of pneumonia among the COPD patients varied according to the different preparations of the ICSs. With adjustment for covariates, the users of fluticasone/salmeterol, fluticasone, and either fluticasone/salmeterol or fluticasone were found to be at a greater risk of pneumonia (OR =1.35, 95% CI =1.28–1.41; OR =1.22, 95% CI =1.10–1.35; and OR =1.33, 95% CI =1.27–1.39, respectively). In contrast, there were no significant differences in the risk of pneumonia among the users of budesonide/formoterol (OR =1.02, 95% CI =0.96–1.08), budesonide (OR =1.06, 95% CI =0.99–1.13), or either budesonide/formoterol or budesonide (OR =1.03, 95% CI =0.99–1.08). A previous studyCitation22 produced similar findings that the rate of serious pneumonia was higher with fluticasone use (RR =2.01, 95% CI =1.93–2.10) and lower with budesonide use (RR =1.17, 95% CI =1.09–1.26). In a subanalysis of the PATHOS study,Citation23 the rate of pneumonia event per 100 patient-years was higher among fluticasone/salmeterol users (10.4–11.8) than among budesonide/formoterol users (6.0–6.4). The different effects of the budesonide-containing preparation and fluticasone-containing preparations may be attributable to the differences in the pharmacokinetic and pharmacodynamic characteristics of fluticasone and budesonide, which may have possible implications. Several studies have demonstrated that fluticasone is a more lipophilic corticosteroid and a more potent immunosuppressant than budesonide; therefore, fluticasone may exhibit a longer period of retention in the airway and a prolonged suppression of local immunity that facilitates bacterial colonization and possible infections.Citation26–Citation30
The results of the present study should be interpreted in light of its both strength and weakness. One of the major strengths is that this investigation was a nationwide population-based cross-sectional study that included nearly all of the COPD patients in Taiwan. Indeed, the NHI covered 99.0% of Taiwan’s population with complete follow-up information regarding mortality for the entire study population. Additionally, the dataset was routinely monitored for diagnostic accuracy by the National Health Insurance Bureau of Taiwan. In this national database study, the majority of the COPD diagnoses were made by physicians on the basis of clinical findings without performing pulmonary function tests. To improve the accuracy of COPD diagnoses, only those patients who had undergone pulmonary function tests within 1 year of the COPD diagnosis were enrolled. In addition to the large sample size, we analyzed the data using rigorous statistical methods. The duration–response relationship may further add to the causal relationship between the use of ICSs and the increasing risk for pneumonia. Finally, the findings of this study were based on real-world practice. Therefore, our study should be representative of Taiwan and thus should provide useful information.
However, our study still had several limitations. First, we did not have detailed data regarding the pulmonary function tests and could not assess the quality of life. Therefore, we could evaluate the severity of COPD, but we could not further ensure that every patient received ICS or ICS/LABA according to the recommendations of guideline. Second, a randomized controlled study design was not used in this study, but instead we used a nested case-control study design. A nested case-control study is a variation of a case-control study in which only a subset of controls from the cohort are compared to the incident cases. In a case-control study, all incident cases in the cohort are compared to a random subset of participants who do not develop the disease of interest. In contrast, in a nested case-control study, controls are selected for each case from the matched risk set of that particular case. By matching for factors such as age and selecting controls from relevant risk sets, the nested case-control model is generally more efficient than a case-cohort design with the same number of selected controls. Therefore, our findings were derived from real-world situations and are more likely to be reflective of common clinical practice. Third, we cannot evaluate the different effects of different inhaler devices based on the NHIRD database. Budesonide is usually delivered by a dry powder inhaler, but fluticasone is delivered by both dry powder inhalers (accuhaler) and MDIs (evohaler). Disabled patients who cannot handle the inhaler by themselves tended to receive prescriptions with MDI through a holding chamber (aerochamber). Therefore, it is possible that the difference in risk could be the result of bias introduced due to differences in inhaler devices.
Conclusion
ICSs are significantly associated with an increased risk of pneumonia among COPD patients. This effect was prominent for fluticasone-containing ICSs but not for budesonide-containing ICSs.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Abbreviations
| ICS | = | inhaled corticosteroid |
| COPD | = | chronic obstructive pulmonary disease |
| OR | = | odds ratio |
| NHRI | = | National Health Research Institutes |
| LABA | = | long-acting β2 agonists |
| LAMA | = | long-acting anti-muscarinic antagonists |
| NHI | = | National Health Insurance |
| DDDs | = | defined daily doses |
| SABA | = | short-acting β2 agonists |
| CCI | = | Charlson Comorbidity Index |
| DM | = | diabetes mellitus |
| CI | = | confidence interval |
| ESRD | = | end-stage renal disease |
| RR | = | relative ratio |
Acknowledgments
This study was supported by grants from National Science Council (NSC 101-2325-B-002-064, NSC 102-2325-B-002-087, NSC 103-2325-B-002-027, and NSC 104-2325-B-002-035) and from National Health Research Institutes (intramural funding). The Taiwan Clinical Trial Consortium for Respiratory Diseases (TCORE) includes Chong-Jen Yu, MD, PhD, (NTUH, Director of Coordinating Center and Core PI of Committee), Hao-Chien Wang, MD, PhD (NTUH, PI of Committee), Chi-Huei Chiang, MD, PhD (Taipei Veterans General Hospital, PI of Committee), Diahn-Warng Perng, MD, PhD (Taipei Veterans General Hospital, PI of Committee), Shih-Lung Cheng, MD, PhD (Far Eastern Memorial Hospital, PI of Committee), Jeng-Yuan Hsu, MD, PhD (Taichung Veterans General Hospital, PI of Committee), Wu-Huei Hsu, MD, PhD (China Medical University Hospital, PI of Committee), Ying-Huang Tsai, MD, PhD (Chang Gung Memorial Hospital, Chia-Yi, PI of Committee), Tzuen-Ren Hsiue, MD, PhD (National Cheng Kung University Hospital, PI of Committee), Meng-Chih Lin, MD, PhD (Chang Gung Memorial Hospital, Kaohsiung, PI of Committee), Hen-I Lin, MD (Cardinal Tien Hospital, PI of Committee), Cheng-Yi Wang, MD, PhD (Cardinal Tien Hospital, PI of Committee), Yeun-Chung Chang, MD, PhD (NTUH, PI of Committee), Ueng-Cheng Yang, PhD (National Yang-Ming University, PI of Committee), Chung-Ming Chen, PhD (NTUH, PI of Committee), Cing-Syong Lin, MD, PhD (Changhua Christian Hospital, PI of Committee), Likwang Chen, PhD (National Health Research Institutes, PI of Committee), Yu-Feng Wei, MD (E-Da Hospital, PI of Committee), Inn-Wen Chong, MD (Kaohsiung Medical University Chung-Ho Memorial Hospital, PI of Committee).
Disclosure
The authors report no conflicts of interest in this work.
References
- Lopez-CamposJLTanWSorianoJBGlobal burden of COPDRespirology2016211142326494423
- LopezADMathersCDEzzatiMJamisonDTMurrayCJGlobal and regional burden of disease and risk factors, 2001: systematic analysis of population health dataLancet200636795241747175716731270
- ManninoDMBuistASGlobal burden of COPD: risk factors, prevalence, and future trendsLancet2007370958976577317765526
- BeranDZarHJPerrinCMenezesAMBurneyPForum of International Respiratory Societies working group collaborationBurden of asthma and chronic obstructive pulmonary disease and access to essential medicines in low-income and middle-income countriesLancet Respir Med20153215917025680912
- BhattSPWellsJMDransfieldMTCardiovascular disease in COPD: a call for actionLancet Respir Med201421078378525298057
- PauwelsRARabeKFBurden and clinical features of chronic obstructive pulmonary disease (COPD)Lancet2004364943461362015313363
- WedzichaJASinghRMackayAJAcute COPD exacerbationsClin Chest Med201435115716324507843
- WedzichaJASeemungalTACOPD exacerbations: defining their cause and preventionLancet2007370958978679617765528
- DecramerMLChapmanKRDahlRINVIGORATE investigatorsOnce-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group studyLancet Respir Med20131752453324461613
- UzunSDjaminRSKluytmansJAAzithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trialLancet Respir Med20142536136824746000
- ZhengJPWenFQBaiCXPANTHEON study groupTwice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trialLancet Respir Med20142318719424621680
- GartlehnerGHansenRACarsonSSLohrKNEfficacy and safety of inhaled corticosteroids in patients with COPD: a systematic review and meta-analysis of health outcomesAnn Fam Med20064325326216735528
- MateraMGCardaciVCazzolaMRoglianiPSafety of inhaled corticosteroids for treating chronic obstructive pulmonary diseaseExpert Opin Drug Saf201514453354125557156
- CummingRGMitchellPLeederSRUse of inhaled corticosteroids and the risk of cataractsN Engl J Med199733718149203425
- GarbeELeLorierJBoivinJFSuissaSInhaled and nasal glucocorticoids and the risks of ocular hypertension or open-angle glaucomaJAMA199727797227279042844
- HubbardRBSmithCJSmeethLHarrisonTWTattersfieldAEInhaled corticosteroids and hip fracture: a population-based case-control studyAm J Respir Crit Care Med200216612 Pt 11563153612406825
- IsraelEBanerjeeTRFitzmauriceGMKotlovTVLaHiveKLeBoffMSEffects of inhaled glucocorticoids on bone density in premenopausal womenN Engl J Med20013451394194711575285
- van BovenJFde Jong-van den BergLTVegterSInhaled corticosteroids and the occurrence of oral candidiasis: a prescription sequence symmetry analysisDrug Saf201336423123623516006
- ChengSLSuKCWangHCPerngDWYangPCChronic obstructive pulmonary disease treated with inhaled medium- or high-dose corticosteroids: a prospective and randomized study focusing on clinical efficacy and the risk of pneumoniaDrug Des Devel Ther20148601607
- NanniniLJPoolePMilanSJKestertonACombined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20138Cd00682623990350
- SuissaSCoulombeJErnstPDiscontinuation of inhaled corticosteroids in COPD and the risk reduction of PneumoniaChest201514851177118326110239
- SuissaSPatenaudeVLapiFErnstPInhaled corticosteroids in COPD and the risk of serious pneumoniaThorax201368111029103624130228
- JansonCLarssonKLisspersKHPneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting beta2 agonist: observational matched cohort study (PATHOS)BMJ2013346f330623719639
- FinneyLBerryMSinganayagamAElkinSLJohnstonSLMalliaPInhaled corticosteroids and pneumonia in chronic obstructive pulmonary diseaseLancet Respir Med201421191993225240963
- KewKMSeniukovichAInhaled steroids and risk of pneumonia for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20143Cd010115
- BrattsandRMiller-LarssonAThe role of intracellular esterification in budesonide once-daily dosing and airway selectivityClin Ther200325Suppl CC28C4114642802
- DalbyCPolanowskiTLarssonTBorgstromLEdsbackerSHarrisonTWThe bioavailability and airway clearance of the steroid component of budesonide/formoterol and salmeterol/fluticasone after inhaled administration in patients with COPD and healthy subjects: a randomized controlled trialRespir Res20091010419878590
- EkALarssonKSiljerudSPalmbergLFluticasone and budesonide inhibit cytokine release in human lung epithelial cells and alveolar macrophagesAllergy199954769169910442524
- RennardSITashkinDPMcElhattanJEfficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trialDrugs200969554956519368417
- ThorssonLEdsbackerSKallenALofdahlCGPharmacokinetics and systemic activity of fluticasone via Diskus and pMDI, and of budesonide via TurbuhalerBr J Clin Pharmacol200152552953811736861