Abstract
Endocan, formerly called endothelial cell-specific molecule 1, is an endothelial cell-associated proteoglycan that is preferentially expressed by renal and pulmonary endothelium. It is upregulated by proangiogenic molecules as well as by pro-inflammatory cytokines, and since it reflects endothelial activation and dysfunction, it is regarded as a novel tissue and blood-based relevant biomarker. As such, it is increasingly being researched and evaluated in a wide spectrum of healthy and disease pathophysiological processes. Here, we review the present scientific knowledge on endocan, with emphasis on the evidence that underlines its possible clinical value as a prognostic marker in several malignant, inflammatory and obstructive disorders of the respiratory system.
Introduction
Endocan, formerly called endothelial cell-specific molecule 1 (ESM-1), is a novel proteoglycan mainly, but not entirely expressed by pulmonary and renal endothelial cells. It is secreted upon stimulation by cytokines, namely tumor necrosis factor-α (TNF-α), interleukin (IL)-1 and microbial lipopolysaccharide, as well as by proangiogenic factors such as vascular endothelial growth factor (VEGF). Via its interaction with intercellular adhesion molecules, endocan exhibits a well-described inhibitory role on leukocyte binding to the vascular endothelium.Citation1–Citation6 These properties have highlighted its potential role as a biomarker of endothelial dysfunction and inflammation, while it has also been shown to be overexpressed in several human tumors and has, therefore, been implicated in the pathogenesis of malignancy and cancer angiogenesis.Citation7–Citation10
Based on its aforementioned tropism to lung endothelium and the accumulating research outcomes, despite the fact that its exact role has yet to be defined, endocan is being increasingly acknowledged as a promising agent in predicting and further understanding several inflammatory and malignant conditions of the respiratory system. In the present review, we will commence by describing endocan’s general structure followed by a brief summary of its molecular and biological properties. We will then proceed with a presentation of the current information concerning its emerging significance in the prognosis and follow-up of patients with various respiratory conditions, as well as of the evidence pointing toward a possible future role in the diagnosis and study of obstructive and inflammatory lung disorders.
Endocan: general knowledge
Structure and expression
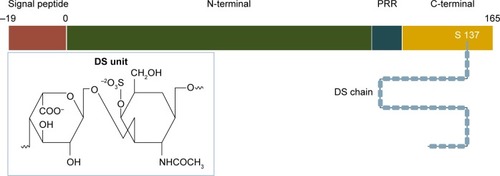
Endocan was first described and molecularly characterized in 1996 by Lassale who isolated an endothelial cell-related molecule in a human umbilical vein endothelial cell cDNA library. In humans, it is encoded by a single gene, the ESM-1 gene, localized on chromosome 5 at the position 5q11.2.Citation1 Originally termed ESM-1, it was shown to exhibit significant cytokine-dependent functions during the inflammatory reaction and to be constitutively expressed in the endothelial cell network. Based on the latter, it was renamed accordingly, receiving its current name in 2001.Citation4 Structurally, it is a 50 kDa soluble dermatan sulfate (DS) proteoglycan (PG) and as such comprises a protein core, covalently attached to a glycosaminoglycan (GAG)-type linear polysaccharide chainCitation3 (). The protein part is composed of 165 amino acids and possesses an N-terminal cysteine-rich region of 110 amino acids, which also includes an endothelial growth factor-like region, a phenylalanine-rich domain and a C-terminal region. It is linked to the GAG chain via serine 137 during posttranslational modification.Citation1,Citation5,Citation6 The GAG chain contains 32 disaccharide residues consisting of an amino sugar (N-acetylglucosamine, glucosamine that is variously N-substituted, or N-acetylgalactosamine) and a uronic acid (glucuronic acid or iduronic acid with a ratio of 30/70).Citation3
Unlike most chondroitin/DS-containing proteoglycans, which are either extracellular matrix (ECM) or cell membrane associated, endocan belongs to a limited category of secreted proteoglycans, but displays no clear structural linkage to any of them. First of all, its small size and single DS chain distinguishes it from most ECM PGs, which are relatively large molecules, possessing several GAG chains. In contrast to the largest class of the ECM PGs, the small leucine-rich PGs, and the hyaluronan- and lectin-binding PGs (hyalectans), endocan does not contain the conserved leucine rich repeats of the former, nor the C-type lectin domains of the latter.Citation11,Citation12 Furthermore, compared to the existing DS PGs, endocan’s DS chain is shorter and characterized by a higher content of non-sulfated and disulfated disaccharides.Citation3,Citation5,Citation6 GAG moieties of proteoglycans are responsible for their molecular interactions and consequently their properties. Accordingly, endocan’s saccharide chain, partly due to the conformational flexibility conferred by its iduronic acid residuesCitation13 and partly due to the binding properties associated with its highly sulfated domains,Citation6 is considered critical for the molecule’s biological functions.
As already mentioned, endocan is secreted by activated endothelial cells, preferentially of the lung and less intensively of the renal vasculature, including tumor endothelial cells.Citation1,Citation10 Nevertheless, its expression is not entirely restricted to the endothelium. More recent studies have demonstrated its active synthesis by several normal, actively proliferative tissues, as well as by tissues undergoing neogenesis.Citation7,Citation8,Citation14 Endocan overexpression has also been clearly demonstrated in malignant tissues such as melanoma, glioblastoma, renal and lung carcinoma, with the level of expression directly correlating to the severity of the disease.Citation3 Tissue activity seems to be a prerequisite for endocan expression as it has not yet been described in quiescent tissues such as major vessels or the spleen.Citation14
Binding properties
Being a proteoglycan, the multifunctionality of endocan arises from its structure and involves intercellular interactions, which to a large extent depend on the interaction of the GAG chain and the protein core with various ligands. The latter has been well documented to bind to the β2 integrin CD11a/CD18, known as lymphocyte function-associated antigen-1 (LFA-1). LFA-1 is expressed in leukocytes and is important in the migration of neutrophils, monocytes and lymphocytes through its binding to the cellular adhesion molecules (CAMs) 1 and 2 that are expressed on vascular endothelium in inflammatory sites.Citation15 Through its interaction with LFA-1, endocan inhibits the aforementioned binding and consequently the LFA-1/intercellular adhesion molecule-1 (ICAM-1)-dependent leukocyte adhesion that normally occurs during inflammatory processes.Citation2
As far as the GAG chain is concerned, it has been shown to mediate several biological functions with iduronic acid being fundamental in the protein binding properties of the molecule.Citation10,Citation16 One of these functions is the promotion of the hepatocyte growth factor/scatter factor-dependent proliferation of endothelial cells, a process that is implicated in angiogenesis, tumorigenesis and tumor progression.Citation4,Citation6,Citation17 Taking into consideration the binding properties exhibited by the DS moiety, possible interactions of endocan with several other proteins cannot be excluded.Citation18,Citation19
Regulation
Consistent with endocan’s role in inflammation and endothelial activation, cytokines that provide activation signals for the functional potentiation of integrins and their conversion from low- to high-affinity state on leukocytes have been shown to induce its expression in vitro. Such cytokines are TNF-α and IL-1, which seem to increase not only the molecule’s m-RNA content of HUVEC cells but also its secretion.Citation20 Endocan m-RNA and its protein expression are also induced by VEGF,Citation21 while transforming growth factor (TGF)-β1 and fibroblast growth factor-2 in vitro stimulate its release in endothelial cells and tumor cell lines.Citation22 At the same time, others such as interferon-γ (IFNγ) and IL-4 seem to play an inhibitory and no role at all, respectively. IFNγ, in particular, interestingly exhibits a stronger inhibitory effect on endocan expression when combined with TNF-α, despite their usual synergistic effect on the expression of various pro-inflammatory factors and adhesion molecules.Citation1,Citation20
Pathophysiological significance
Due to its involvement in the regulation of cellular behavior and in an attempt to explore its potential significance as a biomarker, endocan’s role is increasingly being studied in a spectrum of healthy and disease pathophysiological processes.
Despite a certain controversy in literature in cases of low-grade inflammation, endocan’s blood levels have been found elevated in septic patients with increasing severity of illness as well as in immunocompromised patients with complicating bacterial infections. This underlines a possible future role in the differential diagnosis of the systemic inflammatory response syndrome and a predictive value in terms of clinical outcome.Citation9,Citation20,Citation23 Such findings are, as expected, consistent with the reported higher levels of ICAM-1 and P-selectin in relation to the degree of the syndrome’s severity.Citation24–Citation26 Given the endothelium’s central role in its pathogenesis, endocan has also been studied in relation to Behcet’s-Adamantiades disease and has been found potentially significant as a biomarker.Citation27
The molecule was also recently investigated in relation to essential hypertension, as the pathogenesis of the latter is characterized by inflammation and interactions between the vasculature and circulating leukocytes, and its levels were found to be directly related to the blood pressure values.Citation28 It should be mentioned that hypertensive patients also exhibit elevated serum levels of E-selectin, P-selectin and ICAM-1 compared to normotensive individuals. This evidence is supportive of a protective role against complications, for instance atherosclerosis, through endocan’s competitive affinity for LFA-1 and provides a basis for further investigation in other conditions with underlying endothelial dysfunctions such as cardiovascular diseases and diabetes mellitus. Surprisingly though, despite the fact that obesity is a state of low-grade inflammation and is associated with enhanced release of adipokines and adhesion molecules, namely ICAM-1, circulating levels of endocan are decreased in obese individuals.Citation7,Citation29
As far as endocan’s aforementioned potential role in tumor development and growth is concerned, it has motivated ongoing scientific activity with the majority of studies exploring its future use as a relevant biomarker. Recent studies indicate that endocan not only is upregulated in the presence of proangiogenic factors such as VEGF,Citation21 but that it in turn enhances their mitogenic activity.Citation30 Furthermore, increased vascular expression of endocan has been demonstrated by its immunoreactivity within endothelial cells, and association with intra-tumoral microvascular density has been reported in studies involving glioblastoma, bladder, clear renal cell, colorectal and hepatocellular carcinoma as well as in malignant melanoma cases.Citation22,Citation31–Citation36 Therefore, it is possibly involved in neoangiogenesis with its levels correlating with tumor aggressiveness and progression and could be valuable in monitoring treatment with antiangiogenic agents in cancer patients. This hypothesis is strengthened by the confirmation of its expression and specificity to endothelial tip cells.Citation37 Tip cells are a subpopulation of endothelial cells that are known to mediate vascular growth and to play an important role in neoangiogenesis. The recent identification of endocan’s expression and active secretion by tumor cells, which tend to express endothelial cell-associated genes, also suggests its possible pro-tumorigenic role, with higher degrees of expression being associated with increased tumor invasiveness.Citation22,Citation38 Not surprisingly, given the biological significance of the molecule’s DS, only fully glycanated endocan promotes tumorigenesis, although not without the contribution of its polypeptide.Citation38,Citation39
Besides solid tumors, the molecule might also have a clinical impact in hematological malignancies, as suggested by recent attempts to evaluate it as a prognostic marker in acute myeloid and lymphoid leukemia.Citation40,Citation41 According to the results, its serum levels, as well as its cytoplasmic expression in bone marrow blasts, were associated with chemotherapy outcome, a finding which is again in consistence with VEGF’s reported role in leukemia-associated angiogenesis.Citation42 This rapidly expanding research activity surrounding endocan is facilitated by the relatively simple laboratory methods developed for such purposes, including sandwich enzyme-linked immunosorbent assays, immunohistochemistry and immunoprecipitation. Sandwich enzyme-linked immunosorbent assays are commercially available for diagnostic purposes as well; can be applied in cell culture supernatants, serum or plasma; and could provide a useful tool for quick quantification of endocan levels in various clinical settings.
Endocan and the respiratory system
A considerable spectrum of lung diseases, ranging from atopic asthma and COPD to lung cancers, is invariably characterized by different degrees of endothelial damage and microvascular inflammation with accompanying upregulation of adhesion molecules, the expression of which mediates leukocyte trafficking and adherence to the endothelium. ICAM-1 and LFA-1 are thought to play a crucial role in airway inflammation and lung tumors with certain studies clearly demonstrating their upregulation, while others present with more or less conflicting results.Citation43–Citation50 Considering endocan’s selective expression in activated pulmonary endothelial cells and interaction with LFA-1, its role in lung disorders with underlying endothelial dysfunction is of particular interest as it could reflect the severity of such pathophysiological conditions. Nevertheless, data about the molecule’s role are just emerging since it has, surprisingly, not been studied extensively in clinical settings of the respiratory system ().
Table 1 Studies examining the role of endocan in respiratory disorders
Lung neoplasms
As already mentioned in the previous section, the majority of existing endocan-centered studies have investigated it in relation to tumorigenesis. Taking into consideration the fact that endothelial endocan expression is stimulated by VEGF production by neoplastic cells, these two molecules are strictly connected. Nevertheless, in contrast to the abundant literature assessing VEGF expression and serum levels,Citation51,Citation52 presently only two studies have investigated endocan in relation to primary lung cancer.Citation38,Citation53
In the study conducted by Scherpereel et al,Citation38 the sera of patients with various lung cancers were collected upon diagnosis and prior to specific treatment. The revealed elevated endocan concentrations, directly correlating with the tumoral size, provided the first indication of the molecule’s possible use in the evaluation of the overall prognosis of such cases. The subsequent clinical study by Grigoriu et alCitation53 evaluated endocan’s expression in lung in specimens of 24 non-small cell lung carcinoma (NSCLC) patients undergoing surgery, as well as in 30 previously untreated ones, and demonstrated a VEGF-dependent overexpression of both the molecules’ m-RNA and protein in the tumoral, but not in the distal, healthy lung tissue. This study also suggested a close relationship between the elevated serum endocan levels and poor survival, with the cutoff level between survivors and non-survivors being 1.3 ng/mL. Lung biopsy molecular profiling studies, like the one conducted by Borczuk et al,Citation54 which supports the use of gene expression profiles as predictive information about clinical outcome and has identified the ESM-1 gene among the ones associated with high risk of death within 1 year, represent an attractive future approach.
Acute lung injury/acute respiratory distress syndrome
In addition to lung neoplasms, endocan has also been studied in relation to acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) with more or less contradictory outcomes. These two conditions are characterized by acute onset, alveolar and endothelial injury, pulmonary edema and severe hypoxemia and are major complications of either pulmonary or extra-pulmonary insults.Citation55 Clinical conditions that exert their effects directly on lung cells include diffuse pulmonary infection, aspiration and, less commonly, injurious ventilation, pulmonary contusion and toxic inhalation. On the other hand, indirect lung injury can be the result of sepsis, severe extrathoracic trauma, multiple blood transfusions, as well as several other causes of an acute systemic inflammatory response.Citation56
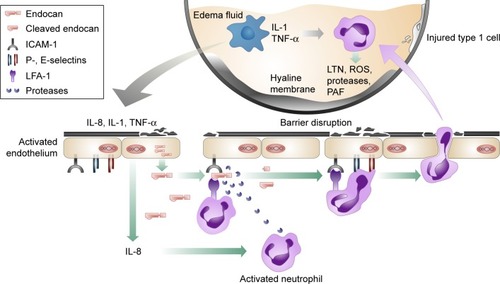
Irrespective of the noxious agent’s nature, it triggers an intense pulmonary inflammatory response with accumulation of pro-inflammatory mediators originating from activated platelets, alveolar macrophages, migrating leukocytes as well as injured tissues.Citation57,Citation58 Such mediators include thromboxane A2, TNF-β, VEGF and TNF-α and initiate a cascade of events leading to the disruption of the pulmonary endothelial functional and structural integrity that characterizes the condition.Citation59 The upregulation of CAMs in particular, with subsequent enhancement of leukocyte and platelet recruitment and aggregation, is of key significance. The adherent platelets further release mediators that lead to endothelial activation, promoting its procoagulant properties, while leukocytes, particularly neutrophils, through their ICAM-1 interaction release reactive oxygen species, which induce oxidative injury.Citation59,Citation60 Studies emphasizing the role of markers denoting pulmonary endothelial dysfunction have been performed in an attempt to investigate the aforementioned pathogenetic mechanisms and have reported, despite a relative confliction, higher levels of VEGF, P-selectin, ICAM-1 and vWF in ALI patients.Citation61–Citation65
Based on the aforementioned pathophysiological processes, endocan has been investigated as a predictive biomarker for the development of ALI or ARDS originating from severe sepsis in a 72-hour prospective study of 21 intensive care unit patients and nine healthy controls.Citation66 Although significantly increased in the severe sepsis and septic shock patients versus the individuals belonging to the controls’ group, the molecule’s levels were found to be lower than expected in those patients who developed either ALI or ARDS at 48 and 72 hours compared to the ones that did not at the same time points. Despite the pathophysiological and clinical differences between ALI of traumatic and non-traumatic etiology, these findings are in accordance with another recent study attempting to associate endocan’s serum levels to the development of ALI triggered by major trauma.Citation67,Citation68 The outcome from both studies supports the hypothesis that endocan-mediated inhibition of leukocyte recruitment may exhibit a protective role against the development of ALI and ARDS () and if further verified, it could represent a future therapeutic approach. The results, at a molecular level, could be further explained by either a reduced release of endocan from pulmonary endothelial cells, or an increase of its proteolysis by neutrophil serine proteases.Citation69
Figure 2 Possible protective role of endocan in ALI/ARDS.
By contrast, significantly elevated plasma endocan levels were observed by Tang et alCitation70 in pneumonia patients developing ARDS compared with ones that did not, and were further associated with an increased incidence of multiple organ dysfunction in these patients. The proposed responsible mechanism is the induction of the molecule’s synthesis by bacterial endotoxins as well as by pro-inflammatory cytokines such as IL-1β and TNF-α.Citation70
The compromise of the pulmonary endothelial cell barrier in response to ALI is also strongly dependent on cytoskeletal remodeling, resulting in the disruption of the cytoskeleton network. These complex mechanisms involved in endothelial cell permeability are not fully explored. Nevertheless, they seem to include signal transduction pathways that involve, among others, protein kinase C, myosin light chain kinase, Rho and p38 kinase signaling, resulting in the phosphorylation of several cytoskeletal proteins.Citation71 Endocan’s role in the aforementioned processes could be an attractive topic for future research. Its regulation by VEGF and the fact that the latter has been shown to stimulate the reorganization of endothelial actin and the formation of stress fibersCitation72 could also be of relevance and should be taken into consideration.
Other clinical settings
At this point, it is worth mentioning that endocan has also been studied in less critical lower respiratory infections. In their study, Kao et al attempted to evaluate endocan’s usefulness in differentiating healthy individuals from patients with pulmonary infections.Citation73 More specifically, endocan levels were measured in 82 hospitalized patients with community-acquired pneumonia (CAP) and compared to those of healthy controls. The molecule’s levels were found to directly correlate to illness severity and response to antibiotic treatment in a more specific manner than to C-reactive protein concentrations or white blood cell counts.
Based on the association of obstructive sleep apnea (OSA) and endothelial dysfunction, endocan was also very recently investigated in a prospective study by Altintas et al.Citation74 The effect of the condition on the molecule’s levels was assessed in moderate-to-severe OSA patients, at baseline and following 3 months of continuous positive airway pressure treatment. According to the results, not only was endocan able to distinguish patients with OSA from individuals with suspected, though not confirmed, diagnosis but also further correlated with the severity of the condition, measured by the apnea-hypopnea index and the therapeutic response. Such data are supportive of OSA having an endothelium-dependent pathology, as well as of endocan’s potential role as a biomarker in the diagnosis and monitoring of affected individuals.
Finally, in a study of patients admitted with pulmonary thromboembolism (PTE), endocan levels demonstrated a significant difference not only between patients and controls but also between the patients’ clinical subclasses according to right ventricular dilatation.Citation75 Endocan clearly showed a positive correlation with the latter and was significantly higher in the submassive and massive PTE cases than in the non-massive ones. As expected, since the molecule reflects endothelial integrity, the results are indicative not only of its possible value as a biomarker in such events but also in the determination of their severity.Citation75
Future perspectives in inflammatory and obstructive lung disorders
Endocan’s properties provide enough ground for further investigation in search of information about its possible value as a marker of several respiratory conditions. For instance, it has been reported that TGF-β and VEGF levels are elevated in complicated parapneumonic effusions and empyemas, with the latter exhibiting a predictive value for the development of residual pleural thickening.Citation76–Citation78 As both growth factorsCitation1,Citation30 induce endocan’s expression we postulate that endocan correlates with parapneumonic effusions as well, but this hypothesis may warrant further investigation. Furthermore, although the demonstration of a causal relationship between endothelial dysfunction and chronic airway disease is challenging, there is accumulating evidence supporting an association between endothelial dysfunction, COPD and asthma.
Both systemic and bronchial inflammation with the observed elevated levels of TNF-α, IL-6, IL-8 and adhesion molecules and the increased bronchial leukocyte counts could be responsible for the atherosclerotic lesions that characterize many of the cardiovascular comorbidities of COPD patients.Citation49,Citation79 The airway vasculature has been assessed in such patients, and findings of altered small airway wall vascularity and reactivity are consistent with endothelial dysfunction, possibly linking bronchial obstruction to the functional changes of the endothelial arterial wall.Citation80,Citation81 In addition, as heightened systemic inflammation is associated with recurrent exacerbations, the inflammatory changes observed during the course of such episodes, including the levels of markers such as TNF-α and IL-6, have been shown to be of potential value in the exacerbation pattern prediction.Citation82–Citation84 As far as asthma is concerned, possibly due to inflammation, the airway circulation in such patients has been shown to be characterized by microvascular hyperpermeability, vascular remodeling that involves the whole bronchial tree, increase in subepithelial blood flow and endothelial dysfunction.Citation85–Citation88
Taking into consideration endocan’s upregulation both in settings of endothelial dysfunction and in inflammatory processes of similar molecular patterns, there is adequate ground for future investigation of its role in the natural history of obstructive pulmonary diseases as well as in the follow-up of affected patients. The relationship of the molecule’s levels and the clinical outcomes of patients could be of importance, possibly in line with recent findings supporting an inverse relation between serum levels of VEGF and disease severityCitation89,Citation90 and is a potentially interesting field for further exploration. Endocan could furthermore provide information about the endothelial function of patients with different exacerbation phenotypes, not only contributing in the ongoing research of a possible causal relationship between endothelial dysfunction and COPD exacerbation pattern, but also in the determination of an optimal and individualized therapeutic approach.
Conclusion
Endocan is a promising novel marker of endothelial dysfunction with recent developments pointing toward possible future applications in the prognosis and staging of several inflammatory and malignant clinical conditions. The identification of its overexpression in NSCLC tissues, paired with the emerging evidence of a key role in respiratory disorders characterized by inflammatory response, underlines the fact that endocan could be considered as a biomarker of pulmonary-activated endothelial cells with promising potential applications. These could include, among others, its adoption as a biomarker in the severity assessment of CAP and PTE, the prediction of development of ALI, the phenotypic classification of COPD patients, as well as its use as possible therapeutic target for these conditions. Endocan could also improve the prognostic evaluation of cancer patients and the identification of those with high-risk tumors, as well as the selection of appropriate chemotherapeutic regimens. Furthermore, it could not only aid the early detection and study of neoangiogenesis but also provide a new target for antiangiogenic therapies. Nevertheless, despite the solid pathophysiological basis, it has not been adequately studied nor evaluated, particularly in non-neoplastic lung diseases. At the moment, this represents, in our opinion, the biggest limitation in its use as a relevant biomarker. Therefore, further investigations are required in order to clarify its exact role in the aforementioned processes and to fully assess its suitability and consistency as a biomarker of respiratory diseases characterized by microvascular inflammation and endothelial damage.
Acknowledgments
We would like to thank Dr Giorgos Ntatsis for his valuable help with graphic designing.
Disclosure
The authors report no conflicts of interest in this work.
References
- LassallePMoletSJaninAESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokinesJ Biol Chem19962713420458204648702785
- BéchardDScherpereelAHammadHHuman endothelial-cell specific molecule-1 binds directly to the integrin CD11a/CD18 (LFA-1) and blocks binding to intercellular adhesion molecule-1J Immunol200116763099310611544294
- DeleheddeMDevenynsLMaurageCAVivèsRREndocan in cancers: a lesson from a circulating dermatan sulfate proteoglycanInt J Cell Biol2013201370502723606845
- BéchardDGentinaTDeleheddeMEndocan is a novel chondroitin sulfate/dermatan sulfate proteoglycan that promotes hepatocyte growth factor/scatter factor mitogenic activityJ Biol Chem200127651483414834911590178
- BishopJRSchukszMEskoJDHeparan sulphate proteoglycans fine-tune mammalian physiologyNature200744671391030103717460664
- SarrazinSLyonMDeakinJACharacterization and binding activity of the chondroitin/dermatan sulfate chain from endocan, a soluble endothelial proteoglycanGlycobiology201020111380138820581009
- JankeJEngeliSGorzelniakKAdipose tissue and circulating endothelial cell specific molecule-1 in human obesityHorm Metab Res2006381283316477537
- WellnerMHerseFJankeJEndothelial cell specific molecule-1 – a newly identified protein in adipocytesHorm Metab Res2004354217221
- PaulusPJenneweinCZacharowskiKBiomarkers of endothelial dysfunction: can they help us deciphering systemic inflammation and sepsis?Biomarkers201116suppl 1S11S2121707440
- SarrazinSAdamELyonMEndocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapyBiochim Biophy Acta2006176512537
- IozzoRVSchaeferLProteoglycan form and function: a comprehensive nomenclature of proteoglycansMatrix Biol201542115525701227
- IozzoRVMatrix proteoglycans: from molecular design to cellular functionAnnu Rev Biochem1998676096529759499
- CasuBPetitouMProvasoliMSinayPConformational flexibility: a new concept for explaining binding and biological properties of iduronic acid-containing glycosaminoglycansTrends Biochem Sci19881362212253076283
- ZhangSMZuoLZhouQExpression and distribution of endocan in human tissuesBiotech Histochem201287317217821526908
- LongEOIntercellular adhesion molecule 1 (ICAM-1): getting a grip on leukocyte adhesionJ Immunol201118695021502321505213
- LyonMDeakinJARahmouneHFernigDGNakamuraTGallagherJTHepatocyte growth factor/scatter factor binds with high affinity to dermatan sulfateJ Biol Chem199827312712789417075
- BhowmickNANeilsonEGMosesHLStromal fibroblasts in cancer initiation and progressionNature2004432701533233715549095
- MaimoneMMTollefsenDMStructure of a dermatan sulfate hexasaccharide that binds to heparin cofactor II with high affinityJ Biol Chem19902653018263182712211700
- MerleBDurusselLDelmasPDClezardinPDecorin inhibits cell migration through a process requiring its glycosaminoglycan side chainJ Cell Biochem199975353854610536375
- BechardDMeigninVScherpereelACharacterization of the secreted form of endothelial-cell-specific molecule 1 by specific monoclonal antibodiesJ Vasc Res200037541742511025405
- RennelEMellbergSDimbergAEndocan is a VEGFA and PI3K regulated gene with increased expression in human renal cancerExp Cell Res200731371285129417362927
- MaurageCAAdamEMinéoJFEndocan expression and localization in human glioblastomasJ Neuropathol Exp Neurol200968663364119458546
- ScherpereelADepontieuFGrigoriuBEndocan, a new endothelial marker in human sepsisCrit Care Med200634253253716424738
- SesslerCNWindsorACSchwartzMCirculating ICAM-1 is increased in septic shockAm J Respir Crit Care Med19951515142014277735595
- KayalSJaïsJPAguiniNChaudièreJLabrousseJElevated circulating E-selectin, intercellular adhesion molecule 1, and von Willebrand factor in patients with severe infectionAm J Respir Crit Care Med19981573 Pt 17767849517590
- EndoSInadaKKasaiTLevels of soluble adhesion molecules and cytokines in patients with septic multiple organ failureJ Inflamm19954642122198878795
- BaltaIBaltaSKoryurekOMSerum endocan levels as a marker of disease activity in patients with Behçet diseaseJ Am Acad of Dermatol201470229129624176522
- TadzicRMihaljMVcevAEnnenJTadzicADrenjancevicIThe effects of arterial blood pressure reduction on endocan and soluble endothelial cell adhesion molecules (CAMs) and CAMs ligands expression in hypertensive patients on Ca-channel blocker therapyKidney Blood Press Res2013372–310311523594880
- FainJNRelease of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a reviewMediators Inflamm2010201051394820508843
- ShinJWHuggenbergerRDetmarMTranscriptional profiling of VEGF-A and VEGF-C target genes in lymphatic endothelium reveals endothelial-specific molecule-1 as a novel mediator of lymphangiogenesisBlood200811262318232618614759
- ZuoLZhangSMHuRLCorrelation between expression and differentiation of endocan in colorectal cancerWorld J Gastroenterol200814284562456818680240
- KimJHParkMYKimCNExpression of endothelial cell-specific molecule-1 regulated by hypoxia inducible factor-1α in human colon carcinoma: impact of ESM-1 on prognosis and its correlation with clinicopathological featuresOncol Rep20122851701170822948784
- ChenLYLiuXWangSLQinCYOver-expression of the endocan gene in endothelial cells from hepatocellular carcinoma is associated with angiogenesis and tumour invasionJ Int Med Res201038249851020515564
- HuangGWTaoYMDingXEndocan expression correlated with poor survival in human hepatocellular carcinomaDig Dis Sci200954238939418592377
- LeroyXAubertSZiniLVascular endocan (ESM-1) is markedly overexpressed in clear cell renal cell carcinomaHistopathology201056218018720102396
- RoudnickyFPoyetCWildPEndocan is upregulated on tumor vessels in invasive bladder cancer where it mediates VEGF-A-induced angiogenesisCancer Res20137331097110623243026
- StrasserGAKaminkerJSTessier-LavigneMMicroarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branchingBlood2010115245102511020154215
- ScherpereelAGentinaTGrigoriuBOverexpression of endocan induces tumor formationCancer Res200363186084608914522939
- DepontieuFGrigoriuBDScherpereelALoss of endocan tumorigenic properties after alternative splicing of exon 2BMC Cancer200881418205914
- XuZZhangSZhouQWangYXiaREndocan, a potential prognostic and diagnostic biomarker of acute leukemiaMol Cell Biochem20143951–211712324934240
- HatfieldKJLassallePLeivaRALindåsRWendelboeØBruserudØSerum levels of endothelium-derived endocan are increased in patients with untreated acute myeloid leukemiaHematology201116635135622183069
- WegielBEkbergJTalasilaKMJaliliSPerssonJLThe role of VEGF and a functional link between VEGF and p27Kip1 in acute myeloid leukemiaLeukemia200923225126118987662
- PopperHHPailerSWurzingerGFeldnerHHesseCEberEExpression of adhesion molecules in allergic lung diseasesVirchows Arch2002440217218011964048
- CorryDBKheradmandFAsthmaZanderDSPopperHHJagirdarJHaqueAKCaglePTBarriosRMolecular Pathology of Lung DiseasesNew YorkSpringer2010549576
- JiangZWodaBASavasLFraireAEExpression of ICAM-1, VCAM-1, and LFA-1 in adenocarcinoma of the lung with observations on the expression of these adhesion molecules in non-neoplastic lung tissueMod Pathol19981112118911929872650
- Jahnz-RózykKChciałowskiAPirozyńskaERogalewskaAExpression of adhesion molecules LFA-1 (CD11a and ICAM-1 (CD54) on lymphocytes and chemokines IL-8 and MCP-1 concentrations in bronchoalveolar lavage of patients with asthma or chronic obstructive pulmonary diseasePol Merkur Lekarski200095264965211144049
- Di StefanoAMaestrelliPRoggeriAUpregulation of adhesion molecules in the bronchial mucosa of subjects with chronic obstructive bronchitisAm J Respir Crit Care Med19941493 Pt 18038107509705
- VignolaAMCampbellAMChanezPHLA-DR and ICAM-1 expression on bronchial epithelial cells in asthma and chronic bronchitisAm Rev Respir Dis199314836896948103654
- Lopez-CamposJLCaleroCArellano-OrdenEIncreased levels of soluble ICAM-1 in chronic obstructive pulmonary disease and resistant smokers are related to active smokingBiomark Med20126680581123227846
- TsoutsouPGGourgoulianisKIPetinakiEICAM-1, ICAM-2 and ICAM-3 in the sera of patients with idiopathic pulmonary fibrosisInflammation200428635936416245079
- YuanAYuCJChenWJCorrelation of total VEGF mRNA and protein expression with histologic type, tumor angiogenesis, patient survival and timing of relapse in non-small-cell lung cancerInt J Cancer200089647548311102890
- PapaioannouAIKostikasKKolliaPGourgoulianisKIClinical implications for vascular endothelial growth factor in the lung: friend or foe?Respir Res20067112817044926
- GrigoriuBDDepontieuFScherpereelAEndocan expression and relationship with survival in human non-small cell lung cancerClin Cancer Res200612154575458216899604
- BorczukACShahLPearsonGDMolecular signatures in biopsy specimens of lung cancerAm J Respir Crit Care Med2004170216717415087295
- BernardGRArtigasABrighamKLThe American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordinationAm J Respir Crit Care Med19941493 Pt 18188247509706
- AtabaiKMatthayMAThe pulmonary physician in critical care. 5: acute lung injury and the acute respiratory distress syndrome: definitions and epidemiologyThorax20025745245811978926
- HasletonPSRobertsTEAdult respiratory distress syndrome-an updateHistopathology199934428529410231395
- WareLBPathophysiology of acute lung injury and the acute respiratory distress syndromeSemin Respir Crit Care Med200627433734916909368
- ManiatisNAOrfanosSEThe endothelium in acute lung injury/acute respiratory distress syndromeCurr Opin Crit Care2008141223018195622
- AlbeldaSMSmithCWWardPAAdhesion molecules and inflammatory injuryFASEB J1994885045128181668
- ThickettDRArmstrongLChristieSJMillarABVascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndromeAm J Respir Crit Care Med200116491601160511719296
- RubinDBWiener-KronishJPMurrayJFElevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndromeJ Clin Invest19908624744802384595
- MossMAckersonLGillespieMKMooreFAMooreEEParsonsPEVon Willebrand factor antigen levels are not predictive for the adult respiratory distress syndromeAm J Respir Crit Care Med1995151115207812545
- SakamakiFIshizakaAHandaMSoluble form of P-selectin in plasma is elevated in acute lung injuryAm J Respir Crit Care Med19951516182118267539327
- MossMGillespieMKAckersonLMooreFAMooreEEParsonsPEEndothelial cell activity varies in patients at risk for the adult respiratory distress syndromeCrit Care Med19962411178217868917025
- LassallePMethods and kits for predicting the risk of respiratory failure, renal failure or thrombopenia in a septic patient by measuring endocan levels in bloodLille, France2012
- CalfeeCSEisnerMDWareLBTrauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disordersCrit Care Med200735102243225017944012
- MikkelsenMEShahCVScherpereelALower serum endocan levels are associated with the development of acute lung injury after major traumaJ Crit Care2012275522
- De Freitas CairesNLegendreBParmentierEIdentification of a 14 kDa endocan fragment generated by cathepsin G, a novel circulating biomarker in patients with sepsisJ Pharm Biomed Anal201378–794551
- TangLZhaoYWangDEndocan levels in peripheral blood predict outcomes of acute respiratory distress syndromeMediators Inflamm2014201462518025132734
- KásaACsortosCVerinADCytoskeletal mechanisms regulating vascular endothelial barrier function in response to acute lung injuryTissue Barriers201531–2e97444825838980
- Morales-RuizMFultonDSowaGVascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase AktCirc Res200086889289610785512
- KaoSJChuangCYTangCHPlasma endothelial cell-specific molecule-1 (ESM-1) in management of community-acquired pneumoniaClin Chem Lab Med201452344545124108208
- AltintasNMutluLCAkkoyunDCEffect of CPAP on new endothelial dysfunction marker, endocan, in people with obstructive sleep apneaAngiology201667436437426076702
- GüzelADuranLKöksalNEvaluation of serum endothelial cell specific molecule-1 (endocan) levels as a biomarker in patients with pulmonary thromboembolismBlood Coagul Fibrinolysis201425327227624509328
- ChengDLeeYCRogersJTVascular endothelial growth factor level correlates with transforming growth factor-β isoform levels in pleural effusionsChest200011861747175311115468
- ThickettDRArmstrongLMillarABVascular endothelial growth factor (VEGF) in inflammatory and malignant pleural effusionsThorax199954870771010413724
- PapaioannouAIKostikasKTsopaPResidual pleural thickening is related to vascular endothelial growth factor levels in parapneumonic pleural effusionsRespiration201080647247920029166
- MaclayJDMcAllisterDAMillsNLVascular dysfunction in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2009180651352019542477
- HashimotoMTanakaHShoshakuAQuantitative analysis of bronchial wall vascularity in the medium and small airways of patients with asthma and COPDChest2005127396597215764783
- MoroLPedoneCScarlataSMalafarinaVFimognariFAntonelli-IncalziREndothelial dysfunction in chronic obstructive pulmonary diseaseAngiology200859335736418388072
- PereraWRHurstJRWilkinsonTMInflammatory changes, recovery and recurrence at COPD exacerbationEur Respir J200729352753417107990
- Pinto-PlataVMLivnatGGirishMSystemic cytokines, clinical and physiological changes in patients hospitalized for exacerbation of COPDChest20071311374317218554
- KoutsokeraAKiropoulosTSNikoulisDJClinical, functional and biochemical changes during recovery from COPD exacerbationsRespir Med2009103691992619121927
- KumarSDEmeryMJAtkinsNDDantaIWannerAAirway mucosal blood flow in bronchial asthmaAm J Respir Crit Care Med199815811531569655722
- LiXWilsonJWIncreased vascularity of the bronchial mucosa in mild asthmaAm J Respir Crit Care Med199715612292339230753
- GreenFHYButtJCJamesALCarrollNGAbnormalities of the bronchial arteries in asthmaChest200613041025103317035434
- WannerAMendesESAirway endothelial dysfunction in asthma and chronic obstructive pulmonary disease. A challenge for future researchAm J Respir Crit Care Med2010182111344135120709816
- Pinto-PlataVCasanovaCMüllerovaHInflammatory and repair serum biomarker pattern. Association to clinical outcomes in COPDRespir Res20121317122906131
- TuderRMYoshidaTArapWPasqualiniRPetracheIState of the art. Cellular and molecular mechanisms of alveolar destruction in emphysema: an evolutionary perspectivePrac Am Thorac Soc200636503510