Abstract
Long-acting muscarinic antagonist (LAMA) or long-acting β2-agonist (LABA) bronchodilators and their combination are recommended for the maintenance treatment of chronic obstructive pulmonary disease (COPD). Although the efficacy of LAMAs and LABAs has been well established through randomized controlled trials (RCTs), questions remain regarding their cardiovascular (CV) safety. Furthermore, while the safety of LAMA and LABA monotherapy has been extensively studied, data are lacking for LAMA/LABA combination therapy, and the majority of the studies that have reported on the CV safety of LAMA/LABA combination therapy were not specifically designed to assess this. Evaluation of CV safety for COPD treatments is important because many patients with COPD have underlying CV comorbidities. However, severe CV and other comorbidities are often exclusion criteria for RCTs, contributing to a lack in external validity and generalizability. Real-world observational studies are another important tool to evaluate the effectiveness and safety of COPD therapies in a broader population of patients and can improve upon the external validity limitations of RCTs. We examine what is already known regarding the CV and cerebrovascular safety of LAMA/LABA combination therapy from RCTs and real-world observational studies, and explore the advantages and limitations of data derived from each study type. We also describe an ongoing prospective, observational, comparative post-authorization safety study of a LAMA/LABA combination therapy (umeclidinium/vilanterol) and LAMA monotherapy (umeclidinium) versus tiotropium, with a focus on the relative merits of the study design.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality, accounting for approximately three million deaths worldwide in 2012.Citation1,Citation2 As COPD is characterized by persistent airflow limitation, long-acting muscarinic antagonists (LAMA) or long-acting β2-agonist (LABA) bronchodilators are recommended treatment options.Citation1 Bronchodilator monotherapy with LAMAs improves lung function and health status, and prevents exacerbations compared with placebo.Citation3–Citation5 Combination therapy with LAMAs and LABAs exert complementary bronchodilatory effects, resulting in greater improvements in lung function compared with long-acting bronchodilator monotherapies.Citation4,Citation6–Citation10 These dual combination bronchodilators also improve symptoms and health status, and reduce exacerbation risk to a greater extent than monotherapies or inhaled corticosteroid/LABA combinations.Citation6,Citation7,Citation10,Citation11
Due to their mechanisms of action, both LAMAs and LABAs have the potential to cause cardiac-related adverse events (AEs). LAMAs suppress parasympathetic control of heart rate (HR) and LABAs stimulate sympathetic control of HR. These effects serve to raise HR with the potential to cause cardiac arrhythmias, myocardial infarction (MI), stroke, and sudden death in susceptible patients.Citation1,Citation12–Citation15 When administered as combination therapy, LAMAs and LABAs are often combined using the same doses as for monotherapy. Thus, hypothetically, an increase in cardiovascular (CV) AEs compared with monotherapy cannot be excluded.
Randomized controlled trials (RCTs) are considered the gold standard study design for gaining evidence for regulatory approval. Real-world observational studies are often non-interventional in nature and may be prospective or retrospective in design. They are conducted in wider patient populations who have a range of demographics and characteristics and are important for monitoring the effects of therapies outside of the controlled setting. This is particularly important in COPD, where CV comorbidities are prevalent,Citation16 but are frequently an exclusion criterion for participation in RCTs,Citation17–Citation20 an issue that has been coined the “COPD trial paradox”.Citation12 Here, we review the available evidence from RCTs and real-world observational studies regarding the CV and cerebrovascular safety of LAMA/LABA combination therapy versus monotherapy in COPD. We also include a special focus on an ongoing prospective, observational, comparative post-authorization safety (PAS) study of the LAMA/LABA combination therapy, umeclidinium (UMEC)/vilanterol (VI), or UMEC monotherapy versus the LAMA monotherapy, tiotropium (TIO).
Evidence from COPD RCTs
Although the CV and cerebrovascular risk of LAMA and LABA monotherapies have been extensively examined in RCTs, relatively few studies have reported on the safety of LAMA/LABA combination therapies. RCTs that have reported on the CV and cerebrovascular safety of LAMA/LABA combination therapy are described in . Furthermore, the studies that do report on the CV and cerebrovascular safety of LAMA/LABA combinations were not necessarily powered to study these outcomes, with the exception of Van de Maele et alCitation21 the primary outcome of which was change from baseline in 24-h mean HR. Overall, the available evidence from RCTs has not shown any clinically significant increase in CV or cerebrovascular risk for the LAMA/LABA combinations UMEC/VI,Citation5,Citation7,Citation8,Citation10,Citation22–Citation26 TIO/olodaterol,Citation17,Citation27,Citation28 aclidinium/formoterol,Citation29,Citation30 or indacaterol/glycopyrroniumCitation6,Citation21,Citation31–Citation33 versus monotherapy or placebo (). Indeed, one study reported that the incidence of atrial arrhythmias was similar between UMEC/VI 125/25 µg and placebo, but had a ≥2% greater incidence with UMEC 125 µg compared with placebo. Additionally, the overall incidence of CV AEs with UMEC/VI 125/25 µg was lower compared with UMEC 125 µg or placebo, although because the event rate was low and the study not powered to detect these treatment differences in this endpoint, these results should be interpreted with caution.Citation8
Table 1 RCTs reporting on the CV and cerebrovascular safety of LAMA/LABA combination therapy
Advantages and limitations of COPD RCTs
RCTs typically have high internal validity due to the random allocation of patients to treatment groups, minimizing indication bias and confounding, as well as reducing biased reporting of endpoints. Additionally, a placebo/control arm can be used to measure the impact and remove any imbalance of the patient- and investigator-related Hawthorne effects (the phenomenon where individuals modify or improve aspects of their behavior in response to being observed)Citation34 across treatment arms. The use of precise inclusion and exclusion criteria in RCTs also minimizes the effect of confounding factors such as patient comorbidities.Citation35 However, exclusion criteria are used to exclude high-risk patients. For example, the TIOtropium Safety and Performance In Respimat (TIOSPIR) safety study targeting comorbid patients excluded the highest risk patients, such as those with previous MI, New York Heart Association class III or IV heart failure, or unstable or life-threatening arrhythmia.Citation36 The high internal validity of RCTs allows treatment effects to be reliably determined. Consequently, RCTs are considered the gold standard for evaluating the efficacy of novel therapies to obtain regulatory approval.Citation37
However, trial results need to be generalizable and suitable for extrapolation to a wider patient population to be clinically useful. There are several reasons why RCTs in COPD have often lacked external validity. COPD studies often enforce exclusion criteria based on age (an upper age limit of 70–75 years is common), disease severity (often excluding patients with mild airflow and very severe obstruction; forced expiratory volume in 1 second [FEV1] >80% predicted and <30% predicted, respectively), comorbid conditions that potentially cause excessive risk for AEs, background maintenance therapy use, or long-term oxygen therapy.Citation38 The resulting patient populations are homogeneous but less generalizable to the wider heterogeneous COPD patient population.Citation39,Citation40
COPD has an established association with CV and other comorbidities. For example, 98% of patients with COPD in a secondary care referral study had ≥1 comorbid condition and over half had ≥4 comorbid conditions.Citation41 Although common in the general COPD patient population, such comorbidities are frequently exclusion criteria in RCTs.Citation12,Citation17–Citation20,Citation35 Thus, there is a mismatch between a real-life COPD patient population and the subset of patients that would fit into the criteria used by RCTs.Citation42–Citation45 A postal survey was conducted in 2002–2005 in patients with COPD in New Zealand to determine eligibility for inclusion in any one of 18 previously conducted RCTs cited in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines. The study found that eligibility ranged from 0% to 20% in all subjects with COPD and from 0% to 9% in those patients receiving treatment for COPD with inhaled or oral steroids, or bronchodilators. These results demonstrate that the external validity of the major RCTs on which the GOLD treatment guidelines was based is low.Citation46 Similarly, two other analyses each demonstrated that just 17% of a real-life COPD patient population would fit the criteria commonly used in RCTs in COPD.Citation42,Citation44
More recently, Kruis et alCitation45 evaluated the external validity of six large industry-sponsored COPD trials (ISOLDE, TRISTAN, TORCH, UPLIFT, ECLIPSE, and POET-COPD), on which current COPD treatment guidelines are largely based. Compared with data from seven large European primary care databases, the population included in these RCTs was younger, predominantly male, with worse lung function and poorer quality of life. There was also a large difference in the disease severity (GOLD) distribution of patients enrolled in the RCTs versus the primary care database. For example, no patients with COPD with mild severity (GOLD I) were included in the RCTs, while patients with severe COPD (GOLD III) were overrepresented in the industry-sponsored studies versus the primary care population (44.5% vs 21%, respectively). Furthermore, baseline data on exacerbations suggested overrepresentation of patients with prior exacerbations in the RCTs compared with the primary care population. Overall, it was shown that the proportion of patients from the primary care population that would be eligible to be included in the industry-sponsored RCTs ranged from 17% (TRISTAN) to 42% (ECLIPSE and UPLIFT).
Other factors that impact the external validity of RCTs relate to the trial setting (primary, secondary, or tertiary care), the health care system, and the country in which the trial is conducted.Citation35 Even where health care systems are similar, other differences such as racial demographics, disease susceptibility, and natural history of the disease can influence external validity.Citation35 Furthermore, differences between health care systems can affect hospital admissions, a factor that defines a COPD exacerbation as severe. However, the threshold for hospitalization differs between countries. For example, an adjusted 10-fold difference in respiratory disease-related hospital admissions has been reported across 31 European countries, being highest in Eastern Europe and Germany, and lowest in France, Portugal, UK, and Scandinavia.Citation47
Evidence from COPD real-world observational studies
Data from real-world observational studies reporting on LAMA/LABA combination therapy in COPD are currently lacking. Recently, a real-world study of patients included in the Registre de Données en Santé Pulmonaire database, which records information on Canadian patients with asthma or COPD, compared AEs associated with LAMA or LABA monotherapy use and LAMA/LABA combination use in patients with COPD. However, the study did not specifically report on CV and cerebrovascular AEs.Citation48 Indeed, to date, we are not aware of any real-world observational studies that have specifically assessed the CV and cerebrovascular risk of LAMA/LABA combination therapy in COPD.
Advantages and limitations of COPD real-world observational studies
Real-world observational studies are designed to more closely reflect routine clinical practice and thus do not exclude patients with comorbidities associated with COPD or have further limitations with inclusion criteria such as upper age limit or smoking history. In contrast to RCTs, observational studies include a wider selection of patients and focus on balancing the risks and benefits of treatments.Citation37 In contrast to the internal validity achieved in RCTs, the main advantage of real-world observational studies is their higher external validity, generally enrolling a wide range of patients across different treatment settings.Citation34,Citation37 Real-world data from observational studies can also provide evidence of effectiveness to support health care decisionsCitation49 and assist health care policy makers in dealing with coverage and reimbursement decisions when evaluating the cost effectiveness of a treatment.Citation50,Citation51 Real-world observational studies also ensure that drug safety is monitored in a broad population of patients.
Prospective non-interventional studies, such as DACCORD registry, or the open-label pragmatic trial called the Salford Lung Study are examples of large observational COPD studies generating real-world evidence.Citation49,Citation52 DACCORD is an ongoing 2- to 4-year real-world study being conducted in over 6,000 patients in ~500 primary and secondary care practices in Germany. Patients fall into two groups, one treated according to standard of care with a glycopyrronium-containing regimen and another group treated according to standard of care without glycopyrronium. The study focuses on patient-related outcomes, time to first exacerbation, frequency of exacerbations, and lung function variables. The main strengths of the DACCORD study are the large size of study population, the long-term follow-up period, the broad inclusion criteria, and the implementation of disease management program criteria, which aims to ensure that only patients with appropriate COPD diagnosis are enrolled.Citation52 The Salford Lung Study consists of two open-label Phase III pragmatic RCTs in asthma and COPD. It was designed to compare the real-world effectiveness of fluticasone furoate/VI via inhaler (plus standard care) versus regular maintenance therapy (plus standard care) for COPD and asthma in routine primary care. Following randomization, patients receive standard care by their physician for 12 months and effectiveness and safety data were collected using electronic health records. The design strengths of the Salford Lung Study are that it is a large, prospective, randomized study with broad inclusion criteria, which allow it to bridge the gap between low external validity RCTs and low internal validity non-randomized observational studies.Citation49,Citation53 One potential limitation of the study is the relatively high level of intervention required by regulatory authorities due to the randomized nature of the trial, as this undermines the real-world design of the study, impacting on the Hawthorne effect.
The main limitations with real-world studies, specifically observational studies (ie, without random subject allocation), are the effects of potential confounding by indication bias, where the most severe patients may preferentially receive certain treatments.Citation39 Additionally, the lack of blinding in real-world studies is generally considered a limitation. However, this reflects clinical practice where patients either receive treatment or nothing at all. Thus, the efficacy of treatment versus placebo, already proven in completed RCTs, is not the target outcome of observational studies. If a comparator intervention already exists, then the usual standard of care may be a more appropriate comparator.Citation37 Observational studies that are retrospective in nature use electronic records and these studies can be limited by the robustness and completeness of their data sources, such as inconsistent reporting of data on disease severity. Another important source of bias in real-world observational studies is attrition bias. For example, in the DACCORD study, of the 6,000 patients initially included in the study, only 4,123 patients remained after 1 year.Citation54
A PAS study of UMEC/VI combination therapy
There are a few studies with large patient populations that have specifically assessed the CV and cerebrovascular safety of LAMAs and LABAs, such as SUMMIT, TORCH, UPLIFT, and TIOSPIR.Citation36,Citation55–Citation57 However, much of the available data assessing CV and cerebrovascular risk have come from non-prespecified AE analyses in RCTs, which may not have been powered for CV AEs.
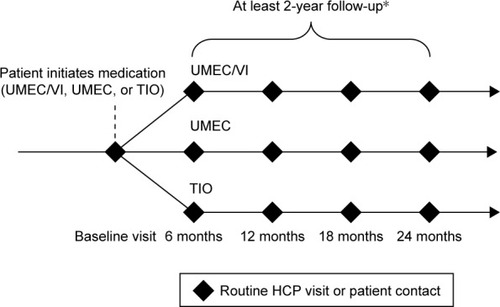
The 201038 PAS study is a prospective real-world observational cohort study that aims to reflect the real-world experience of patients with COPD treated with UMEC/VI or UMEC in the post-approval setting. The primary objectives of the study are to demonstrate the non-inferiority of UMEC/VI and UMEC alone versus TIO for the risks of MI, stroke, or heart failure, each based on an analysis of time to first event. The study will also quantify the incidence rate and frequency of MI, stroke, and heart failure for new users of UMEC/VI, UMEC, and TIO. The primary and secondary safety outcomes are presented in .
Table 2 Primary and secondary study safety outcomes in the 201038 Post-authorization Safety Study of UMEC/VI combination therapy
Study design
This study is a non-randomized, observational study being carried out in several European Union (EU) and non-EU countries that have UMEC/VI, UMEC, and TIO available on prescription. Patients are enrolled in the study at the time of receiving a new prescription for UMEC/VI, UMEC, or TIO. The decision to initiate treatment with UMEC/VI, UMEC, or TIO is made independently by the patient and their physician and is not mandated by the study design or protocol. All patients are followed from initiation of treatment until the required number of CV events has been observed in the study population. All patients are observed over a minimum of 24 months, or until withdrawal of consent, loss to follow-up, or death (). The estimated maximum duration of follow-up is between 2 and 5 years. Data on patients are collected at routine visits at least twice yearly, as well as at unscheduled visits as per normal standard of care. All data will be collected via electronic case report forms (eCRFs) from information routinely recorded in patient’s medical records or through patient self-report. Hospital discharge summaries will be requested by the investigator or site staff for all hospitalizations of enrolled patients. Data from these summaries will be captured on the eCRF and also used for adjudication of CV and cerebrovascular events. Written, informed consent was and will be obtained from all patients who participate in the study. The study was approved by Sächsische Landesärztekammer, Dresden, Germany and other relevant national, regional, or investigational center Ethics Committee/Institutional Review Boards, and is and will be performed in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice (ICH-GCP) guidelines and all applicable patient privacy requirements and the ethical principles outlined in the Declaration of Helsinki, 2013.
Figure 1 Study design for the new 201038 Post-authorization Safety Study of UMEC/VI combination therapy.
Abbreviations: COPD, chronic obstructive pulmonary disease; HCP, health care practitioner; TIO, tiotropium; VI, vilanterol; UMEC, umeclidinium.
Study population
The study is enrolling ~7,800 patients from 700 study centers in selected EU member states and other non-EU countries where UMEC/VI, UMEC, and TIO are available on prescription. Enrollment in non-EU countries is capped at ~50%. Eligible patients are enrolled by primary care physicians and pulmonologists and are aged ≥18 years, with a clinical diagnosis of COPD verified by spirometry (defined as a post-bronchodilator FEV1/forced vital capacity ratio of <0.7) and initiating treatment with either UMEC/VI, UMEC, or TIO. Key exclusion criteria include current participation in any interventional clinical trials; hypersensitivity to UMEC, VI, TIO, or excipients; and maintenance treatment (defined as ≥60 days of continuous use) with a LAMA-containing medication during the 12 months prior to enrollment. At enrollment, detailed data on patient demographics, baseline characteristics, modified Medical Research Council and COPD assessment test questionnaire scores, disease comorbidities, and concomitant medication use are collected using an eCRF. Information is provided by the treating physician based on a combination of self-reported information from the enrolled patient and where available, supplemented by the patient’s electronic medical records. The study is event-driven, requiring at least 98 events for each of MI, stroke, and heart failure for each pair of treatments (UMEC/VI vs TIO; UMEC vs TIO). Therefore, the number of patients that need to be enrolled will be updated as necessary throughout the study. The study has a 90% power to reject a non-inferiority margin of a hazard ratio of 2.0 for each treatment pair for each outcome (MI, stroke, and heart failure). This is based on the requirement that the 95% confidence interval for the hazard ratio excludes 2.0.
Advantages and limitations of the study design
The study incorporates several important design features that aim to minimize the potential limitations of observational studies, such as potential confounding by indication bias or attrition bias, and the lack of blinding. Observational studies are often retrospective; however, enrollment in this study is prospective, allowing for robust data collection through eCRFs. The study aims to recruit a wide population of patients with COPD from different care settings. Eligible patients are enrolled by both primary care physicians and pulmonologists, which helps to ensure representation from different care settings. Patients “new” to therapy are defined as not having received LAMA maintenance therapy for ≥60 consecutive days during the previous 12 months. This should minimize enrolled patient “survival” bias, where prevalent users of LAMAs that have survived are disproportionately represented in any treatment groups.Citation58 This is particularly important for assessing whether mortality risk is affected by treatment. Specifically, it minimizes the bias of prescription of two LAMAs (eg, UMEC/VI added to TIO), which may unfortunately occur in error. However, this is also a potential limitation of the study as it excludes many patients, particularly as LAMA maintenance is the most frequently prescribed treatment for COPD in some countries, such as Germany.Citation59 The requirement that patients must not have received LAMA maintenance therapy for ≥60 consecutive days during the prior 12 months also limits the patients included, meaning that the enrolled population will not be fully representative of the wider COPD patient population. However, patients can continue on existing maintenance treatments, which helps to reduce the potential for excluding patients with more severe disease. Also the factors that may be associated with treatment choice (and with risk of primary events) are documented to account for potential confounding in propensity score analyses. Additionally, as follow-up does not require patients to return to their study center except for routine care, follow-up bias whereby patients with AEs are more or less likely to return to see their study physician is minimized by maintaining a low lost to follow-up rate (a rate of <5% is anticipated). Furthermore, to standardize the reporting of primary CV events (MI, stroke, or heart failure) between centers (which may adopt different interpretations and event definitions), only events confirmed by the blinded adjudication committee are included. The expected event rates for MI, stroke, and heart failure are 98, 108, and 168,Citation60 although as the study is event-driven, recruitment rates will be adjusted once the actual event rate in this population is known.
Conclusion
Both RCTs and real-world observational studies contribute important data regarding the efficacy, safety, and effectiveness of COPD treatments. When evaluating a treatment effect it is important for health care practitioners to consider the generalizability of study findings to their patients. As both study types have inherent limitations, data from the pivotal COPD RCTs are complemented by real-world observational study data, which should both be evaluated to elucidate evidence of any treatment benefits and safety concerns. More research is needed to determine the effects of COPD treatments in patients who have been underrepresented in RCTs, such as women and patients with mild and very severe disease. Furthermore, the CV safety of COPD treatments in real-life patients, including those with comorbidities, should be further investigated, both in observational studies and more inclusive pragmatic RCTs. As there is hypothetically a potential for higher CV risk with dual bronchodilator treatment, the new 201038 PAS study documented here will provide data on the CV and cerebrovascular risks of the LAMA/LABA combination UMEC/VI, and the LAMA monotherapies UMEC and TIO in a real-world setting. The study design aims to reduce some of the common limitations of observational studies and to provide safety data for dual bronchodilator therapy in a more vulnerable COPD population than those included in RCTs.
Author contributions
PK, SW, DS, MR-R, HM, and DEN contributed to the study concept and design. All authors were involved in the preparation and review of the manuscript, approved the final version submitted and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank the members of the 201038 Post-authorisation Safety Study (PAS) Steering Scientific Committee for their contribution to the study design. They would also like to thank Alex Lowe, PhD, from Fishawack Indicia Ltd, who provided editorial assistance with developing this manuscript (in the form of writing assistance, including development of the initial draft, assembling tables and figures, collating authors comments, grammatical editing, and referencing), funded by GSK. This study was funded by GSK (GSK study: 201038).
Disclosure
DEN has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards and research grants from various pharmaceutical companies including AstraZeneca, BMS, Boehringer Ingelheim, GSK, Novartis, and Pfizer.
DS has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards and research grants from various pharmaceutical companies including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GSK, Glenmark, Johnson & Johnson, Merck, NAPP, Novartis, Pfizer, Skyepharma, Takeda, Teva, Theravance, and Verona.
MR-R has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards and research grants from various pharmaceutical companies including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, Novartis, Pfizer, ROVI, and Teva.
PK received honoraria for lecturing and advisory boards from the following pharmaceutical companies: AstraZeneca, Boehringer Ingelheim, Chiesi, Menarini, Mundipharma, Novartis, Roche, Takeda, and Teva; and sponsorship for German national and international conferences from AstraZeneca, Boehringer Ingelheim, Menarini, and Novartis.
SW and HM are employees of GSK and hold stocks/shares in GSK.
The authors report no other conflicts of interest in this work.
References
- GOLDGlobal strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease Available from: http://www.goldcopd.com/Accessed August 12, 2015
- WHOChronic Obstructive Pulmonary Disease Available from: http://www.who.int/mediacentre/factsheets/fs315/en/Accessed November, 2015
- BarrRGBourbeauJCamargoCARamFSInhaled tiotropium for stable chronic obstructive pulmonary diseaseCochrane Database Syst Rev20052CD00287615846642
- DonohueJFMaleki-YazdiMRKilbrideSMehtaRKalbergCChurchAEfficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPDRespir Med2013107101538154623830094
- CelliBCraterGKilbrideSOnce-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled studyChest2014145598199124385182
- BatemanEDFergusonGTBarnesNDual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE studyEur Respir J20134261484149423722616
- DecramerMAnzuetoAKerwinEEfficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trialsLancet Respir Med20142647248624835833
- DonohueJFNiewoehnerDBrooksJO’DellDChurchASafety and tolerability of once-daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: results from a 52-week, randomized, double-blind, placebo-controlled studyRespir Res2014157825015176
- FarneHACatesCJLong-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary diseaseCochrane Database Syst Rev201510CD008989
- Maleki-YazdiMRKaelinTRichardNZvarichMChurchAEfficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24-week, randomized, controlled trialRespir Med2014108121752176025458157
- WedzichaJABanerjiDChapmanKRIndacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPDN Engl J Med2016374232222223427181606
- LahousseLVerhammeKMStrickerBHBrusselleGGCardiac effects of current treatments of chronic obstructive pulmonary diseaseLancet Respir Med20164214916426794033
- GershonACroxfordRCalzavaraACardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary diseaseJAMA Intern Med2013173131175118523689820
- SalpeterSROrmistonTMSalpeterEECardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysisChest200412562309232115189956
- SinghSLokeYKEnrightPFurbergCDPro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medicationsThorax201368111411622764216
- ChenWThomasJSadatsafaviMFitzGeraldJMRisk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysisLancet Respir Med20153863163926208998
- BuhlRMaltaisFAbrahamsRTiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4)Eur Respir J201545496997925573406
- ChapmanKRBeehKMBeierJA blinded evaluation of the efficacy and safety of glycopyrronium, a once-daily long-acting muscarinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 studyBMC Pulm Med201414424438744
- FukuchiYSamoroRFassakhovRBudesonide/formoterol via Turbuhaler® versus formoterol via Turbuhaler® in patients with moderate to severe chronic obstructive pulmonary disease: phase III multinational study resultsRespirology201318586687323551359
- GelbAFTashkinDPMakeBJZhongXGarcia GilECaractaCLAS-MD-35 study investigatorsLong-term safety and efficacy of twice-daily aclidinium bromide in patients with COPDRespir Med2013107121957196523916502
- Van de MaeleBFabbriLMMartinCHortonRDolkerMOverendTCardiovascular safety of QVA149, a combination of Indacaterol and NVA237, in COPD patientsCOPD20107641842721166630
- HuCJiaJDongKPharmacokinetics and tolerability of inhaled umeclidinium and vilanterol alone and in combination in healthy Chinese subjects: a randomized, open-label, crossover trialPLoS One2015103e012126425816315
- MaltaisFSinghSDonaldACEffects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomized, double-blind clinical trialsTher Adv Respir Dis20148616918125452426
- NaccarelliGFinkleJChopraBBrooksJHarrisSChruchACardiovascular safety of Umeclidinium/Vilanterol in COPD: Results from eight randomized clinical trialsPresented at: San Diego, California: ATS ConferenceMay 16–21, 2014
- SilerTMKerwinESousaARDonaldAAliRChurchAEfficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: Results of two randomized studiesRespir Med201510991155116326117292
- DonohueJFSinghDMunzuCKilbrideSChurchAMagnitude of umeclidinium/vilanterol lung function effect depends on monotherapy responses: Results from two randomised controlled trialsRespir Med2016112657426797016
- SinghDFergusonGTBolitschekJTiotropium + olodaterol shows clinically meaningful improvements in quality of lifeRespir Med2015109101312131926320402
- ZuWallackRAllenLHernandezGTingNAbrahamsREfficacy and safety of combining olodaterol Respimat(®) and tiotropium HandiHaler(®) in patients with COPD: results of two randomized, double-blind, active-controlled studiesInt J Chron Obstruct Pulmon Dis201491133114425342898
- D’UrzoADRennardSIKerwinEMMergelVLeselbaumARCaractaCFAUGMENT COPD study investigatorsEfficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD studyRespir Res20141512325756831
- SinghDJonesPWBatemanEDEfficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised studyBMC Pulm Med20141417825404569
- MahlerDADecramerMD’UrzoADual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: the BLAZE studyEur Respir J20144361599160924176997
- WedzichaJADahlRBuhlRPooled safety analysis of the fixed-dose combination of indacaterol and glycopyrronium (QVA149), its monocomponents, and tiotropium versus placebo in COPD patientsRespir Med2014108101498150725135743
- MahlerDAKerwinEAyersTFLIGHT1 and FLIGHT2: Efficacy and safety of QVA149 (Indacaterol/glycopyrrolate) versus its mono-components and placebo in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201519291068107926177074
- BartlettSJBarnesTMcIvorRAIntegrating patients into meaningful real-world researchAnn Am Thorac Soc201411Suppl 2S112S11724559023
- RothwellPMExternal validity of randomised controlled trials: “to whom do the results of this trial apply?Lancet20053659453829315639683
- WiseRAAnzuetoACottonDTiotropium Respimat inhaler and the risk of death in COPDN Engl J Med2013369161491150123992515
- FreemantleNStrackTReal-world effectiveness of new medicines should be evaluated by appropriately designed clinical trialsJ Clin Epidemiol201063101053105819880285
- HalpinDMLessons from the major studies in COPD: problems and pitfalls in translating research evidence into practicePrim Care Respir J201019217017920352172
- AlbertRK“Lies, damned lies …” and observational studies in comparative effectiveness researchAm J Respir Crit Care Med2013187111173117723725614
- CarolanBJSutherlandERClinical phenotypes of chronic obstructive pulmonary disease and asthma: recent advancesJ Allergy Clin Immunol20131313627634 quiz 63523360757
- VanfleterenLESpruitMAGroenenMClusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187772873523392440
- HerlandKAkselsenJPSkjonsbergOHBjermerLHow representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease?Respir Med2005991111915672843
- TraversJMarshSWilliamsMExternal validity of randomised controlled trials in asthma: to whom do the results of the trials apply?Thorax200762321922317105779
- ScichiloneNBasileMBattagliaSBelliaVWhat proportion of chronic obstructive pulmonary disease outpatients is eligible for inclusion in randomized clinical trials?Respiration2014871111724281343
- KruisALStallbergBJonesRCPrimary care COPD patients compared with large pharmaceutically-sponsored COPD studies: an UNLOCK validation studyPLoS One201493e9014524598945
- TraversJMarshSCaldwellBExternal validity of randomized controlled trials in COPDRespir Med200710161313132017113277
- SocietyERThe European Lung White Book: Respiratory Health and Disease in Europe2nd edSheffield, UKEuropean Respiratory Society2013
- RodrigueCBeauchesneMFSavariaFAdverse events among COPD patients treated with long-acting anticholinergics and beta2-agonists in an outpatient respiratory clinicRespir Med2016113657326896922
- NewJPBakerlyNDLeatherDWoodcockAObtaining real-world evidence: the Salford Lung StudyThorax201469121152115424603195
- GarrisonLPJrNeumannPJEricksonPMarshallDMullinsCDUsing real-world data for coverage and payment decisions: the ISPOR Real-World Data Task Force reportValue Health200710532633517888097
- van BovenJFRoman-RodriguezMKocksJWSorianoJBPostmaMJvan der MolenTPredictors of cost-effectiveness of selected COPD treatments in primary care: UNLOCK study protocolNPJ Prim Care Respir Med2015251505126247130
- KardosPVogelmeierCBuhlRCrieeCPWorthHThe prospective non-interventional DACCORD study in the national COPD registry in Germany: design and methodsBMC Pulm Med201515225578330
- BakerlyNDWoodcockANewJPThe Salford Lung Study protocol: a pragmatic, randomised phase III real-world effectiveness trial in chronic obstructive pulmonary diseaseRespir Res20151610126337978
- KardosPBuhlRCriéeC-PVogelmeierCMailaenderCWorthHP46 Frequency of COPD exacerbations in the German DACCORD RegistryThorax201570A98A99
- CalverleyPMAndersonJACelliBSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med2007356877578917314337
- TashkinDPLeimerIMetzdorfNDecramerMCardiac safety of tiotropium in patients with cardiac events: a retrospective analysis of the UPLIFT® trialRespir Res2015166526031308
- VestboJAndersonJBrookRDThe study to understand mortality and morbidity in COPD (SUMMIT) study protocolEur Respir J20134151017102223018908
- RayWAEvaluating medication effects outside of clinical trials: new-user designsAm J Epidemiol2003158991592014585769
- WorthHBuhlRCrieeCPKardosPMailanderCVogelmeierCThe ‘real-life’ COPD patient in Germany: the DACCORD studyRespir Med2016111647126775251
- JaraMWentworthC3rdLanesSA new user cohort study comparing the safety of long-acting inhaled bronchodilators in COPDBMJ Open201223 pii:e000841
