Abstract
Objective
To determine the prevalence, change in breathlessness status over time, and risk factors for disabling and persistent disabling breathlessness in relation to treatments in chronic obstructive pulmonary disease (COPD).
Materials and methods
Longitudinal analysis of data from the Swedish National Register of COPD with breathlessness measured using modified Medical Research Council (mMRC) scores at two subsequent visits. Prevalence of disabling breathlessness (mMRC ≥2 at baseline) and persistent disabling breathlessness (disabling breathlessness at baseline and follow-up) was investigated in relation to COPD treatment. Risk factors for disabling breathlessness, change from non-disabling to disabling breathlessness, and persistent disabling breathlessness were analyzed using multiple logistic regression.
Results
A total of 1,689 patients were included in the study with a median follow-up of 12 months (interquartile range: 4 months). Prevalence of disabling breathlessness was 54% at baseline. Persistent disabling breathlessness was present in 43% of patients despite treatment and in 74% of patients despite combined inhaled triple therapy and physiotherapy. Risk factors for disabling breathlessness or change to disabling breathlessness were higher age, lower lung function, frequent exacerbations, obesity, heart failure, depression, and hypoxic respiratory failure (all P<0.05). Persistent disabling breathlessness was associated with lower lung function and ischemic heart disease (all P<0.05).
Conclusion
Disabling breathlessness is common in COPD despite treatment, which calls for improved symptomatic treatments and consideration of factors influencing disabling breathlessness. Factors influencing disabling breathlessness should be considered for COPD management.
Introduction
Breathlessness is a cardinal symptom in patients with chronic obstructive pulmonary disease (COPD).Citation1,Citation2 Breathlessness is measured using the modified Medical Research Council (mMRC) dyspnea scale,Citation3 is strongly associated with impaired health-related quality of life,Citation4,Citation5 shorter survival,Citation6,Citation7 and predicts mortality even stronger than measures of health statusCitation7 and lung functionCitation6 in COPD. According to the current Global Initiative of Obstructive Lung Disease (GOLD) recommendations, breathlessness is an important factor for assessing disease severity and a major target for pharmacological and non-pharmacological treatment of COPD.Citation1 Disabling breathlessness is commonly defined as an mMRC score of ≥2,Citation1 which means having breathlessness when walking slower than others or stopping when walking at own pace on level ground, stopping every 100 m or after a few minutes, or being too breathless to leave the house or being breathless on washing or dressing. An mMRC cut-off score of ≥2 has been found to be optimal for prediction of increased mortalityCitation7 and is used in the current GOLD severity classification of COPD.Citation1
Many patients have persistent disabling breathlessness despite optimal treatment of the underlying disease(s).Citation8,Citation9 Knowledge from real-life studies is limited on the prevalence, course, and risk factors for disabling breathlessness. In particular, the prevalence of persistent disabling breathlessness despite treatments for COPD in clinical practice is unknown. This information is important for management of COPD and for development of strategies to obtain relief from persistent disabling breathlessness.
The aim of this study was to determine the prevalence, change in breathlessness status over time, and risk factors associated with disabling and persistent breathlessness, particularly in relation to different treatment options for COPD.
Materials and methods
Study design and subjects
This was a longitudinal analysis of the Swedish National Registry of COPD.Citation10 The register was started in 2009 and is run on behalf of the Swedish Respiratory Society with governmental funding. Outpatient data are entered at patient visits in both primary care and hospital-based outpatient care, including patient demographics, lung function, health status (COPD Assessment Test), breathlessness (mMRC), smoking habits, exacerbations, treatments, and comorbid conditions. In the most recent annual report from 2015, the national coverage of all patients with severe and very severe COPD was valued to be 75%.Citation11 The calculation was based on a recent population-based study of prevalence of different COPD stages in Sweden.Citation12
Inclusion criteria were a ratio of forced expiratory volume in one second to the forced expiratory volume (FEV1/FVC) below 0.7, age 35 years and above, and having at least two registered visits (baseline and follow-up) separated by a minimum of 2 months during the period between January 1, 2013 and February 22, 2016.
Methods
The following data were obtained from the Swedish National Register of COPD: mMRC score, sex, age, smoking habits, spirometry data, body mass index (BMI), number of exacerbations, and hospitalizations due to exacerbations in the recent year, pharmacological or non-pharmacological treatment, and a doctor’s diagnosis of comorbid heart failure, ischemic heart disease, depression, and hypoxemia. Generally, post-bronchodilator spirometry values were taken for the study, or if missing, pre-bronchodilator spirometry values were used.
Disabling breathlessness was defined as an mMRC score of ≥2. Persistent disabling breathlessness was defined as disabling breathlessness at both baseline and follow-up (duration ≥2 months). Frequent exacerbations were defined according to the GOLD criteria as having at least two exacerbations or one severe exacerbation leading to hospitalization in the recent year.Citation1 Smoking was categorized as current daily smokers or nonsmokers. Lung function was measured by FEV1 as percentage of the predicted normal (FEV1%pred), reported as mean FEV1%pred and GOLD stages I to IV,Citation1 and analyzed as the effect of 10% change. Hypoxic respiratory failure was defined as oxygen saturation levels ≤88%Citation13 or use of long-term oxygen therapy (LTOT). COPD treatment groups included long-acting muscarinic antagonists (LAMA), long-acting beta-2-agonists (LABA), combined bronchodilators (LAMA + LABA), combined long-acting beta-2-agonists and inhalation corticosteroids (LABA + ICS), triple therapy (LAMA + LABA + ICS), per-oral steroids, LTOT, and physiotherapy (in recent 12 months). Maximal inhaled therapy was defined as inhaled triple therapy with LAMA, LABA, and ICS, and maximal treatment was defined as combined inhaled triple therapy and physiotherapy. Chronic breathlessness was defined as disabling breathlessness at both baseline and follow-up (duration ≥2 months) despite triple inhaled therapy and physiotherapy.
Analyses
Differences in baseline characteristics between patients with or without disabling breathlessness were analyzed using cross-tabulations. The proportions of patients with persistent disabling breathlessness and those worsening from non-disabling to disabling breathlessness during follow-up were calculated. Persistent disabling breathlessness was investigated in the main population as well as within different COPD treatment groups.
Logistic regression with disabling breathlessness at follow-up as dependent variable and patient baseline characteristics as independent variables was performed. Sex, age, and all statistically significant factors from univariate analyses were included in the multivariate model.
Risk factors for persistent disabling breathlessness and change from non-disabling to disabling breathlessness over time were analyzed using conditional change multivariate logistic regression model with adjustment for breathlessness status at baseline (disabling or not) in addition to covariates from the main analysis. Almost all patients with hypoxic respiratory failure had disabling breathlessness status at baseline, and thus hypoxic respiratory failure could not be included in the conditional change model. All multiple logistic regression analyses were repeated with further adjustment for pharmacological and non-pharmacological treatments. A P-value below 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 22.0 (SPSS Inc, Chicago, IL, USA).
Ethics
The study protocol was approved by the Regional Ethical Review Board of Gothenburg (Dnr 393-15). Patients included in the National Register of COPD were informed verbally by the caregivers and by pamphlets and announcements at the clinics. Oral consents were received at inclusion from all patients who participated in the study. The Regional Ethical Review Board of Gothenburg did not require written informed consent to be obtained from the participants, because registration implied consent if not specifically expressed otherwise by the patients, and because the database used for analyses was anonymous to the authors of the study.
Results
Population characteristics
A total of 1,689 patients with mMRC scores at baseline and follow-up were included. The mean FEV1%pred was 51.3 among included patients and 53.8 among excluded patients without mMRC scores at both baseline and follow-up (n=817; P=0.001). The patients had a mean age of 70 years, mean FEV1 of 51% of predicted (57% post-bronchodilator values and 43% pre-bronchodilator values), 27% of the patients were frequent exacerbators with either two exacerbations in total or one exacerbation requiring hospitalization in the recent year, and 53% were women. Patient characteristics by presence of disabling breathlessness are shown in . Lung function was stable over time, with mean FEV1%pred of 51.3 at baseline and 50.1 at follow-up.
Table 1 Patient characteristics at baseline
Prevalence and predictors of disabling breathlessness
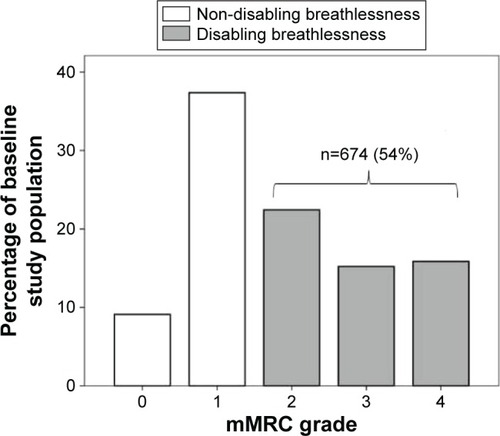
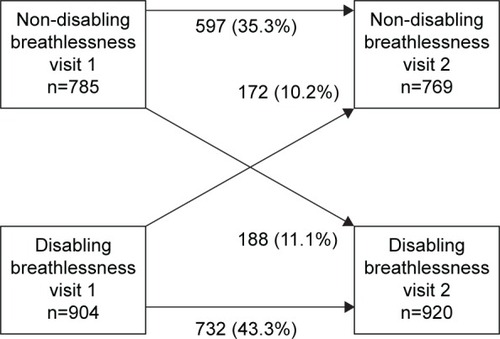
Disabling breathlessness was experienced by 54% of the patients at baseline. The distribution of mMRC scores is shown in . The change of breathlessness status (disabling or not) from baseline to follow-up, during a median time of 12 (interquartile range: 4) months, is shown in . In longitudinal univariate logistic regression analyses, disabling breathlessness at follow-up was more common in patients with higher age, frequent exacerbations, obesity, heart failure, ischemic heart disease, depression, or hypoxic respiratory failure. Disabling breathlessness was less common with increasing FEV1%pred and in current smokers (). All patients, but one, with hypoxic respiratory failure had disabling breathlessness. In multivariate analysis, disabling breathlessness was more common with higher age, decreasing FEV1%pred, and in patients with frequent exacerbations, obesity, heart failure, depression, and hypoxic respiratory failure (). The results did not substantially change after further adjustment for groups receiving pharmacological treatment and physiotherapy (data not shown).
Table 2 Risk factors for disabling breathlessness in longitudinal analyses
Change in breathlessness over time
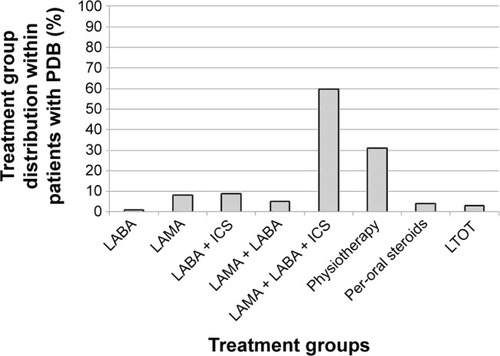
The prevalence of persistent disabling breathlessness at follow-up according to COPD treatments at baseline is shown in and . More intense COPD treatment was driven by higher breathlessness. The prevalence of persistent disabling breathlessness increased from about 20% in patients on a single long-acting bronchodilator to >50% in patients with two long-acting bronchodilators and/or ICS, and was even higher in patients with per-oral steroids and LTOT (). Chronic breathlessness (persistent disabling breathlessness despite inhaled triple therapy and physiotherapy) was present in 74% of patients (). Among patients with persistent disabling breathlessness, 40% had not received maximal inhaled triple therapy and 69% had not received physiotherapy (), and as many as 79% had not been prescribed the maximal combination of inhaled triple therapy and physiotherapy.
Table 3 Persistent disabling breathlessness despite COPD treatment
Figure 3 Persistent disabling breathlessness (PDB) in treatment groups.
Note: Data presented as percentages of PDB in different treatment groups (n=1,689).
Abbreviations: LAMA, long-acting muscarinic antagonists; LABA, long-acting beta- 2-agonists; ICS, inhalation corticosteroids; LTOT, long-term oxygen therapy.
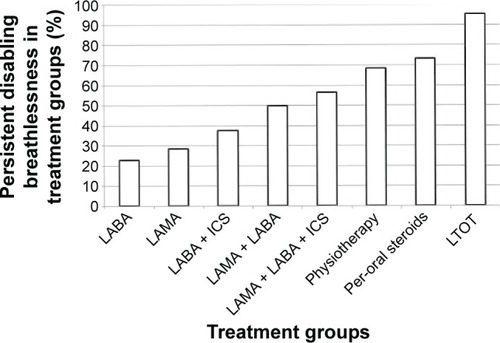
Figure 4 Distribution of treatment groups within patients with persistent disabling breathlessness (PDB).
Abbreviations: LAMA, long-acting muscarinic antagonists; LABA, long-acting beta-2-agonists; ICS, inhalation corticosteroids; LTOT, long-term oxygen therapy.
Predictors of persistent disabling breathlessness and worsening over time
The presence of persistent disabling breathlessness was predicted by lower FEV1%pred and was about twice as likely in patients with ischemic heart disease (). Predictors of developing disabling breathlessness over time were higher age, lower FEV1%pred, frequent exacerbations, obesity, and comorbid depression (). The results did not substantially change after further adjustment for groups receiving pharmacological treatment and physiotherapy (data not shown).
Table 4 Risk factors for change in disabling breathlessness over time
Discussion
Main findings
The first finding is that disabling breathlessness is common in COPD patients despite treatment. The risk of disabling breathlessness over a median of 12 months was higher in patients with higher age, lower FEV1%pred, frequent exacerbations, obesity, heart failure, depression, and hypoxic respiratory failure.
The second important finding is that most patients remained in the same status of breathlessness over time, with almost half of the patients having persistent disabling breathlessness.
Third, although some patients with persistent disabling breathlessness were not maximally treated, the majority of patients had chronic breathlessness, ie, persistent disabling breathlessness despite intensive COPD treatment including triple inhaled therapy and physiotherapy.
What does this study add?
The major novelty of this study is that it includes longitudinal analyses of predictors of disabling breathlessness, change in breathlessness status over time, and persistent disabling breathlessness related to therapy.
In previous studies, the prevalence of disabling breathlessness with mMRC ≥2 has differed from 39.5% to 60.2% in different European countries, so the proportion found in this study is consistent with international findings.Citation14 Previous studies of associations with breathlessness have mainly been cross-sectional analyses, with findings of associations with higher age,Citation15 lower lung function,Citation15,Citation16 frequent exacerbations,Citation15,Citation16 comorbid underweight,Citation17,Citation18 obesity,Citation15,Citation16,Citation19,Citation20 heart failure,Citation16 and depression.Citation16 The importance of depression as a risk factor for breathlessness, rather than the reverse that breathlessness causes depression and anxiety, has been shown and discussed previously,Citation21 and is confirmed in the present study. However, this study shows that age, FEV1%pred, exacerbations, obesity, and heart failure are associated with disabling breathlessness even in a longitudinal analysis, and these data are important as they indicate a causal relation. It is speculated that the association of hypoxic respiratory failure with disabling breathlessness is mainly a marker for more severe disease, but hypoxemia could also potentially be associated with increased ventilatory drive in some patients.
As for the analyses of change in breathlessness status, it is previously known that the mean mMRC score worsens over time,Citation22,Citation23 but this study also distinguishes different courses over time and adds analyses of persistent disabling breathlessness in relation to treatment alternatives. As for the analysis of persistent disabling breathlessness, it is speculated that the association of ischemic heart disease with persistent disabling breathlessness may indicate that treatment of ischemic heart disease has not been paid attention to the same extent as comorbid heart failure, and that optimized treatment of comorbid heart disease is a potential way to decrease the disability from breathlessness in COPD. The conclusion is that treatment of comorbid diseases, which themselves cause breathlessness, is very important for COPD patients.
As breathlessness is a major symptom and cause of disability in COPD, symptomatic management is of utter importance to improve symptom control, mastery, and quality of life in patients with persistent disabling breathlessness. The need for focusing clinical management and research on chronic breathlessness, a syndrome that impacts the patients’ function or well-being despite maximal treatment for the underlying disease, has recently been pointed out.Citation24,Citation25 There is good evidence for reducing breathlessness by both pharmacological treatment with bronchodilatorsCitation26,Citation27 and by physical training,Citation28 and the importance of a symptom-based therapy has recently been proposed.Citation29 This study confirms the results from a Spanish study where breathlessness was an important determinant for stepping up treatment.Citation30 The clear association of disabling breathlessness with more advanced treatment could partly be explained by treatment choice, being based on symptoms as well as lung function and exacerbation frequency.Citation1 However, patients with disabling breathlessness may also consult their doctors more often and subsequently receive more advanced treatments. However, there is obviously a room for improvement in COPD management, as not all patients with disabling breathlessness received maximal treatment. Unfortunately, physiotherapy in the recent 12 months was even less common than pharmacological therapy. Swedish national guidelines and treatment recommendations did not include the GOLD ABCD classification until 2015, which means that previous pharmacological treatments could have been more guided by lung function stage than by grade of breathlessness. However, even more interesting is that a high proportion of patients had chronic breathlessness despite intensive COPD treatment, with 74% of patients receiving both inhaled triple therapy and physiotherapy. This is an important finding which indicates that available treatments are insufficient and that improved management strategies for symptom relief are needed.
Strengths and limitations
The major strengths of this study are the national multi-center design and that analyses of breathlessness were multivariate, longitudinal, and investigated persistent disabling breathlessness related to COPD therapy. The study uses real-life data from clinical practice in both primary and secondary care, which should ensure a good external validity. The prevalence of disabling breathlessness correspond well to an average of mMRC scores in COPD patients in five different European countries.Citation14 In the same study, the mean age was slightly lower than in this study and the majority of patients were men, but in another large primary care database in UK,Citation31 the mean age and proportion of men were similar to this study. In addition, the sex and age distribution of this study is consistent with other Swedish observational COPD studies, with either primaryCitation32 or secondaryCitation33 COPD patients, and in a regional observational database with patients from both primary and secondary care.Citation34 Therefore, the generalizability of the study findings is likely to be good.
Important limitations were that no data on treatment of comorbidity and symptomatic treatment with opioids or benzodiazepines was included. Previous meta-analyses have shown that opioids but not benzodiazepines are effective as symptomatic treatment for breathlessness, and information on the impact of opioids would have been of great interest for the analyses.Citation35,Citation36 Breathlessness in COPD patients may be influenced by comorbid conditions. The analyses were adjusted for comorbid heart failure and ischemic heart disease, but lacked more detailed information on parameters such as left ventricular ejection fractions, pulmonary hypertension, and hemoglobulin levels. Furthermore, data registration was not complete for all variables. However, according to the most recent annual report from the register, the national coverage of severe and very severe COPD is acceptable.Citation11 Another issue is that the exact durations of medical treatment are unknown, but as reported treatment was prescribed at the latest at visit 1 and the follow-up time was minimum 2 months; it is reasonable to believe that a treatment effect on breathlessness should have been achieved. Finally, the attrition analysis showed that the included patients had statistically significant lower lung function than the patients excluded due to the absence of baseline and follow-up data on mMRC, however it is believed that the difference was not clinically significant.
Implications
The most important implications of this study are that persistent disabling breathlessness is common, and that although many patients do not have maximal COPD treatment, there is a considerable proportion of patients having chronic breathlessness despite maximal treatment. Several factors influencing disabling breathlessness, such as comorbid conditions and their treatment, should be considered and optimized for COPD management.
Important research implications are that future studies of breathlessness in COPD should include persistent breathlessness as this state is an important clinical problem in COPD, and also consider treatment of comorbid conditions. As COPD is an irreversible disease, future clinical management and research need to focus on symptomatic treatment.
Conclusion
Disabling breathlessness is influenced by age, FEV1%pred, exacerbations, and several comorbid conditions, which should be considered in managing COPD. Persistent disabling breathlessness is common despite treatment, which calls for development of new treatment options and strategies for symptom relief in COPD.
Acknowledgments
The authors thank the National Register of COPD for supplying data to the study. The study was supported by the Region Örebro County through funding by ALF (Avtal om Läkarutbildning och Forskning [Agreement concerning Cooperation on Medical Education and Research]).
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease Available from: http://www.goldcopd.comAccessed June 14, 2016
- MiravitllesMFerrerJBaroELleonartMGaleraJDifferences between physician and patient in the perception of symptoms and their severity in COPDRespir Med2013107121977198523890959
- MahlerDAWellsCKEvaluation of clinical methods for rating dyspneaChest19889335805863342669
- BestallJCPaulEAGarrodRGarnhamRJonesPWWedzichaJAUsefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary diseaseThorax199954758158610377201
- WilkeSJonesPWMullerovaHOne-year change in health status and subsequent outcomes in COPDThorax201570542042525782757
- NishimuraKIzumiTTsukinoMOgaTDyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPDChest200212151434144012006425
- CasanovaCMarinJMMartinez-GonzalezCDifferential effect of modified Medical Research Council Dyspnea, COPD Assessment Test, and Clinical COPD Questionnaire for symptoms evaluation within the new GOLD staging and mortality in COPDChest2015148115916825612228
- ParshallMBSchwartzsteinRMAdamsLAn official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspneaAm J Respir Crit Care Med2012185443545222336677
- JohnsonMJCurrowDCChronic refractory breathlessness is a distinct clinical syndromeCurr Opin Support Palliat Care20159320320526125309
- HenochIStrangSLöfdahlC-GEkberg-JanssnAHealth-related quality of life in a nationwide cohort of patients with COPD related to other characteristicsEur Clin Respir J201633145927238360
- Luftvägsregistret, Årsrapport2015Swedish National Register of COPD, Annual report 2015 Available from: http://www.registercentrum.se/sites/default/files/dokument/luftvagsregistret_arsrapport_2015.pdfAccessed October 30, 2016
- BackmanHErikssonBRonmarkEDecreased prevalence of moderate to severe COPD over 15 years in northern SwedenRespir Med201611410311027109819
- QaseemAWiltTJWeinbergerSEDiagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory SocietyAnn Intern Med2011155317919121810710
- PunekarYSMHSmallMHolbrookTWoodRNayaIValleMPrevalence and burden of dyspnoea among patients with chronic obstructive pulmonary disease in five European countriesPulm Ther201625972
- MullerovaHLuCLiHTabbererMPrevalence and burden of breathlessness in patients with chronic obstructive pulmonary disease managed in primary carePloS One201491e8554024427316
- PerezTBurgelPRPaillasseurJLModified Medical Research Council scale vs Baseline Dyspnea Index to evaluate dyspnea in chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2015101663167226316740
- KatsuraHYamadaKKidaKBoth generic and disease specific health-related quality of life are deteriorated in patients with underweight COPDRespir Med200599562463015823461
- SahebjamiHSathianpitayakulEInfluence of body weight on the severity of dyspnea in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20001613 Pt 188689010712338
- LaunoisCBarbeCBertinEThe modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot studyBMC Pulm Med2012126123025326
- Garcia-RioFSorianoJBMiravitllesMImpact of obesity on the clinical profile of a population-based sample with chronic obstructive pulmonary diseasePloS One201498e10522025153331
- NeumanAGunnbjornsdottirMTunsaterADyspnea in relation to symptoms of anxiety and depression: a prospective population studyRespir Medicine20061001018431849
- OgaTNishimuraKTsukinoMSatoSHajiroTMishimaMLongitudinal deteriorations in patient reported outcomes in patients with COPDRespir Med2007101114615316713225
- OgaTTsukinoMHajiroTIkedaANishimuraKAnalysis of longitudinal changes in dyspnea of patients with chronic obstructive pulmonary disease: an observational studyRespir Res20121318523006638
- CurrowDCAbernethyAPAllcroftPThe need to research refractory breathlessnessEur Respir J201647134234326721965
- CurrowDCJohnsonMJChronic breathlessness: silent and deadlyCurr Opin Support Palliat Care201610322122227359078
- KarnerCChongJPoolePTiotropium versus placebo for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20147CD009285
- KewKMMavergamesCWaltersJALong-acting beta2-agonists for chronic obstructive pulmonary diseaseCochrane Database Syst Rev201310CD010177
- McCarthyBCaseyDDevaneDMurphyKMurphyELacasseYPulmonary rehabilitation for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20152CD003793
- CabreraCCasanovaCMartinYAgreement between a simple dyspnea-guided treatment algorithm for stable COPD and the GOLD guidelines: a pilot studyInt J Chron Obstruct Pulmon Dis2016111217122227354780
- Lopez-CamposJLAbad ArranzMCalero AcunaCDeterminants for changing the treatment of COPD: a regression analysis from a clinical auditInt J Chron Obstruct Pulmon Dis2016111171117827330285
- JonesRCPriceDChavanessNHMulti-component assessment of chronic obstructive pulmonary disease: an evaluation of the ADO and DOSE indices and the global obstructive lung disease categories in international primary care data setsNPJ Prim Care Respir Med2016261601027053297
- StallbergBJansonCJohanssonGManagement, morbidity and mortality of COPD during an 11-year period: an observational retrospective epidemiological register study in Sweden (PATHOS)Prim Care Respir J2014231384524346825
- SundhJJohanssonGLarssonKComorbidity and health-related quality of life in patients with severe chronic obstructive pulmonary disease attending Swedish secondary care unitsInt J Chron Obstruct Pulmon Dis20151017318325653516
- SundhJStallbergBLisspersKKampeMJansonCMontgomerySComparison of the COPD Assessment Test (CAT) and the Clinical COPD Questionnaire (CCQ) in a clinical populationCOPD201519
- EkstromMNilssonFAbernethyAACurrowDCEffects of opioids on breathlessness and exercise capacity in chronic obstructive pulmonary disease. A systematic reviewAnn Am Thorac Soc20151271079109225803110
- SimonSTHigginsonIJBoothSHardingRBauseweinCBenzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adultsCochrane Database System Rev20101CD00735420091630