Abstract
Patients with COPD and other chronic respiratory diseases are especially vulnerable to viral and bacterial pulmonary infections, which are major causes of exacerbations, hospitalization, disease progression, and mortality in COPD patients. Effective vaccines could reduce the burden of respiratory infections and acute exacerbations in COPD patients, but what is the evidence for this? This article reviews and discusses the existing evidence for pneumococcal vaccination efficacy and its changing role in patients with chronic respiratory diseases, especially COPD. Specifically, the recent Community-Acquired Pneumonia Immunization Trial in Adults (CAPITA) showed the efficacy of pneumococcal conjugate vaccine in older adults, many of whom had additional risk factors for pneumococcal disease, including chronic lung diseases. Taken together, the evidence suggests that pneumococcal and influenza vaccinations can prevent community-acquired pneumonia and acute exacerbations in COPD patients, while pneumococcal vaccination early in the course of COPD could help maintain stable health status. Despite the need to prevent pulmonary infections in patients with chronic respiratory diseases and evidence for the efficacy of pneumococcal conjugate vaccine, pneumococcal vaccine coverage and awareness are low and need to be improved. Respiratory physicians need to communicate the benefits of vaccination more effectively to their patients who suffer from chronic respiratory diseases.
Introduction
Patients with chronic respiratory diseases (CRDs), especially those with COPD, exhibit altered lung defenses and frequently have multiple comorbidities, which include cardiovascular disease, diabetes, metabolic syndrome, chronic anemia, osteoporosis, depression, and cancer.Citation1–Citation3 Such adverse conditions make these patients especially vulnerable to viral and bacterial pulmonary infections, which are major causes of exacerbations, hospitalization, and disease progression.Citation1,Citation4,Citation5 Pneumonic infections in COPD patients have a major impact on morbidity, mortality, and health-care costs.Citation6,Citation7 Since vaccines are among the most effective means of preventing infectious diseases and their consequences, preventing pulmonary infections in COPD patients through vaccination could reduce the burden of COPD and its complications, as well as further declines in patient health. For many years, pneumococcal vaccination in adults has relied primarily on polysaccharide vaccines. More recently, however, a large study demonstrated the efficacy of pneumococcal conjugate vaccine (PCV) in older adults (Community-Acquired Pneumonia Immunization Trial in Adults [CAPITA]), in which many of the participants had additional risk factors for pneumococcal disease.Citation8 Following these new developments, a symposium was held at the 2016 European Respiratory Society International Congress (September 5, 2016, London, UK) to examine and discuss the changing role of pneumococcal vaccination in patients with CRD, especially COPD. One major aim of these discussions was to document whether vaccination with PCV should be included among other strategies known to benefit COPD patients, such as smoking cessation, respiratory therapy, trigger avoidance, and influenza vaccination, to reduce the risk of exacerbations and respiratory events.
Pneumococcal diseases in patients with chronic respiratory diseases
The global burdens of respiratory diseases remain among the highest of all diseases and the most frequent causes of death.Citation9,Citation10 COPD and lower respiratory tract infections (LRTIs) are the third- and fourth-leading causes of death, respectively, after heart disease and stroke,Citation9,Citation11 with ~55% of all LRTI deaths attributable to pneumococcal pneumonia.Citation10 In Europe, pneumonia is the leading cause of respiratory hospitalizations and the second leading cause of death due to respiratory disease after lung cancer.Citation12 The situation is compounded by the nearly 23 million Europeans with moderate–severe COPD who account for 1.1 million hospitalizations and 150,000 deaths each year.Citation12 COPD is frequently associated with comorbidities that contribute greatly to patient mortality. Among these, coronary artery disease and congestive heart failure play a major role.Citation2 Hospitalizations and deaths due to COPD can be expected to rise in the future as the disease progresses in the 17 million additional persons in the EU, who are estimated to be living with mild-stage COPD.Citation12 Currently, pneumonia, COPD, and asthma account for 55.7% of respiratory hospital admissions and 47.3% of respiratory deaths in the EU.Citation12
Patients with COPD, other CRDs, or other underlying chronic conditions are at high risk of community-acquired pneumonia (CAP), which imposes a substantial disease burden on the European population. Each year, there are an estimated 3.37 million cases of CAP, almost a third of which require hospitalization.Citation12 A retrospective cohort analysis of health-care claims in the US determined the risk of pneumococcal pneumonia to be 2.7 times higher in adults ≥65 years old compared to adults 50−64 years old ().Citation13 In general, the incidence of pneumococcal pneumonia increased after the age of 65 years and was severalfold higher in persons of all ages living with chronic diseases, such as diabetes, heart disease, asthma, or lung disease, or with underlying conditions, such as smoking or alcoholism, compared to those with no known risk conditions. Among adults ≥65 years old, the incidence of pneumococcal pneumonia was 7.7-fold higher in persons with CRD compared to those without comorbidity (). Further, incidence increased exponentially in patients who had multiple comorbidities (“risk stacking”). In patients with three at-risk conditions, incidence was 9–16 times higher than in healthy adults without any known risk factors and twice as high as in immunocompromised patients, who are considered to be at high risk.Citation13
Figure 1 Incidence of pneumococcal pneumonia in adults with underlying conditions in the US, 2007–2010.
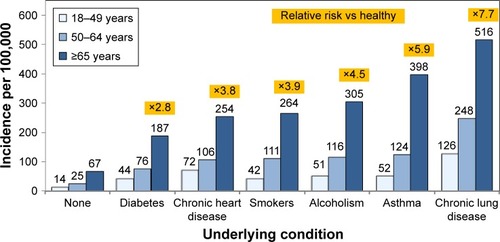
The prevalence of chronic conditions that put people at risk is also high, as shown in another US study in which 32.1% of adults 45−64 years old and 61.6% of adults ≥65 years of age had at least two chronic conditions and were considered at high risk of CAP.Citation14 As the mean age of the population continues to increase, the prevalence of persons with multiple chronic conditions is also expected to rise.Citation14 In Europe, the incidence of CAP was found to be more than 20-fold higher in persons with COPD (22.4 per 1,000 person-years) than in the general population (1.07−1.2 per 1,000 person-years), with the incidence dramatically higher in persons with severe COPD (forced expiratory volume in 1 second [FEV1] <50% of expected).Citation15,Citation16 In Sweden, the risk of severe/invasive pneumococcal disease (IPD) was fivefold higher in persons with COPD or pulmonary fibrosis and twice as high in persons with asthma than in the general population.Citation17
COPD patients may be more susceptible to respiratory infections, because their mucociliary clearance mechanisms are impaired, and production of the specific cell adhesion molecules that mediate attachment of bacteria and viruses is increased in their airways. Expression of platelet adhesion factor receptor (a cell adhesion molecule for Streptococcus pneumoniae and untypable Haemophilus influenza) and more recently ICAM1 (a cell adhesion molecule for rhinovirus) have been found to be significantly elevated in COPD patients and smokers compared to normal controls.Citation18,Citation19 Elevated levels of these proteins may increase the risk of respiratory infection and bacterial colonization. In addition, certain treatments, such as inhaled corticosteroids, can further increase the risk of pneumonia in COPD patients. This risk appears to increase with the dose of the drug, regardless of disease severity, although it does not appear to be associated with a concomitant increase in mortality.Citation20
Preventing exacerbations in COPD patients is a major treatment objective. Among the predominant triggers of exacerbations are LRTI, environmental contaminants, pollution, and poor compliance with pulmonary rehabilitation regimes or with long-term oxygen therapy.Citation21 However, approximately two-thirds of exacerbations are associated with bacterial or viral LRTI or bacterial colonization of the lungs,Citation1,Citation5,Citation22,Citation23 and the spectrum of microorganisms associated with these infections appears to vary with disease severity.Citation24 In a study of hospitalized COPD patients, Streptococcus spp. were more prevalent in patients with less severe airway obstruction, whereas Haemophilus spp. were more prevalent in moderate disease and Enterobacteriaceae and Pseudomonas spp. in severe disease.Citation24 This finding suggests that pneumococcal vaccination should be envisaged particularly in patients with mild airflow obstruction.
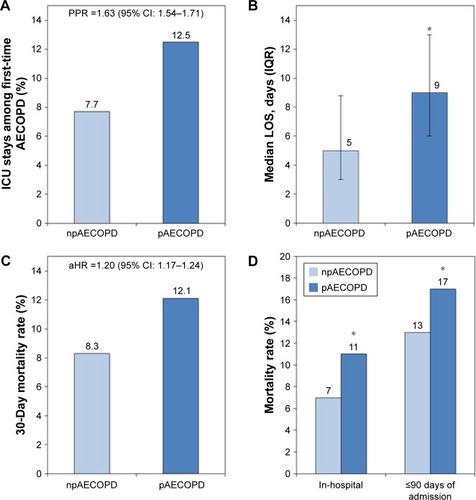
Although there has been some debate on whether pneumonia should be considered a cause or differential diagnosis of acute exacerbation of COPD (AECOPD), another important observation is that pneumonic AECOPD tends to be more severe than nonpneumonic AECOPD. In analyses of COPD-related hospitalizations, pneumonic AECOPD in Denmark accounted for 36.1% of all first-time hospitalizations for AECOPD and resulted in more ICU admissions (12.5% vs 7.7%), longer median hospitalization stay (9 vs 5 days), and higher 30-day mortality (12% vs 8%) than nonpneumonic AECOPD ().Citation25,Citation26 The severity of pneumonic AECOPD is further substantiated by a UK study that found in-hospital mortality and 90-day mortality to be significantly higher in patients with pneumonic AECOPD compared to patients with nonpneumonic AECOPD ()Citation27 and by a Serbian study that found pneumonia to be the second-most frequent autopsy-confirmed cause of death (28%) after heart failure (37%) in COPD patients who died within 24 hours of hospital admission.Citation28
Figure 2 Intensive care unit (ICU) admissions, stays, and mortality of COPD patients with pneumonic and nonpneumonic exacerbations.
Pneumonia not only bodes poorly for the outcome of COPD patients but also has profound implications for health-care systems. Direct medical costs and productivity costs associated with hospitalization for CAP are twice as high in patients with COPD compared to those in patients with no comorbidity.Citation29 COPD patients with CAP are also ninefold more likely to be hospitalized and fourfold more likely to visit the ER, both of which drive the first-year health-care costs for these patients to an excess of US$22,348 over the costs of COPD patients without CAP.Citation6 Excess costs in COPD patients with CAP also remain high over the second year after hospitalization and have been estimated to be $6,000.Citation7
In summary, the global burden of COPD is substantial, and the risk and frequency of pneumococcal pneumonia in these patients, as well as in others with multiple comorbidities, is many times higher than in healthy adults without comorbidities. Viral and bacterial pneumonic infections are a major cause of respiratory events in COPD patients. The outcomes of these infections tend to be more severe and more deadly than in COPD patients with nonpneumonic AECOPD, leading to longer, more expensive hospitalizations and greater overall costs. Preventing pneumonia in COPD patients could provide substantial benefits to these patients, as well as to the community.
Pneumococcal vaccines: clinical evidence of efficacy and recommendations
Multivalent pneumococcal polysaccharide vaccines (PPVs) have existed for many years, and the 23-valent vaccine (PPV23) is recommended by many public health agencies for adults ≥65 years of age and other high-risk groups. Its use comes with possible caveats, however, because the immunoresponses to PPV23 tend to wane over time, and demonstrations of clinical efficacy against CAP and IPD in at-risk populations have been inconsistent.Citation30–Citation32
However, in a recently updated Cochrane meta-analysis published after our symposium, pneumococcal vaccination (primarily with PPV23) was associated with statistically significant efficacy against CAP and AECOPD in COPD patients.Citation33 In contrast, another recent review found the evidence for the clinical efficacy of PPV23 in high-risk and at-risk populations to be inconsistent, despite some short-term protection.Citation34 In view of the historically uncertain and variable clinical efficacy of PPV23, the World Health Organization and public health agencies in Europe and the US recognize that there remains an unmet medical need to protect older adults and high-risk groups against pneumococcal pneumonia.Citation35–Citation38
Overall, PCVs tend to be more consistently immunogenic than PPVs and to increase the duration and memory of antipneumococcal immunoresponses. Vaccination with PPV produces an immunoresponse that is limited to B-cell stimulation and antibody production.Citation39,Citation40 In contrast, PCVs contain pneumococcal polysaccharide antigens covalently linked to an immunogenic carrier protein that together induce T-cell-dependent humoral immunoresponses () and stimulate T cells to help B cells produce antibodies to the vaccine and generate immune memory.Citation40–Citation43 These characteristics can improve the magnitude and duration of the initial immunoresponse and potentially allow the immune system to respond more effectively to subsequent exposure to vaccine-type (VT) pneumococcal strains. In studies that have compared humoral immunoresponses to PCV and PPV in normal vaccine-naïve adults or older adults, short-term immunoglobulin and functional antibody responses were generally superior with PCV.Citation40,Citation44–Citation46 This has also been demonstrated in COPD patients, in whom superior functional antibody responses persisted for at least 2 years.Citation47,Citation48 In some studies, however, the in vitro B-cell-specific responses of older adults vaccinated with PCV or PPV23 did not differ significantly immediately after vaccinationCitation49,Citation50 or after 6 months.Citation45 In healthy adults, long-term immunoresponses with PPV23 remain above baseline for as long as 10 years, though booster immunizations are generally recommended after 5 years.Citation51 To our knowledge, the long-term immunogenicity of PCV in normal healthy adults beyond 2 years has not been reported, but significant immunoresponses are known to persist for up to 5 years in HIV-positive adults.Citation52
Figure 3 Immunogenic effects of pneumococcal vaccines.
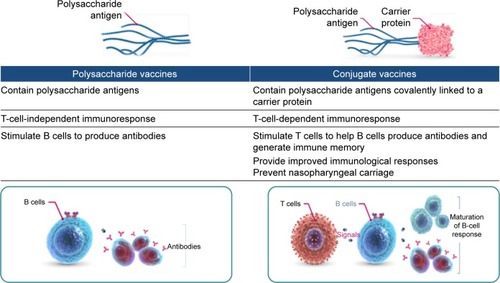
PCVs are recommended for all children and have been shown to prevent nasopharyngeal carriage of VT strains and to protect children against mucosal diseases, IPD, and otitis media.Citation53–Citation57 Childhood vaccination programs that include PCV have also demonstrated significant reductions in pneumococcal diseases in adults (herd protection) as an indirect consequence of lower pneumococcal carriage in children.Citation58,Citation59
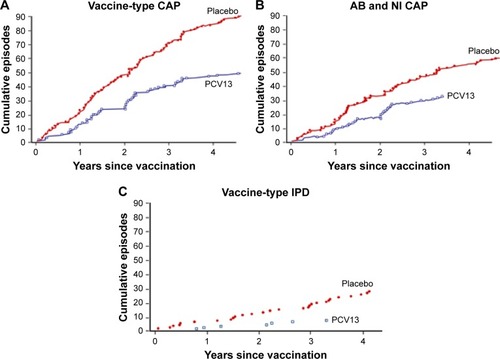
CAPITA was conducted to assess the clinical efficacy of PCV13 in older adults. It was a large prospective randomized, placebo-controlled trial in >84,000 participants ≥65 years of age.Citation8 The primary outcome was the efficacy of preventing a first episode of VT CAP. With up to 5 years of follow-up, vaccine efficacy in the per-protocol population was 45.6% against VT CAP and 45% against abacteremic, noninvasive VT CAP (). Vaccine efficacy was 30.6% against pneumococcal CAP and 75% against VT IPD. In an exploratory post hoc analysis of the at-risk population in the study, which included over 42% of the participants in each group, 10% of which had lung disease, vaccine efficacy was similar: 40.3% against VT CAP and 30.2% against pneumococcal CAP.Citation97 Furthermore, the magnitude and duration of humoral immunoresponses in at-risk adults assessed by opsonophagocytic assay geometric mean antibody titers were comparable to those of the overall population and persisted for at least 2 years.Citation60
Figure 4 Efficacy of PCV13 in older adults: results of the CAPITA study.
Abbreviation: CAPITA, Community-Acquired Pneumonia Immunization Trial in Adults.
Finally, another post hoc analysis predicted that vaccine efficacy would decline from 65% for adults 65 years of age to 40% for adults 75 years of age, suggesting that patients with indications of PCV should be vaccinated as early as possible.Citation61 While these results are encouraging, post hoc analyses should be viewed with caution, and the efficacy of PCV13 for preventing VT CAP in CRD patients has yet to be demonstrated in a randomized clinical trial designed to address this issue specifically. It should be noted that in such a hypothetical trial, the necessary sample size to demonstrate efficacy is likely to be very large, and recruiting the minimum number of CRD patients for the treatment and control arms would be extremely difficult, especially in the context of populations benefiting from herd immunity arising from pediatric vaccination programs.
Recently published studies have also investigated the efficacy of PPV23 in older adults. A meta-analysis of 17 eligible studies in adults ≥60 years of age found pooled efficacies of PPV23 against IPD (45%−73%) and pneumococcal pneumonia (48%−64%) by any serotype.Citation62 Another meta-analysis of studies in adults ≥50 years of age also found evidence of significant vaccine efficacy for PPV23 against IPD (50%−54%) and against all-cause CAP (4%−17%).Citation63 Both studies concluded that efficacies of PPV23 and PCV13 against VT pneumococcal disease were comparable. In a multicenter prospective study in adults ≥65 years of age treated for CAP, PPV23 efficacy was 27.4% (95% CI: 3.2%–45.6%) against all pneumococcal pneumonia.Citation64
The US Advisory Committee on Immunization Practices currently recommends PCV13 followed by PPV23 for all adults ≥65 years of age who have not previously received pneumococcal vaccine and in persons ≥19 years of age who are at high risk of pneumococcal disease due to underlying medical conditions.Citation65,Citation66 Several European scientific societies also recommend PCV13 for immunocompromised adults and all persons ≥65 years of age, along with at least one dose of PPV23 given 2−6 months afterward.Citation67 PCV13 is now being progressively implemented into national programs across Europe. As of August 2016, PCV13 is recommended for use in high-risk populations in >25 countries, for use in at-risk populations in >16 countries, and for use in older adults (>50 or >65 years of age) in >14 countries.
PCV13 is recommended to be given before PPV23.Citation66 This schedule is based on the observation that the responses to the serotypes common to both vaccines were stronger if PCV13 were administered first.Citation68 Of note, PCV13 can be given concomitantly with a seasonal inactivated influenza vaccine without impacting immunoresponses to either vaccine, which can help avoid missed opportunities for vaccination.Citation69,Citation70
In summary, PCV13 has been developed to provide improved protection for older adults and adults with chronic conditions, such as COPD, who are at high risk of pneumococcal infection and pneumonia. In the pivotal CAPITA study, PCV13 induced specific and efficacious immunoresponses in adults ≥65 years of age with or without underlying chronic conditions that elevate the risk of pneumonia, and vaccine efficacy against VT CAP and VT IPD persisted for up to 5 years. As a result, an increasing number of countries across Europe are including PCV13 in their vaccination recommendations.
Role of pneumococcal vaccination in patients with chronic respiratory diseases
Current European Respiratory Society and European Society of Clinical Microbiology and Infectious Diseases guidelines were published before the recent results on PCV, and thus recommended only PPV23 for older adults and adults with risk factors for pneumococcal disease, such as age ≥65 years, congestive heart failure, COPD, and a previous history of pneumonia.Citation71 Specific recommendations on pneumococcal vaccination are lacking in many guidelines on CRDs from international societies. For COPD, pneumococcal vaccination is recommended in current GOLD guidelines, which mention the increased immunogenicity of PCV13, but do not propose a clear position on whether it should be preferred over PPV23.Citation72
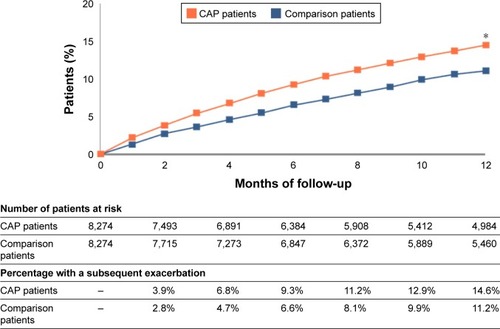
Because LRTIs are an important trigger for exacerbations, preventing them in COPD patients could also help prevent exacerbations.Citation5 Early prevention of exacerbations may also be critically important for preventing disease progression and subsequent events, as exacerbations can indicate or precede a feed-forward pattern of increased susceptibility to exacerbation and in some cases a frequent exacerbation phenotype.Citation21 In the natural history of COPD, each exacerbation is associated with an increased risk of subsequent exacerbations, which tend to occur with greater frequency and severity. It is well known that patients can enter a vicious cycle of infection, exacerbation, and disease progression.Citation3,Citation4,Citation73,Citation74 With each exacerbation, further tissue destruction and functional impairment can occur along with worsening mucociliary clearance and increased bacterial colonization, all of which increase the risk of developing an LRTI and another exacerbation. The reality of this concept was further demonstrated by comparing the exacerbation frequencies of COPD patients after a CAP episode to those in COPD patients who had not had CAP (). The cumulative risk of exacerbation in the first year after CAP was 1.5 times higher in patients who had had CAP (17%) compared to those who had not (11.4%; P<0.001).Citation96
Figure 5 Community-acquired pneumonia (CAP) and occurrence of subsequent exacerbations in COPD patients in the US, 2010.
Clinical evidence of pneumococcal vaccine efficacy in COPD
In principle, pneumococcal vaccination should prevent LRTI and acute exacerbations in COPD patients, but interpreting the existing evidence for the clinical efficacy of PPV in these high-risk patients has been somewhat problematic. In a 2010 Cochrane systematic review and meta-analysis of seven studies,Citation32 the overall vaccine effect against pneumonia was of borderline significance compared to placebo, with an OR of 0.72 (95% CI: 0.51–1.01), with no significant effects on either exacerbations or cardiorespiratory mortality. While these results suggested that PPV may confer some protection in COPD patients, the lack of statistical significance for any outcome and the heterogeneity of the studies rendered the evidence inconclusive. The evidence for vaccine efficacy improved somewhat in the 2017 update of this Cochrane review, which included a reanalysis of the same studies used in the 2010 meta-analysis of pneumococcal vaccination efficacy (primarily PPV23) against CAP, and concluded that vaccination was associated with significant reductions in the likelihood of experiencing CAP (OR: 0.62, 95% CI: 0.43–0.89) or AECOPD (OR: 0.6, 95% CI: 0.39–0.9).Citation33
A recent review of pneumococcal vaccine efficacy studies in persons with underlying risk factors, such as COPD or a CRD, vaccine efficacy against any-cause CAP was found to vary widely between −338% and 43%, with an overall efficacy of 29% (95% CI: −39%–63%) in adults aged ≥50 years with CRD.Citation34 In a randomized controlled trial in 596 COPD patients, PPV23 efficacy against CAP was significant at 76% (95% CI: 20–93, P=0.013) in patients <65 years of age, nearly significant at 48% (95% CI: 27–80, P=0.076) in those with severe airflow obstruction (<40% FEV1), and strongest for both factors combined (91% [95% CI: 35–99, P=0.002]).Citation75 However, vaccine efficacy was not significant for the overall cohort (24% [95% CI: −24–54, P=0.333]).
In a study of the combined efficacy of PPV23 and influenza vaccination in preventing acute exacerbation in adult CRD patients, acute exacerbation frequency was significantly lower in the PPV23 + influenza vaccination group than in the influenza vaccination-alone group (P=0.022), and there was a significant additive effect of PPV23 in COPD patients (P=0.037).Citation76 In view of the limited numbers of studies available to date, the evidence on the role of PPV for protecting COPD patients from CAP can be considered inconsistent.
In contrast, seasonal influenza vaccine has demonstrated significant efficacy in preventing exacerbations in COPD patients compared to placebo, with a reported weighted mean difference between treatments of −0.37 (95% CI: −0.64 to −0.11, P=0.006) in a meta-analysis of two randomized trials.Citation77 If influenza vaccine is administered concomitantly with PPV23, the two vaccines provide an additive effect in reducing the risk of hospitalization for pneumonia, death, death from influenza, and death from pneumonia.Citation78–Citation80
Another potential benefit of an effective pneumococcal vaccination could be a reduction in antibiotic treatments. This has not been studied in COPD patients, but has been estimated in children by comparing antibiotic treatment days for S. pneumoniae infections in countries with high childhood PCV coverage versus countries without such coverage.Citation81 Globally, an estimated 11.4 million antibiotic treatment days were avoided in countries with PCV coverage, a reduction of 47%. Reducing antibiotic treatment in COPD patients could also help delay or prevent colonization of the lungs by antibiotic resistant bacteria.
Real-life vaccine coverage of the target population
Despite the availability of effective pneumococcal vaccines and recommendations from most national health authorities, coverage remains suboptimal in many settings. Recent coverage rates have been fairly high in the UK (70% in persons ≥65 years of age in 2014−2015) and in Spain (76% in persons ≥60 years of age in 2010), lower in Ireland (36% in persons ≥65 years of age and 18% in high-risk adults in 2013), and very low in Norway (15%−30% in persons ≥65 years of age in 2014−2015), Germany (15% in high-risk persons in 2014), and France (~5% in persons ≥65 years of age in 2010−2011).Citation82–Citation87
An indication of the reasons behind these suboptimal coverage rates may lie in the perceptions and vaccine awareness of older adults. A recent survey (PneuVUE, Pfizer, Paris, France) measured public awareness of pneumonia and vaccine perceptions in adults ≥50 years of age across nine EU countries.Citation88 The survey found that 85% of these adults trusted vaccines, 27% avoided vaccines (for safety reasons), and 29% feel they “didn’t need” vaccines because they were not at risk. However, high proportions of respondents reported that they did follow their physician’s advice (92%) and that they preferred to obtain additional medical information from their doctor (92%). Overall, pneumococcal vaccine awareness was low, and the most frequent reason given (55%) for not being vaccinated was that it was never offered by their doctor.
To summarize, evidence-based guidelines recommend pneumococcal vaccination in persons with CRD, and PCV13 has already been included in a number of national recommendations, including those in the US, France, Portugal, Spain, Italy, and others.Citation66,Citation89–Citation92 Some countries, such as Germany and the UK, continue to consider the evidence and favor the broader serotype coverage of PPV23.Citation93,Citation94 In patients with COPD, preventing LRTIs, which frequently involve S. pneumoniae, appears critical, because they can alter disease status and speed disease progression, thus increasing the risk of subsequent exacerbation. Including pneumococcal vaccination in early care for COPD may improve the long-term natural history of the disease, although further evidence is needed. Despite recommendations, overall pneumococcal vaccination coverage is poor across Europe and public awareness and knowledge of pneumococcal disease and vaccines is low. Since patients rely on their physicians as the main source of health information, physicians need to do a better job of informing their at-risk patients about the dangers of pneumonia and the benefits of pneumococcal vaccination.
Conclusion
Global CRD burdens are high and contribute to substantial excess health-care use, hospitalizations, and global morbidity and mortality.Citation72 Patients with COPD and other CRDs are at high risk of CAP, IPD, and exacerbations that further increase health-care burdens and mortality.Citation12,Citation13 In COPD, airway infections are important triggers of exacerbations and hasten disease progression. There is evidence for the efficacy of PPV23 in older adults,Citation62,Citation63 but evidence for protection in CRD patients has been considered inconclusive.Citation32 The CAPITA study provided clinical evidence that PCV13 is effective at preventing CAP in older adults, including those with comorbidities.Citation8 Post hoc analyses of CAPITA are consistent with PCV13 efficacy in patients with comorbidities, but these results should be viewed with caution. Indeed, vaccine efficacy in adults is likely to vary according to the pneumococcal vaccines used in pediatric vaccination programs and efficacy may be low or difficult to demonstrate in settings where pediatric uptake of pneumococcal vaccine is high and the consequent burden of disease in adults is reduced.Citation95 However, given the substantially higher risk of respiratory infection and dire consequences of such infections in patients with COPD or CRD, vaccination is warranted in these patients, regardless of the level of herd protection present in the community.
Influenza and pneumococcal infections are important contributors to CAP and acute exacerbations in COPD patients, and pneumococcal and influenza vaccinations together may have an additive preventive effect.Citation78–Citation80 Vaccination early in the course of COPD could help maintain stable health status, although further demonstration is needed.Citation5,Citation21 Despite the great burden of pneumonia and pneumococcal infection, the need for vaccination in patients with CRD–COPD, the evidence on pneumococcal vaccine efficacy, and widespread national recommendations, improvements are needed in pneumococcal vaccine coverage, knowledge, and awareness.Citation88 As the main source of medical information for the public, physicians need to communicate more effectively the benefits of vaccination with PCV to their patients, especially those with CRDs.
Acknowledgments
The content of this article is based on a symposium (sponsored by Pfizer Inc., Paris, France) held at the ERS International Congress in London in September 2016. The authors take full responsibility for the content of this article and thank Dr Kurt Liittschwager (4Clinics, Paris, France) for scientific writing services and assistance in preparing the manuscript in close collaboration with the authors. The authors also thank Dr Tin Tin Htar (Pfizer) for valuable comments and suggestions. Financial support for the preparation of this manuscript was provided by Pfizer.
Disclosure
FF, NR, and FB received personal fees from Pfizer to conduct the symposium. FF has also received grants, personal fees, and nonfinancial support from Pfizer; personal fees and nonfinancial support from MSD; personal fees from Sanofi Pasteur and Novartis; and nonfinancial support from Sanofi, AstraZeneca, Bayer, and Bial. NR has received grants and personal fees from Pfizer, Boehringer Ingelheim, and Novartis, and personal fees from Teva, GSK, AstraZeneca, Chiesi, Mundipharma, Cipla, Sanofi, Sandoz, 3M, and Zambon. FB has received grants and personal fees from Bayer, Chiesi, Guidotti, Menarini, Pfizer, Teva, and Zambon, and personal fees from Dompé, AstraZeneca, and Novartis. The authors report no other conflicts of interest in this work.
References
- CelliBRBarnesPJExacerbations of chronic obstructive pulmonary diseaseEur Respir J20072961224123817540785
- DivoMCoteCde TorresJPComorbidities and risk of mortality in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2012186215516122561964
- SethiSInfection as a comorbidity of COPDEur Respir J20103561209121520513910
- SethiSMurphyTFInfection in the pathogenesis and course of chronic obstructive pulmonary diseaseN Engl J Med2008359222355236519038881
- WedzichaJAMackayAJSinghRCOPD exacerbations: impact and preventionBreathe201396434440
- LinJLiYTianHCosts and health care resource utilization among chronic obstructive pulmonary disease patients with newly acquired pneumoniaClinicoecon Outcomes Res2014634935625075195
- RyanMSuayaJAChapmanJDStasonWBShepardDSThomasCPIncidence and cost of pneumonia in older adults with COPD in the United StatesPLoS One2013810e7588724130749
- BontenMJHuijtsSMBolkenbaasMPolysaccharide conjugate vaccine against pneumococcal pneumonia in adultsN Engl J Med2015372121114112525785969
- VosTAllenCAroraMGlobal, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015Lancet2016388100531545160227733282
- WangHNaghaviMAllenCGlobal, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015Lancet2016388100531459154427733281
- World Health OrganizationThe top 10 causes of death2014 Available from: http://www.who.int/mediacentre/factsheets/fs310/enAccessed December 31, 2016
- European Respiratory SocietyEuropean Lung White BookLausanne, SwitzerlandERS2016
- SheaKMEdelsbergJWeyckerDFarkouhRAStruttonDRPeltonSIRates of pneumococcal disease in adults with chronic medical conditionsOpen Forum Infect Dis201411ofu02425734097
- WardBWBlackLIState and regional prevalence of diagnosed multiple chronic conditions among adults aged ≥18 years: United States, 2014MMWR Morb Mortal Wkly Rep2016652973573827467707
- MullerovaHChigboCHaganGWThe natural history of community-acquired pneumonia in COPD patients: a population database analysisRespir Med201210681124113322621820
- TorresAPeetermansWEViegiGBlasiFRisk factors for community-acquired pneumonia in adults in Europe: a literature reviewThorax201368111057106524130229
- InghammarMEngströmGKahlmeterGLjungbergBLöfdahlCGEgestenAInvasive pneumococcal disease in patients with an underlying pulmonary disorderClin Microbiol Infect201319121148115423464817
- ShuklaSDMahmoodMQWestonSThe main rhinovirus respiratory tract adhesion site (ICAM-1) is upregulated in smokers and patients with chronic airflow limitation (CAL)Respir Res2017181628056984
- ShuklaSDMullerHKLathamRSohalSSWaltersEHPlatelet- activating factor receptor (PAFr) is upregulated in small airways and alveoli of smokers and COPD patientsRespirology201621350451026662379
- YawnBPLiYTianHZhangJArconaSKahlerKHInhaled corticosteroid use in patients with chronic obstructive pulmonary disease and the risk of pneumonia: a retrospective claims data analysisInt J Chron Obstruct Pulmon Dis2013829530423836970
- WedzichaJABrillSEAllinsonJPDonaldsonGCMechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary diseaseBMC Med20131118123945277
- SapeyEStockleyRACOPD exacerbations – 2: aetiologyThorax200661325025816517585
- PapiABellettatoCMBraccioniFInfections and airway inflammation in chronic obstructive pulmonary disease severe exacerbationsAm J Respir Crit Care Med2006173101114112116484677
- EllerJEdeASchabergTNiedermanMSMauchHLodeHInfective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung functionChest19981136154215489631791
- AndreassenSLLiaaenEDStenforsNHenriksenAHImpact of pneumonia on hospitalizations due to acute exacerbations of COPDClin Respir J201481939923889911
- SøgaardMMadsenMLøkkeAHilbergOSørensenHTThomsenRWIncidence and outcomes of patients hospitalized with COPD exacerbation with and without pneumoniaInt J Chron Obstruct Pulmon Dis20161145546527042038
- MyintPKLoweDStoneRABuckinghamRJRobertsCMU.K. National COPD Resources and Outcomes Project 2008: patients with chronic obstructive pulmonary disease exacerbations who present with radiological pneumonia have worse outcome compared to those with non-pneumonic chronic obstructive pulmonary disease exacerbationsRespiration201182432032721597277
- ZvezdinBMilutinovSKojicicMA postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbationChest2009136237638019318666
- PolskyDBonafedeMSuayaJAComorbidities as a driver of the excess costs of community-acquired pneumonia in U.S. commercially-insured working age adultsBMC Health Serv Res20121237923113880
- AndrewsNJWaightPAGeorgeRCSlackMPMillerEImpact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and WalesVaccine201230486802680823000122
- ArtzASErshlerWBLongoDLPneumococcal vaccination and revaccination of older adultsClin Microbiol Rev200316230831812692100
- WaltersJASmithSPoolePGrangerRHWood-BakerRInjectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary diseaseCochrane Database Syst Rev201011CD00139021069668
- WaltersJATangJNPoolePWood-BakerRPneumococcal vaccines for preventing pneumonia in chronic obstructive pulmonary diseaseCochrane Database Syst Rev20171CD00139028116747
- HtarMTStuurmanALFerreiraGEffectiveness of pneumococcal vaccines in preventing pneumonia in adults, a systematic review and meta-analyses of observational studiesPLoS One2017125e017798528542347
- No authors listedPneumococcal vaccines WHO position paper: 2012Wkly Epidemiol Rec2012871412914424340399
- KhoieTTiernanRdeVoreNVaccines and Related Biological Products Advisory CommitteeFDA Briefing Document – Prevnar 13 (CV13): pneumococcal 13-valent conjugate vaccine (diphtheria CRM197 protein)2011 Available from: https://pdfs.semanticscholar.org/d394/d5e91b28e01e28c89a81c312dd79b02007b9.pdfAccessed October 11, 2017
- deVoreNSummary basis for regulatory action [Prevnar 13/pneumococcal 13-valent conjugate vaccine (diphtheria CRM197 protein)]2011 Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM287412.pdfAccessed October 1, 2017
- European Medicines AgencyCHMP variation assessment report [Prevenar 13]: type II variation EMEA/H/C/001104/II/00282011 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/001104/WC500119784.pdfAccessed October 1, 2017
- ClutterbuckEALazarusRYuLMPneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cellsJ Infect Dis201220591408141622457293
- de RouxASchmöle-ThomaBSiberGRComparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memoryClin Infect Dis20084671015102318444818
- ClutterbuckEASaltPOhSMarchantABeverleyPPollardAJThe kinetics and phenotype of the human B-cell response following immunization with a heptavalent pneumococcal-CRM conjugate vaccineImmunology2006119332833717067312
- PollardAJPerrettKPBeverleyPCMaintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccinesNat Rev Immunol20099321322019214194
- SiegristCAVaccine immunologyPlotkinSAOrensteinWOffittPAVaccines5th edAmsterdam, the NetherlandsElsevier20081736
- GoldblattDSouthernJAndrewsNThe immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50–80 yearsClin Infect Dis20094991318132519814624
- GrabensteinJDManoffSBPneumococcal vaccines in adults: assessing the evolving evidenceVaccine201129376149615421763386
- LazarusRClutterbuckEYuLMA randomized study comparing combined pneumococcal conjugate and polysaccharide vaccination schedules in adultsClin Infect Dis201152673674221367726
- DransfieldMTNahmMHHanMKSuperior immune response to protein-conjugate versus free pneumococcal polysaccharide vaccine in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2009180649950519556517
- DransfieldMTHarndenSBurtonRLLong-term comparative immunogenicity of protein conjugate and free polysaccharide pneumococcal vaccines in chronic obstructive pulmonary diseaseClin Infect Dis2012555e35e4422652582
- BaxendaleHEJohnsonMKeatingSMCirculating pneumococcal specific plasma and memory B cells in the elderly two years after pneumococcal conjugate versus polysaccharide vaccinationVaccine201028426915692220708698
- BaxendaleHEKeatingSMJohnsonMSouthernJMillerEGoldblattDThe early kinetics of circulating pneumococcal-specific memory B cells following pneumococcal conjugate and plain polysaccharide vaccines in the elderlyVaccine201028304763477020471437
- GrabensteinJDManoffSBPneumococcal polysaccharide 23-valent vaccine: long-term persistence of circulating antibody and immunogenicity and safety after revaccination in adultsVaccine201230304435444422542818
- ChengAChangSYTsaiMSLong-term immune responses and comparative effectiveness of one or two doses of 7-valent pneumococcal conjugate vaccine (PCV7) in HIV-positive adults in the era of combination antiretroviral therapyJ Int AIDS Soc20161912063126829360
- Ben-ShimolSGivon-LaviNLeibovitzERaizSGreenbergDDaganRNear-elimination of otitis media caused by 13-valent pneumococcal conjugate vaccine (PCV) serotypes in southern Israel shortly after sequential introduction of 7-valent/13-valent PCVClin Infect Dis201459121724173225159581
- GreenbergDGivon-LaviNBen-ShimolSZivJBDaganRImpact of PCV7/PCV13 introduction on community-acquired alveolar pneumonia in children <5 yearsVaccine201533364623462926116251
- GriffinMRMitchelEMooreMRWhitneyCGGrijalvaCGDeclines in pneumonia hospitalizations of children aged <2 years associated with the use of pneumococcal conjugate vaccines: Tennessee, 1998–2012MMWR Morb Mortal Wkly Rep2014634499599825375070
- LauWCMurrayMEl-TurkiAImpact of pneumococcal conjugate vaccines on childhood otitis media in the United KingdomVaccine201533395072507926297875
- MaromTTanAWilkinsonGSPiersonKSFreemanJLChonmaitreeTTrends in otitis media-related health care use in the United States, 2001–2011JAMA Pediatr20141681687524276262
- LooJDConklinLFleming-DutraKESystematic review of the indirect effect of pneumococcal conjugate vaccine dosing schedules on pneumococcal disease and colonizationPediatr Infect Dis J201433Suppl 2S161S17124336058
- van HoekAJSheppardCLAndrewsNJPneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in EnglandVaccine201432344349435524657717
- Schmoele-ThomaBJacksonLAGreenbergRNImmunogenicity of 13-valent pneumococcal conjugate vaccine in immunocompetent older adults with stable underlying medical conditionsJ Vaccines Immun201532712
- van WerkhovenCHHuijtsSMBolkenbaasMGrobbeeDEBontenMJThe impact of age on the efficacy of 13-valent pneumococcal conjugate vaccine in elderlyClin Infect Dis201561121835183826265498
- FalkenhorstGRemschmidtCHarderTHummers-PradierEWichmannOBogdanCEffectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal disease in the elderly: systematic review and meta-analysisPLoS One2017121e016936828061505
- Kraicer-MelamedHO’DonnellSQuachCThe effectiveness of pneumococcal polysaccharide vaccine 23 (PPV23) in the general population of 50 years of age and older: a systematic review and meta-analysisVaccine201634131540155026899372
- SuzukiMDhoubhadelBGIshifujiTSerotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design studyLancet Infect Dis201717331332128126327
- Centers for Disease Control and PreventionUse of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Morb Mortal Wkly Rep2012614081681923051612
- KobayashiMBennettNMGierkeRIntervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Morb Mortal Wkly Rep2015643494494726334788
- EspositoSBonanniPMaggiSRecommended immunization schedules for adults: clinical practice guidelines by the ESCMID Vaccine Study Group (EVASG), European Geriatric Medicine Society (EUGMS) and the World Association for Infectious Diseases and Immunological Disorders (WAIDID)Hum Vaccin Immunother20161271777179427135390
- GreenbergRNGurtmanAFrenckRWSequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults 60–64 years of ageVaccine201432202364237424606865
- SchwarzTFFlamaingJRumkeHCA randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged ≥65 yearsVaccine201129325195520221619909
- FrenckRWJrGurtmanARubinoJRandomized, controlled trial of a 13-valent pneumococcal conjugate vaccine administered concomitantly with an influenza vaccine in healthy adultsClin Vaccine Immunol20121981296130322739693
- WoodheadMBlasiFEwigSGuidelines for the management of adult lower respiratory tract infections: full versionClin Microbiol Infect201117Suppl 6E1E59
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary DiseaseBethesda, MDGOLD2017
- MurphyTFSethiSBacterial infection in chronic obstructive pulmonary diseaseAm Rev Respir Dis19921464106710831416398
- WilsonRA vicious circle hypothesis operating during infective exacerbations of chronic bronchitisMonaldi Arch Chest Dis19944921591648049702
- AlfagemeIVazquezRReyesNClinical efficacy of anti-pneumococcal vaccination in patients with COPDThorax200661318919516227328
- FurumotoAOhkusaYChenMAdditive effect of pneumococcal vaccine and influenza vaccine on acute exacerbation in patients with chronic lung diseaseVaccine200826334284428918585831
- PoolePJChackoEWood-BakerRWCatesCJInfluenza vaccine for patients with chronic obstructive pulmonary diseaseCochrane Database Syst Rev20061CD00273316437444
- ChristensonBPauksenKSylvanSPEffect of influenza and pneumococcal vaccines in elderly persons in years of low influenza activityVirol J200855218442371
- GilchristSANanniALevineOBenefits and effectiveness of administering pneumococcal polysaccharide vaccine with seasonal influenza vaccine: an approach for policymakersAm J Public Health2012102459660522397339
- NicholKLThe additive benefits of influenza and pneumococcal vaccinations during influenza seasons among elderly persons with chronic lung diseaseVaccine199917Suppl 1S91S9310471189
- LaxminarayanRMatsosoPPantSAccess to effective antimicrobials: a worldwide challengeLancet20163871001416817526603918
- BraeterUSchulzMGoffrierBSchulzMIhlePBätzing-FeigenbaumJPneumococcal vaccine in GKV-insured persons in the age range of 60 to 64 years: regionalized analysis on the implementation of the recommendations of the Standing Committee on Vaccination Commission based on national contract medical billing data2016 German. Available from: https://www.versorgungsatlas.de/themen/alle-analysen-nach-datum-sortiert/?tab=6&uid=74Accessed October 11, 2017
- Carreno-IbanezLVEsteban-VasalloMDDominguez-BerjonMFCoverage of and factors associated with pneumococcal vaccination in chronic obstructive pulmonary diseaseInt J Tuberc Lung Dis201519673574125946369
- GieseCMereckieneJDanisKO’DonnellJO’FlanaganDCotterSLow vaccination coverage for seasonal influenza and pneumococcal disease among adults at-risk and health care workers in Ireland, 2013: the key role of GPs in recommending vaccinationVaccine201634323657366227255466
- GuthmannJPNational vaccination coverage survey, France, January 2011. Influenza vaccination coverage in target groups and measure of vaccine effectiveness: diphtheria-tetanus-poliomyelitis (dTP) and antipneumococcal vaccination coverage in persons aged 65 years old or above2011 French. Available from: http://invs.santepubliquefrance.fr/Publications-et-outils/Rapports-et-syntheses/Maladies-infectieuses/2011/Enquete-nationale-de-couver-ture-vaccinale-France-janvier-2011Accessed October 1, 2017
- SteensAVestrheimDFde BlasioBFPneumococcal vaccination in older adults in the era of childhood vaccination: public health insights from a Norwegian statistical prediction studyEpidemics201511243125979279
- Public Health EnglandPneumococcal polysaccharide vaccine (PPV) coverage report, England, April 2014 to March 20152015 Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/448406/hpr2615_ppv.pdfAccessed October 1, 2017
- IpsosPneuVUE: a new view into pneumonia among older adults2016 Available from: https://www.ipsos-mori.com/researchpublications/publications/1816/PneuVUE-A-New-View-into-Pneumonia-Among-Older-Adults.aspxAccessed January 12, 2017
- BlasiFAlibertiSBonanniPManteroMOdoneASignorelliCPneumococcal vaccination in adults: recommendations from the Italian Society of Respiratory Medicine (SIMeR) and the Italian Society of Hygiene, Preventive Medicine and Public Health (SItI)Epidemiol Prev2014386 Suppl 2147151 Italian25759360
- Portugal Directorate of Disease Prevention and Health Promotion ServicesVaccination against Streptococcus pneumoniae infections in groups at increased risk for invasive pneumococcal disease (IPD): adults (≥18 years of age)2015 Portuguese. Available from: https://www.dgs.pt/directrizes-da-dgs/normas-e-circulares-normativas/norma-n-0112015-de-23062015.aspxAccessed October 11, 2017
- Gonzalez-RomoFPicazoJJGarcia RojasAConsensus document on pneumococcal vaccination in adults at risk by age and underlying clinical conditions: 2017 updateRev Esp Quimioter2017302142168 Spanish28198169
- France Ministry of Social Affairs and HealthCalendar of vaccinations and vaccine recommendations 20172017 French. Available from: http://social-sante.gouv.fr/vaccins-vaccinationAccessed October 1, 2017
- Joint Committee on Vaccination and ImmunisationMinutes of the meeting on 4 February 20152015 Available from: https://app.box.com/s/iddfb4ppwkmtjusir2tc/1/2199012147/27417264008/1Accessed October 1, 2017
- Standing Committee on Vaccination (STIKO)Recommendations of the Standing Committee on Vaccination (STIKO) at the Robert Koch Institute – effective: August 20162016 Available from: http://www.rki.de/EN/Content/infections/Vaccination/recommandations/34_2016_engl.pdfAccessed October 1, 2017
- KimYKLaFonDNahmMHIndirect effects of pneumococcal conjugate vaccines in national immunization programs for children on adult pneumococcal diseaseInfect Chemother201648425726628032483
- WeyckerDBornheimerRSheaKMSatoRPeltonSIExcess exacerbations following pneumonia in adults with pre-existing heart failure (HF) or chronic obstructive pulmonary disease (COPD)Poster presented at: 10th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD)June 26–30, 2016Glasgow, Scotland
- SuayaJAJiangQBontenMPost-hoc analysis of the 13-valent polysaccharide conjugate vaccine efficacy against vaccine-serotype pneumococcal community acquired pneumonia in at-risk older adultsPoster 634, presented at: the 10th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD)June 29, 2016Glasgow. Scotland
