Abstract
Background
Blood eosinophil measurements may help to guide physicians on the use of inhaled corticosteroids (ICS) for patients with chronic obstructive pulmonary disease (COPD). Emerging data suggest that COPD patients with higher blood eosinophil counts may be at higher risk of exacerbations and more likely to benefit from combined ICS/long-acting beta2-agonist (LABA) treatment than therapy with a LABA alone. This analysis describes the distribution of blood eosinophil count at baseline in Japanese COPD patients in comparison with non-Japanese COPD patients.
Methods
A post hoc analysis of eosinophil distribution by percentage and absolute cell count was performed across 12 Phase II–IV COPD clinical studies (seven Japanese studies [N=848 available absolute eosinophil counts] and five global studies [N=5,397 available eosinophil counts] that included 246 Japanese patients resident in Japan with available counts). Blood eosinophil distributions were assessed at baseline, before blinded treatment assignment.
Findings
Among Japanese patients, the median (interquartile range) absolute eosinophil count was 170 cells/mm3 (100–280 cells/mm3). Overall, 612/1,094 Japanese patients (56%) had an absolute eosinophil count ≥150 cells/mm3 and 902/1,304 Japanese patients (69%) had a percentage eosinophil ≥2%. Among non-Japanese patients, these values were 160 (100–250) cells/mm3, 2,842/5,151 patients (55%), and 2,937/5,155 patients (57%), respectively. The eosinophil distribution among Japanese patients was similar to that among non-Japanese patients. Within multi-country studies with similar inclusion criteria, the eosinophil count was numerically lower in Japanese compared with non-Japanese patients (median 120 vs 160 cells/mm3).
Interpretation
The eosinophil distribution in Japanese patients seems comparable to that of non-Japanese patients; although within multi-country studies, there was a slightly lower median eosinophil count for Japanese patients compared with non-Japanese patients. These findings suggest that blood eosinophil data from global studies are of relevance in Japan.
Introduction
Post hoc analyses of two clinical trials showed that there is evidence that a higher blood eosinophil count may be predictive of a higher risk of exacerbations for chronic obstructive pulmonary disease (COPD) patients with an exacerbation history who were treated with long-acting beta2-agonists (LABA), without inhaled corticosteroids (ICS).Citation1,Citation2 Recent data suggest that patients with higher blood eosinophil levels may be more likely to benefit from combined ICS/LABA treatment than therapy with a LABA alone.Citation1,Citation3–Citation7 Moreover, the use of eosinophils as a potential biomarker to identify specific disease subtypes – or endotypes – for which treatment can be tailored to individual patients has recently been discussed for COPD and other chronic lung conditions.Citation8 For these reasons, blood eosinophil measurements have the potential to help guide physicians on the use of ICS with regard to exacerbation prevention, but, as pointed out in the recent Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, the data are not conclusive, and prospective clinical trials are needed before this can be recommended for daily clinical practice.Citation9
Ethnicity has been reported to have little impact on eosinophil levels,Citation10 but blood eosinophil levels can be elevated within and above the normal range in a number of conditions, including allergies and parasitic/fungal infections,Citation11,Citation12 and by external stimuli, such as smoking and exercise.Citation13,Citation14 However, blood eosinophil levels do not seem to be influenced by the use of ICS.Citation15,Citation16 As these external factors can vary between countries, it is possible that there may be differences in the distribution of eosinophil levels in patients with COPD between countries, although, in a post hoc analysis, the distribution of blood eosinophil counts in COPD patients in UK trials was similar to the distribution in patients from the rest of the world.Citation17
As current evidence supports eosinophils as a potential biomarker, the primary objective of this analysis was to investigate the distribution of blood eosinophil counts at baseline in randomized, controlled trials recruiting Japanese patients with COPD and compare the eosinophil distribution at baseline in Japanese versus non-Japanese patients.
Methods
Study design
A post hoc analysis of blood eosinophil distribution at baseline was performed using data from the GlaxoSmithKline COPD clinical trial database. All individual trials examined in this post hoc analysis were approved by the appropriate regulatory organizations.
The inclusion criteria used to select studies for this analysis were any GlaxoSmithKline Research and Development or local GlaxoSmithKline-sponsored Phase II–IV randomized clinical trials conducted from 1999 to 2016 that recruited Japanese patients and included any of GSK961081, fluticasone furoate, fluticasone propionate, salmeterol, umeclidinium, vilanterol, alone, or as a dual combination as a randomized study drug for COPD, and for which individual patient baseline or screening blood eosinophil counts were available. As individual patient data were not available in the published papers, non-GlaxoSmithKline and investigator-sponsored studies were not included. We also excluded studies reporting data from a single country only (outside Japan).
Across the individual studies included in this analysis, patient inclusion/exclusion criteria were mainly based on COPD diagnosis, forced expiratory volume in 1 second (FEV1) at entry, prior exacerbation history, previous/current diagnosis of asthma, smoking history, and reversible airflow limitation. None of the included studies used blood eosinophil count as an inclusion or exclusion criterion. A summary of the criteria for each trial is provided in Table S1 and further information on the designs of the trials is available on ClinicalTrials.gov.
The included studies were categorized into Japan-only studies and multi-country studies that recruited Japanese patients living in Japan. This is a post hoc analysis of study data, and, although the studies themselves all received ethical approval, the current analysis did not require additional ethical approval.
Assessments
Blood samples for eosinophil assessments were taken according to routine laboratory screening as described in each individual study protocol. The earliest individual patient pre-randomization eosinophil values, prior to any ICS or oral corticosteroid run-in, were selected for use in the analysis, although not all patients were ICS naïve at the time of recruitment. Data on the number of patients who were not ICS naïve at the time of recruitment in each study or in each group were not available for inclusion in these analyses. Values were presented as absolute blood eosinophil counts and percentage blood eosinophils.
Statistical analyses and data presentation
No formal statistical testing was performed. Japanese patient data from all of the studies were the primary focus of the analysis. Histograms of baseline eosinophil counts were produced. Summary tables of the proportion of patients by baseline absolute eosinophil subgroups (<100, ≥100; <150, ≥150; <300, ≥300 cells/mm3) and percentage eosinophil subgroups (<2%, ≥2%) were produced for Japanese patients only, both overall (Group 1: Japan-only studies combined with Japanese data from multi-country studies) and by study.
A cumulative density function was produced to enable a visual comparison of the distribution of absolute eosinophils. A line for Group 1 and each of the four following groups was overlaid into a single plot; Group 2 – all patient data from Japan-only studies; Group 3 – all patient data from multi-country studies; Group 4 – Japanese patient data from multi-country studies; Group 5 – non-Japanese patient data from multi-country studies.
Summary tables for Groups 1–5, based on absolute eosinophil count (<150, ≥150 cells/mm3) and percentage eosinophils (<2%, ≥2%), were also produced to compare baseline demographics and COPD disease characteristics (GOLD 2006 distribution [I–IV], St George’s Respiratory Questionnaire [SGRQ] score, and history of exacerbation). Data are presented from all Japanese patients (Group 1) alongside data from non-Japanese patients recruited to multi-country studies (Group 5).
Results
Included studies
In total, 12 studies (seven Japan-only; five multi-country) met the inclusion criteria and were included in this analysis (Table S1). The seven Japan-only studies were: SCO100646 (NCT0269126), SCO100648 (NCT0269087), HZC114156 (NCT1192191), DB2115362 (NCT1376388), AC4115361 (NCT1702363), SCO116571 (NCT1607398),Citation18 and SCO116717 (COSMOS-J; NCT1762800).Citation19 Study SCO116717 did not include data for absolute eosinophil count. The five multi-country studies included were: HZC112206 (NCT1053988),Citation20 HZC112207 (NCT1054885),Citation19 DB2113361 (NCT1313637),Citation21 DB2113373 (NCT1313650),Citation22 and AC4115408 (NCT1387230).Citation23
Trial participants
Absolute blood eosinophil count data were available from 6,245 trial participants, comprising 1,094 Japanese patients (848 from Japan-only studies; 246 from multi-country studies) and 5,151 non-Japanese patients from multi-country studies. Percentage blood eosinophil data were available from 6,459 patients, comprising 1,304 Japanese patients (1,058 from Japan-only studies; 246 from multi-country studies) and 5,155 non-Japanese patients.
Baseline demographics and disease characteristics of all Japanese patients and non-Japanese patients from multi-country studies are shown in patients with available absolute blood eosinophil count in and by percentage blood eosinophils and absolute eosinophils in Table S2. Baseline demographics and disease characteristics of patients in Japan-only studies according to absolute eosinophils are shown in Table S3. Baseline characteristics were broadly comparable among Japanese and non-Japanese patients for most categories (), as well as comparable across percentage eosinophil subgroups (<2%, ≥2%) within each of these two patient groups (Table S2). However, a higher proportion of Japanese patients were male, ≥65 years of age, or were exacerbation-free in the 12 months prior to the study, compared with non-Japanese patients, and slightly more non-Japanese patients had GOLD stage III disease than Japanese patients ( and S2). In addition, the SGRQ total score seemed lower in Japanese than in non-Japanese patients, although it should be noted that SGRQ data were only available for three of the multi-country studies, and patient numbers were low.
Table 1 Patient demographics
There was no apparent relationship between baseline eosinophil levels and exacerbation burden (Table S2), but no formal correlation analysis was performed.
Blood eosinophil distribution
A summary of absolute and percentage blood eosinophil counts in all five patient groups is shown in . Among Japanese patients, the median absolute blood eosinophil count was 170 cells/mm3, and 56% had an absolute blood eosinophil count ≥150 cells/mm3, with 69% having a percentage blood eosinophil ≥2%; among non-Japanese patients, these values were 160 cells/mm3, 55%, and 57%, respectively ().
Table 2 Summary of absolute and percentage blood eosinophil count across the five patient groups
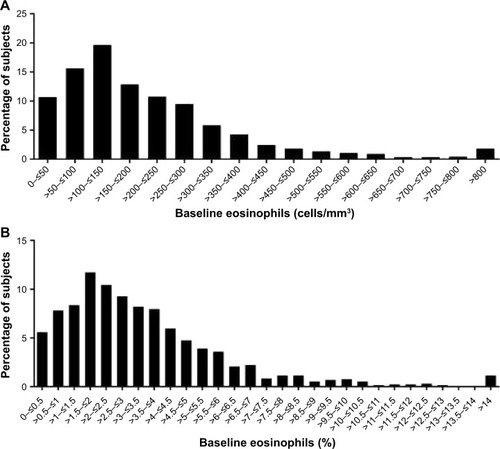
The distribution of absolute blood eosinophil count among Japanese patients was comparable to the distribution among non-Japanese patients (). However, the absolute blood eosinophil count was numerically lower in Japanese patients than in non-Japanese patients in the multi-country studies (median 120 cells/mm3 vs 160 cells/mm3, respectively) (). The distributions of absolute blood eosinophil count and percentage blood eosinophils among all Japanese patients are shown in .
Figure 1 Japan-specific data distribution for absolute blood eosinophil count compared with other groups.
Notes: An empirical cumulative distribution function plot was produced to evaluate the distribution of absolute blood eosinophil count data, with a line for each of the five patient groups overlaid into a single plot. The lines show the cumulative probability for subjects in the specified group. Japan only studies: AC4115361 (NCT1702363), DB2115362 (NCT1376388), HZC114156 (NCT1192191), SCO100646 (NCT0269126), SCO100648 (NCT0269087), and SCO116571 (NCT1607398). The absolute eosinophil values were not available for the Japan-only study, SCO116717 (NCT1762800). Multi-country studies: AC4115408 (NCT1387230), DB2113361 (NCT1313637), DB2113373 (NCT1313650), HZC112206 (NCT1053988), and HZC112207 (NCT1054885).
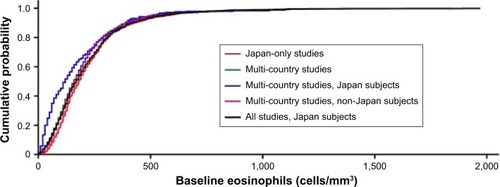
Figure 2 Distribution of (A) absolute blood eosinophil count and (B) percentage blood eosinophils among Japanese patients with COPD.
Abbreviations: COPD, chronic obstructive pulmonary disease; ITT, intention-to-treat.
Discussion
The primary objective of the current analysis was to investigate the distribution of baseline blood eosinophil counts in Japanese COPD patients across GlaxoSmithKlineResearch and Development or local GlaxoSmithKline sponsored Phase II–IV randomized clinical trials of COPD therapies conducted from 1999 to 2016 that recruited Japanese patients and had available individual patient baseline/screening blood eosinophil counts. We also evaluated blood eosinophil distribution in non-Japanese COPD patients in multi-country studies. While no formal statistical testing was performed, we found that the distribution of absolute blood eosinophil counts in Japanese COPD patients across all studies was similar to the distribution in non-Japanese patients. However, when considering the multi-country studies only, the median blood eosinophil count was numerically lower in Japanese than in non-Japanese COPD patients. This difference may represent a more reliable reflection of the differences between Japanese patients resident in Japan and non-Japanese patients recruited elsewhere in the world, as the inclusion criteria for Japanese and non-Japanese patients were the same within each multi-country trial. However, it should be noted that the sample size for this analysis was small.
One reason for the observed small difference in eosinophil count between Japanese and non-Japanese patients may be the differences in the way COPD patients are diagnosed in Western countries and in Japan. In Japan, there is a tendency to exclude patients with any features suggestive of asthma from a COPD diagnosis, and there is a lower diagnosis rate for COPD, especially in younger patients, with milder disease.Citation24–Citation27 Consistent with this, our analysis found that, compared with non-Japanese patients, a numerically higher proportion of Japanese patients overall and those in Japan-only studies were older (≥65 years), male, and with no recent history of exacerbations requiring corticosteroids and/or antibiotics. The greater predominance of older Japanese male COPD patients is typical of other Japanese studies in clinical settings.Citation26–Citation28 However, the data from the Japanese population in the current study show that, despite the exclusion of patients with asthma, a proportion of patients have high eosinophil counts. Of note, one included study (the Japanese COSMOS-J trial; Table S1),Citation18 of 400 patients with COPD, did not exclude patients with asthma.
Another possible reason for differences in eosinophil count between Japanese and non-Japanese patients may be due to local differences in the use of macrolides. Long-term prophylactic use of macrolides has been reported to reduce the frequency of exacerbations in patients with COPD,Citation29 and macrolides can reduce eosinophil count.Citation30 Unfortunately no data on the use of macrolides were available for patients in the included studies.
Data from two previous randomized, controlled trials indicate that patients with a blood eosinophil count ≥2% have a higher risk of exacerbation than patients with lower eosinophil levels.Citation1,Citation31 In the current analysis, there was no difference in the proportions of Japanese or non-Japanese patients with a prior history of COPD exacerbations between the <2% and ≥2% eosinophil count subgroups. Blood eosinophil levels have been proposed as a potential biomarker of responsiveness to ICS in patients with COPD.Citation1–Citation3 The ≥2% cutoff for raised eosinophil levels in COPD patients falls within what has traditionally been considered the normal range.Citation32 While the purpose of the current analysis was not to examine associations between ICS response and blood eosinophil counts in Japanese patients, the similar distributions observed in both Japanese and non-Japanese patients suggest that the same cutoff may be used in Japanese populations. However, this requires more formal testing in a comparative analysis of eosinophil response to ICS in Japanese patient populations.
A strength of the current analysis is that it included data from a relatively large sample of Japanese and non-Japanese patients across several studies. This may provide a more representative assessment of blood eosinophil levels than individual studies, as a variety of different studies with different inclusion criteria were included in the analysis. Furthermore, the data from the multi-country studies allowed for a direct comparison of Japanese and non-Japanese patients in studies that used comparable inclusion/exclusion criteria and with measurements obtained over similar periods of time.
The current analysis was limited in that it was a post hoc analysis of several trials with no formal statistical analysis and not a single directly comparative, randomized, clinical trial. The trials also included patients with a wide range of COPD severity and inflammatory conditions. Previous studies have indicated that combined ICS/LABA therapy may benefit COPD patients with higher eosinophil counts.Citation1,Citation3–Citation7 However, the current study populations mainly comprised patients with infrequent exacerbations and so may not be directly comparable to the populations examined in previous studies. Eosinophil counts may be influenced by smoking history and body mass index, but data on these variables were not available in all trials included in the analysis. A further study limitation is that the clinical diagnosis of COPD used to identify patients for inclusion into these trials was at the discretion of the investigator at the study site, as such there may be differences in the patient characteristics between Japan and other countries. As with all data obtained from randomized, controlled trials with stringent eligibility criteria, the current data may also not be truly generalizable to the wider population of COPD patients in Japan. Not all of the factors that can affect eosinophil cell distribution were investigated, nor were those that can affect eosinophil level (eg, comorbidity, atopy) in each study considered in a statistical manner. Finally, this analysis provides no measure of the stability of blood eosinophil levels in Japanese patients. Despite these limitations, the outcomes of this analysis suggest that blood eosinophil measurements are potential predictors of ICS responsiveness in COPD in Japanese patients.
Conclusion
Overall, the distribution of blood eosinophil counts in Japanese patients seems comparable with that of non-Japanese patients; although there was a slightly lower median eosinophil count for Japanese patients in multi-country studies compared with non-Japanese patients in the same studies. The current post hoc analysis provides support for further exploration of the utility of blood eosinophil counts in Japanese patients. Prospective studies examining the predictive validity of blood eosinophil counts as a marker of ICS responsiveness in Japanese COPD patients are warranted.
Previous presentation of data
These data were presented at a scientific meeting, the Japanese Respiratory Society 2017, held between 21 and 23 April, in a presentation: “Blood eosinophil levels in Japanese COPD patients” by Takeo Ishii, Dawn Midwinter, Mark James, Emma Hilton, Paul Jones, and Neil Barnes.
Author contributions
The authors were fully responsible for the decision to submit the article for publication and for all content and editorial decisions, were involved at all stages of manuscript development, approved the final version for submission, and have met the criteria for authorship as established by the ICMJE.
Acknowledgments
The authors are grateful to the participants in all studies for their participation and also thank all trial center staff and investigators. The authors would also like to thank the GlaxoSmithKline team members for their input into this manuscript. We also gratefully acknowledge Sandra Williams (Veramed, Stockley Park, UK) for assistance with programming.
All included studies (SCO100646 [NCT0269126], SCO100648 [NCT0269087], HZC114156 [NCT1192191], DB2115362 [NCT1376388], AC4115361 [NCT1702363], SCO116571 [NCT1607398], SCO116717 [NCT1762800], HZC112206 [NCT1053988], HZC112207 [NCT1054885], DB2113361 [NCT1313637], DB2113373 [NCT1313650], and AC4115408 [NCT1387230]), as well as the current post hoc analysis, were funded by GlaxoSmithKline.
The authors would like to acknowledge Angela Rogers, PhD, of Gardiner-Caldwell Communications, Macclesfield, UK, for medical writing support during the development of this manuscript, which was funded by GlaxoSmithKline.
Disclosure
All authors, except NH, are full-time employees of Glaxo SmithKline and hold GlaxoSmithKline shares. NH declares research grants to his department from GlaxoSmithKline, as well as consulting fees from GlaxoSmithKline and other pharmaceutical companies. The authors report no other conflicts of interest in this work.
References
- PascoeSLocantoreNDransfieldMTBarnesNCPavordIDBlood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trialsLancet Respir Med20153643544225878028
- SiddiquiSHGuasconiAVestboJBlood eosinophils: a bio-marker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2015192452352526051430
- PavordIDLettisSLocantoreNBlood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPDThorax201671211812526585525
- BafadhelMMcKennaSTerrySBlood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trialAm J Respir Crit Care Med20121861485522447964
- BafadhelMDaviesLCalverleyPMAaronSDBrightlingCEPavordIDBlood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysisEur Respir J201444378979124925917
- BarnesNSharmaRLettisSCalverleyPMBlood eosinophils as a marker of response to inhaled corticosteroids in COPDEur Respir J20164751374138226917606
- WatzHTetzlaffKWoutersEFBlood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trialLancet Respir Med20164539039827066739
- HizawaNClinical approaches towards asthma and chronic obstructive pulmonary disease based on the heterogeneity of disease pathogenesisClin Exp Allergy201646567868727009427
- Global Strategy for the Diagnosis, Management and Prevention of COPD [homepage on the Internet]Global Initiative for Chronic Obstructive Lung Disease (GOLD)2017 Available from: http://goldcopd.orgAccessed January 16, 2017
- BainBSeedMGodslandINormal values for peripheral blood white cell counts in women of four different ethnic originsJ Clin Pathol19843721881936693578
- ScottKAWardlawAJEosinophilic airway disordersSemin Respir Crit Care Med200627212813316612763
- MurphyKJaneway’s Immunobiology8th edNew YorkGarland Science2012
- SzeflerSJWenzelSBrownRAsthma outcomes: biomarkersJ Allergy Clin Immunol20121293 SupplS9S2322386512
- ChristensenRDHillHRExercise-induced changes in the blood concentration of leukocyte populations in teenage athletesAm J Pediatr Hematol Oncol1987921401422954481
- KreindlerJLLocantoreNWatkinsMLLettisSTal-SingerRMinimal effect of inhaled corticosteroids on blood eosinopil count in steroid-naïve COPD patients. Abstract No. 853109ERS International ConferenceAmsterdam2015
- KreindlerJLWatkinsMLLettisSTal-SingerRLocantoreNEffect of inhaled corticosteroids on blood eosinophil count in steroid-naïve patients with COPDBMJ Open Respir Res201631e000151
- HiltonEComptonCMidwinterDBarnesNThe distribution of blood eosinophil count in a COPD clinical trials database: comparing the UK with the rest of the worldThorax201671Suppl 3A159A160
- AsaiKKobayashiAMakiharaYJohnsonMAnti-inflammatory effects of salmeterol/fluticasone propionate 50/250 mcg combination therapy in Japanese patients with chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis20151080381125945045
- BetsuyakuTKatoMFujimotoKA study to assess COPD symptom-based management and to optimise treatment strategy in Japan (COSMOS-J) based on GOLD 2011Int J Chron Obstruct Pulmon Dis2013845345924124358
- SvedsaterHDalePGarrillKWalkerRWoepseMWQualitative assessment of attributes and ease of use of the ELLIPTA™ dry powder inhaler for delivery of maintenance therapy for asthma and COPDBMC Pulm Med2013137224314123
- CelliBCraterGKilbrideSOnce-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled studyChest2014145598199124385182
- DonohueJFMaleki-YazdiMRKilbrideSMehtaRKalbergCChurchAEfficacy and safety of once-daily umeclidinium/vilanterol62.5/25 mcg in COPDRespir Med2013107101538154623830094
- TrivediRRichardNMehtaRChurchAUmeclidinium in patients with COPD: a randomised, placebo-controlled studyEur Respir J2014431728123949963
- TakahashiTIchinoseMInoueHShiratoKHattoriTTakishimaTUnderdiagnosis and undertreatment of COPD in primary care settingsRespirology20038450450814629656
- FukuchiYNishimuraMIchinoseMCOPD in Japan: the Nippon COPD epidemiology studyRespirology20049445846515612956
- OmoriHNonamiYMiharaSMarubayashiTMorimotoYAizawaHPrevalence of airflow limitation on medical check-up in Japanese subjectsJ UOEH200729320921917900001
- OnishiKYoshimotoDHaganGWJonesPWPrevalence of airflow limitation in outpatients with cardiovascular diseases in JapanInt J Chron Obstruct Pulmon Dis2014956356824920894
- YoshimotoDNakanoYOnishiKHaganGJonesPThe relationship between the COPD Assessment Test score and airflow limitation in Japan in patients aged over 40 years with a smoking historyInt J Chron Obstruct Pulmon Dis201491357136325525353
- NiWShaoXCaiXProphylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysisPLoS One201510e012125725812085
- AmayasuHYoshidaSEbanaSClarithromycin suppresses bronchial hyperresponsiveness associated with eosinophilic inflammation in patients with asthmaAnn Allergy Asthma Immunol20008459459810875487
- BarnesNPavordIJonesPWBlood eosinophil count as a predictor of response to inhaled corticosteroids (ICS) in COPDAm J Respir Crit Care Med2015191A3975
- GeorgeLBrightlingCEosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary diseaseTher Adv Chronic Dis201671345126770668
