Abstract
Background
Respiratory parameters are important predictors of prognosis in the COPD population. Global Initiative for Obstructive Lung Disease (GOLD) 2017 Update resulted in a vertical shift of patients across COPD categories, with category B being the most populous and clinically heterogeneous. The aim of our study was to investigate whether respiratory parameters might be associated with increased all-cause mortality within GOLD category B patients.
Methods
The data were extracted from the Czech Multicentre Research Database, a prospective, noninterventional multicenter study of COPD patients. Kaplan–Meier survival analyses were performed at different levels of respiratory parameters (partial pressure of oxygen in arterial blood [PaO2], partial pressure of arterial carbon dioxide [PaCO2] and greatest decrease of basal peripheral capillary oxygen saturation during 6-minute walking test [6-MWT]). Univariate analyses using the Cox proportional hazard model and multivariate analyses were used to identify risk factors for mortality in hypoxemic and hypercapnic individuals with COPD.
Results
All-cause mortality in the cohort at 3 years of prospective follow-up reached 18.4%. Chronic hypoxemia (PaO2 <7.3 kPa), hypercapnia (PaCO2 >7.0 kPa) and oxygen desaturation during the 6-MWT were predictors of long-term mortality in COPD patients with forced expiratory volume in 1 second ≤60% for the overall cohort and for GOLD B category patients. Univariate analyses confirmed the association among decreased oxemia (<7.3 kPa), increased capnemia (>7.0 kPa), oxygen desaturation during 6-MWT and mortality in the studied groups of COPD subjects. Multivariate analysis identified PaO2 <7.3 kPa as a strong independent risk factor for mortality.
Conclusion
Survival analyses showed significantly increased all-cause mortality in hypoxemic and hypercapnic GOLD B subjects. More important, PaO2 <7.3 kPa was the strongest risk factor, especially in category B patients. In contrast, the majority of the tested respiratory parameters did not show a difference in mortality in the GOLD category D cohort.
Introduction
COPD is a major health problem affecting 11.7% of the global population and causing the death of about 3 million persons annually.Citation1 Currently, the Global Initiative for Obstructive Lung Disease (GOLD) introduced a new approach in COPD classification by using separate evaluations of spirometric values (stages 1–4) and the presence of symptoms and exacerbations (categories A–D).Citation1 Application of the new GOLD 2017 recommendations profoundly affected the distribution of patients in the A–D groups. An obvious consequence of the new classification is a vertical shift of a large portion of COPD patients from the C to the A group and from the D to the B group. Thus, more than half of the real-life COPD population represents substantially heterogeneous B category.Citation2
Several risk factors predictive of poor outcome have been identified for stratification of stable COPD patients. Lung function, represented by forced expiratory volume in 1 second (FEV1), has been the most widely used prognostic factor and still has an important role in the assessment of COPD patients.Citation1,Citation3–Citation6 Other prognostic factors associated with an increased risk of death include low exercise tolerance, a high degree of functional breathlessness and a low body mass index (BMI).Citation5 In 2004, Celli et al published an integrative, multidimensional prognostic model for COPD patients named the BODE index (BMI, Obstruction, Dyspnea and Exercise).Citation5 Subsequently, the COTE (COPD-specific comorbidity test) index, involving the BODE index and comorbidities assessment, has been introduced.Citation4 Other scoring instruments may also be predictive of poor outcomes. In a Swedish multicenter study, a Clinical COPD Questionnaire score higher than 2 was associated with a prognosis of higher mortality.Citation7 Since 2011, GOLD recommends stratification of COPD patients into A–D categories;Citation8 however, prognostic values of BODE and COTE indices have been found superior to GOLD 2011 A–D categories.Citation4 Two important studies revealed the shortcomings of GOLD 2011 classification. In the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study as well as in the Copenhagen City Heart Study, no advantages over GOLD 1–4 classification were demonstrated with the introduction of GOLD A–D categories in better predicting long-term mortality.Citation9,Citation10
The latest GOLD 2017 Update does not recommend the use of elementary respiratory parameters for disease classification or for mortality risk assessment or treatment strategy improvement.Citation1 However, chronic respiratory failure is a frequent feature (or rather consequence) of the disease, and the presence of chronic hypoxemia and/or hypercapnia is associated with remarkably higher mortality and morbidity.Citation3 Hypoxemia is a state in which partial pressure of oxygen in arterial blood (PaO2) is decreased below the reference values adjusted for age. Limits between normal and abnormal PaO2 decrease with age. Hypercapnia is defined by partial pressure of arterial carbon dioxide (PaCO2) >6 kPa.Citation11,Citation12
Various respiratory parameters have been assessed as potential risk factors for mortality by a large number of studies. However, there is limited evidence of how respiratory parameters affect outcome in specific subgroups of COPD subjects. The general purpose of the Czech Multicentre Research Database (CMRD) of the COPD project was to analyze the association among respiratory parameters, clinical phenotypes, GOLD categories and all-cause mortality in COPD individuals. The primary aim of the presented study was to assess selected respiratory parameters as potential predictors of death in COPD patients, classified according to the new GOLD 2017 strategy with emphasis on the largest population of COPD individuals: GOLD 2017 category B patients.
Methods
Study design
All patients for the study were recruited from the CMRD of COPD. This project (registered by the State Institute for Drug Control under the identifier 1301100001 and at ClinicalTrials.gov as NCT1923051) was initiated in August 2013.Citation13 The prospective CMRD study is being conducted in full accordance with Czech and European Union laws. The CMRD study and its protocol were approved by The Multicentre Ethical Committee of Masaryk University, Brno, Czech Republic (approval date: JAN-16-2013, protocol code: CHOPN) as well as ethics and regional review boards of all individual participating centers.Citation13 All COPD participants signed a written consent form before study enrolment.
Basic criteria for patient enrolment were respiratory physician’s diagnosis of COPD at least 12 months before enrolment, post-bronchodilator FEV1 ≤60%, exacerbation-free period for at least 8 weeks and patient’s written consent. We used the GOLD definition of COPD case, that is, a patient with confirmed post-bronchodilator airflow limitation (FEV1/forced vital capacity <0.70). Patient recruitment finished in December 2016. Longitudinal and prospective follow-up (in regular 6-month periods) of patients is planned for 5 consecutive years and will be finished in 2021.Citation13 At each control, the patients completed pulmonary function tests, an elementary physical examination, measurement of respiratory and nonrespiratory symptoms and systematic assessment of the patient’s history. Completing a 6-minute walking test (6-MWT) and/or an arterial blood gas (ABG) analysis was optional (not mandatory).Citation13 If done, ABG analysis and/or a 6-MWT were performed without oxygen supplementation in all cases. Any changes in medication, onset of new comorbidities or number of exacerbations were recorded. The prospective nature of the project enabled assessment of various outcomes, including long-term mortality and exacerbation rates, to follow the development of multiple comorbidities as well as to understand the natural evolution of the disease and its manifestations (represented by various clinical phenotypes and GOLD categories).Citation13
In this particular study, the following respiratory parameters were selected for mortality analyses: PaO2, PaCO2, arterial potential of hydrogen (pH), basal peripheral capillary oxygen saturation (SpO2), minimal SpO2 during a 6-MWT, greatest decrease in SpO2 during a 6-MWT and the presence of desaturation (ie, at least 4% drop and/or decrease of SpO2 <90% during 6-MWT).
Study population
Inclusion criteria for our analyses were regular follow-up in the CMRD. Exclusion criteria were the presence of sleep apnea syndrome or systolic pulmonary arterial hypertension (PAH) >60 mmHg (in patient history and/or echocardiographic finding of PAH >60 mmHg during the study enrolment).
Statistical analyses
For a basic description of the study population, categorical parameters are presented as absolute (relative) frequencies. Relative frequencies are calculated from valid N. Continuous variables are described by valid N, using mean with SD and median supplemented by 5th and 95th percentiles.
Kaplan–Meier curves illustrating 3-year survival were calculated for survival analysis of patients in the complete cohort along with groups A–D according to the GOLD 2016 and GOLD 2017 guidelines for these parameters: PaO2 (oxemia), PaCO2 (capnemia), arterial pH, basal SpO2, minimal SpO2 during (after) 6-MWT, greatest decrease of SpO2 during 6-MWT and the presence of desaturation during 6-MWT. The figures are supplemented by numerical data showing proportion of survival at 6, 12, 24 and 36 months of follow-up. Differences in survival between groups were tested by log-rank test.
Correlations of blood gases (PaO2, PaCO2, basal SpO2, minimal SpO2 during (after) 6-MWT and greatest decrease of SpO2 during 6-MWT) were analyzed by Spearman’s coefficient of correlation. In addition, the best calculated cutoff values of oxemia, capnemia, blood pH, basal SpO2, minimal SpO2 during (after) 6-MWT and greatest decrease of SpO2 during 6-MWT were calculated for prediction for mortality.
A Cox proportional hazard model was used to assess risk factors for mortality. Multivariate models analyzed other potential predictors of all-cause mortality for patient groups with hypoxemia, three levels of capnemia and desaturation during a 6-MWT.
Analyses were performed using SPSS Statistics 24.0 software with the level of significance at α=0.05.
Results
Of the 784 patients included in the CMRD (by December 2016), 53 patients were excluded because of the presence of sleep apnea syndrome and six patients because of severe PAH (). Of the remaining 725 patients, the inclusion criteria for the proposed analyses of ABGs were met in 391 patients and for SpO2 in 552 patients.
Figure 1 Flow chart of patients.
Abbreviation: CMRD, Czech Multicentre Research Database.
Patients’ characteristics
Basic demographic characteristics included sex, age at inclusion, age at COPD diagnosis, BMI and smoking status (). Seventy-two percent of the study population were men, 89% were past or current smokers, median age was 67.1 years and median BMI was 26.5. The most frequently reported symptoms were cough (72%), expectoration (58%) and fatigue (47%). Mean exacerbation rate was 1.2 events per year, one-third (0.4 event per year) of these requiring hospitalization. Lung function tests showed that the median FEV1 was 46% of predicted value (pred), and median transfer factor for carbon monoxide was 51% pred, whereas median distance covered during a 6-MWT was 359.5 m ().
Table 1 Basic characteristics of the study cohort – COPD patients (n=725)
Respiratory parameters
summarizes the results of ABG analyses and the results of pulse oximetry performed at rest and during a 6-MWT. Correlation analyses of respiratory parameters showed significant and strong correlation between PaO2 and basal and minimal SpO2 and a significant negative correlation between PaO2 and PaCO2 for the complete study cohort and for GOLD 2017 group B ().
Table 2 Respiratory parameters (n=725)
Table 3 Correlation of respiratory parameters (all patients and GOLD 2017 B patients)
Survival analyses
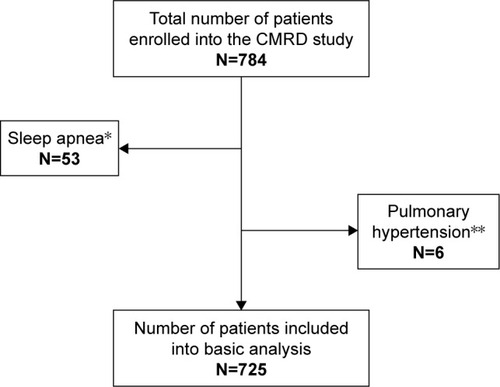
Of the tested respiratory parameters, minimal SpO2 during the 6-MWT yielded the highest ability to predict mortality (area under curve 0.631; p<0.001; ). Survival analyses showed significant differences in long-term all-cause mortality in relation to the selected respiratory parameters (PaO2, PaCO2 and desaturation during a 6-MWT; , and ).
Table 4 Prediction of all-cause mortality by respiratory parameters
Figure 2 (A) Long-term survival according to PaO2 (all patients); (B) long-term survival according to PaO2 (GOLD 2017 group B COPD subjects); (C) long-term survival according to PaO2 (GOLD 2017 group D COPD subjects).
Notes: *Number of patients with known follow-up. p-values <0.001, 0.001 respectively in bold represent significant survival difference between presence of severe hypoxemia and absence of severe hypoxemia in total COPD cohort, and in GOLD 2017 B category.
Abbreviations: GOLD, Global Initiative for Obstructive Lung Disease; PaCO2, partial pressure of arterial carbon dioxide.
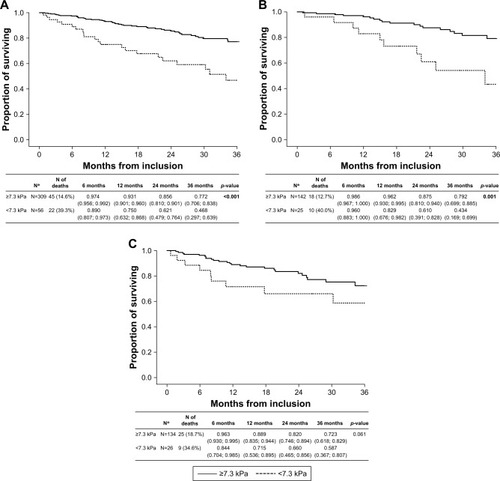
Figure 3 (A) Long-term survival according to PaCO2 (all patients); (B) long-term survival according to PaCO2 (GOLD 2017 group B COPD subjects); (C) long-term survival according to PaCO2 (GOLD 2017 group D COPD subjects).
Note: p-value <0.001 in bold represents significant survival difference between hypocapnic, normocapnic, and hypercapnic patients in GOLD 2017 B category only.
Abbreviations: GOLD, Global Initiative for Obstructive Lung Disease; PaCO2, partial pressure of arterial carbon dioxide.
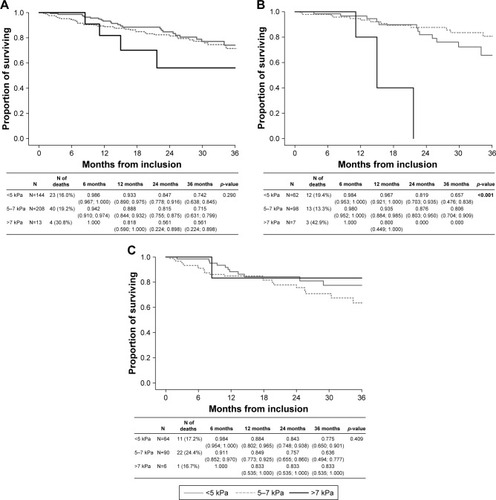
Figure 4 (A) Long-term survival according to desaturation (all patients); (B) long-term survival according to desaturation (GOLD 2017 group B COPD subjects); (C) long-term survival according to desaturation (GOLD 2017 group D COPD subjects).
Abbreviation: GOLD, Global Initiative for Obstructive Lung Disease.
PaO2
Significant association has been found between severe hypoxemia (PaO2 <7.3 kPa) and all-cause mortality in the complete COPD cohort (p<0.001; ) as well as in the GOLD 2017 B category (p=0.001; ). In contrast, severe hypoxemia (PaO2 <7.3 kPa) did not result in a significant difference in all-cause mortality in the COPD 2017 D category (p=0.061; ).
PaCO2
In GOLD 2017 category B patients, the highest survival rate was observed if the level of PaCO2 was 5–7 kPa. The presence of hypercapnia (PaCO2 >7 kPa) significantly increased (p<0.001) the mortality rate in this group. Interestingly, there was no effect of capnemia on all-cause mortality in the complete cohort (p=0.290) or in the GOLD 2017 category D group (p=0.409) ().
pH
pH was not found to be associated with increased risk for mortality in any of the tested patient groups.
Desaturation during 6-MWT
Desaturation was present in 46.4% of the study cohort (Table S1). The presence of desaturation was associated with increased mortality in the complete cohort (p=0.004) and in the GOLD 2017 B category (p=0.022). Desaturation was not associated with higher mortality in the GOLD 2017 D category (p=0.175; ).
Univariate analyses
Univariate analyses using the Cox model of proportional risk showed that different levels of oxemia and capnemia along with desaturation during a 6-MWT were risk factors for mortality in the complete cohort () as well as in the GOLD 2017 group B (). In contrast, we found no relationship between the tested parameters and mortality risk in the GOLD 2017 D category. Severe hypoxemia (PaO2 <7.3 kPa) has been identified as a strong predictor of all-cause mortality in the complete cohort (hazard ratio [HR] 3.064; p<0.001) as well as in the GOLD 2017 B category (HR 3.532; p=0.001). Similarly, severe hypercapnia (PaCO2 >7 kPa) has been identified as a strong predictor of all-cause mortality in the GOLD B category (HR 10.185; p=0.001). Blood pH was not associated with increased risk of death in any of the tested patient groups.
Table 5A Prediction of all-cause mortality by respiratory parameters – all patients
Table 5B Prediction of all-cause mortality by respiratory parameters – GOLD 2017 B patients
Multivariate analyses
A multivariate analysis containing patients with PaO2 ≤7.3 kPa identified PaO2 ≤7.3 kPa as a single and very strong independent risk factor for all-cause, long-term mortality (HR 2.398; 95% CI: 1.245–4.630) (). In an analysis containing three PaCO2 categories (<5, 5–7 and >7 kPa), none of the tested parameters showed as a significant, independent predictor of death (Table S2A). In a multivariate analysis containing desaturation during 6-MWT, only the BODE index was identified as an independent risk factor for all-cause mortality with HR 1.310 (95% CI: 1.168–1.470) (Table S2B).
Table 6 Prediction of all-cause mortality – multivariate analysis containing PaO2 (kPa) ≤7.3
Additional note: When ideal cutoff values for each of the studied parameter were calculated, PaO2 level <7.1 kPa yielded the highest sensitivity and specificity. By using the PaO2 level <7.1 kPa for a multivariate analysis, the HR of this independent risk factor was as high as 5.135 (95% CI: 2.415–10.917) (Table S2C and D). However, oxemia levels of 7.3 and 8.0 kPa are more relevant for clinicians; therefore, only these are further discussed in the paper.
Additional analyses: assessment of the role of comorbidities
The relationships between comorbidities and respiratory parameters in the CMRD study were also assessed (as complementary analyses). The self-reported history of heart failure, tumor and depression was associated with a greater probability of death during 3 years of follow-up (Table S3A). The presence of atopy was associated with higher levels of PaO2 (Table S3B). Personal history of heart failure, coronary artery disease and diabetes was associated with significantly lower PaO2 levels. Finally, levels of PaCO2 > or <5–7 kPa were associated with atopy and/or bronchial asthma, heart failure and/or diabetes (Table S3C).
Discussion
The most important finding of our study was that chronic hypoxemia (PaO2 <7.3 kPa) was a distinctive and very strong prognosis-modifying pattern associated with increased risk of long-term mortality in COPD group B patients. We observed significant differences in all-cause long-term mortality supported by results from univariate and multivariate analyses in the complete cohort and in groups B and D (GOLD 2017). However, the association was the strongest for COPD group B. This finding is very important for clinicians because group B currently represents the largest portion of COPD patients in real-life studies.Citation2 For this group, novel and easy-to-obtain parameters predictive of poor outcome are warranted. In our study, oxemia <7.3 kPa was the strongest independent predictor of mortality in COPD group B patients (using the GOLD 2017 Update). Where arterial blood gasometry is not available, desaturation during a 6-MWT – as an easier-to-obtain parameter (or a simple screening method) – may be used instead of ABG analysis, according to our results. This finding might be important in emergency cases or within areas where ABG analyzers are unavailable.
Our study cohort comprised 71.7% men; median age was 67.1 years, median FEV1 was 46% pred and median PaO2 was 8.8 kPa. Compared to other large cohorts (ECLIPSE, POPE, COPDGene, COCOMICS), the main differences were lower FEV1 and lower proportion of group A patients in favor of groups B and D.Citation14,Citation15 Moreover, a greater proportion of men were enrolled in our study cohort. The differences in rates of COPD groups A–D along with lower median FEV1 are consequences of different inclusion criteria for enrolment in the CMRD study; only patients with FEV1 ≤60% pred were included.Citation13 The CMRD study is focused on all-cause long-term mortality of COPD patients. Patients with a more pronounced impairment of lung function (FEV1 ≤60% pred) are at higher risk of death compared to patients with mild COPD. All 14 centers of the CMRD project represent university or tertiary-type hospitals taking care of nonmild COPD patients.Citation13 In addition, the absence of COPD patients with FEV1 >60% reduces the chance of mistaken enrolment of patients with transient, mild bronchial obstruction (eg, smoking asthmatics who can normalize lung function within a few months). Moreover, globally, milder COPD patients are often underdiagnosed.Citation2,Citation13 On the other hand, the FEV1 threshold in the CMRD study set at 60% pred allowed us to analyze also a substantial portion of GOLD stage 2 patients, not just GOLD stage 3 and 4 subjects. Thus, we believe that the CMRD study cohort (including GOLD stage 2–4 individuals) is representative of a real-life setting. The low number of patients and of deaths in groups A and C (resulting from the abovementioned facts) did not allow us to perform mortality analyses for these groups.
The proportions of GOLD categories (groups) in our study cohort were represented as follows: groups A+C 10.2%, group B 20.2% and group D 69.6% when GOLD 2016 classification was used. Application of the GOLD 2017 Update resulted in major shifts in the distribution of patients across A–D groups, that is, groups A+C 10.2% and group D 36.7%, whereas group B, at 53.0%, represented the most numerous disease category in our cohort. Similar results were published recently by Tudoric et al and Koblizek et al, who demonstrated the consequences of application of the GOLD 2017 Update on the POPE study population, comprising 3,361 COPD patients.Citation2,Citation15 The authors observed major shifts in the distribution of COPD groups A–D, resulting in making group B the most abundant. According to the GOLD 2016 guideline, group B was represented in 30.5% and group D in 57.3%.Citation2,Citation15 When applying the classification approach presented in the GOLD 2017 Update, 50.8% of patients were classified in B and 36.9% in group D.Citation2,Citation15 Importantly, the authors pointed out that 71.5% of the patients who shifted from group D to B used inhaled corticosteroids (ICS), and 18.4% of these group D-to-B shifters had severe airflow limitation (GOLD 4). In consequence, the shift to stage B in these patients may result in discontinuation of ICS treatment and/or in reduction of dual bronchodilator therapy to single bronchodilator use with potentially harmful consequences (ie, unstable COPD and risk of disease progression).Citation2 The authors concluded that the GOLD 2017 Update is relatively closer to the phenotypic approach in the disease management.Citation2 However, the above-mentioned shortcomings of the GOLD 2017 Update stress the need for identifying group B patients at higher risk of rapid disease progression and poor outcome.
Our data showed that a negative correlation exists among PaO2, basal SpO2 and minimal SpO2 on the one hand and PaCO2 on the other hand. This finding is in accordance with the differences in pathophysiology of both types of respiratory failure. Interestingly, some patients with the same disease develop only hypoxemia, whereas others also develop hypercapnia. Hypercapnia alone is rather a rare condition in the COPD population. Although both types of respiratory failure may coexist in a single patient (with increased probability in certain diseases, eg, in COPD patients), the underlying mechanisms of development of hypoxemia and hypercapnia exhibit differences. Hypercapnia is the respiratory expression of alveolar hypoventilation, and in COPD, it results dominantly from severe airflow limitation and hyperinflation.Citation16 Hypercapnia and respiratory acidosis may augment the decrease in respiratory muscle function because of the deleterious effect on mitochondrial function.Citation16 The most important mechanisms underlying the development of chronic hypoxemia include ventilatory/respiratory mismatch, right-to-left shunt, decreased/impaired diffusion, alveolar hypoventilation and hypoxia due to low oxygen intake.Citation11,Citation17 Hypoxemia increases ventilatory drive to increase PaO2 (thus decreasing PaCO2), induces regional pulmonary vasoconstriction and peripheral vasodilation (thus increasing heart rate and cardiac output) and stimulates erythropoiesis, resulting in enhanced oxygen-transporting capacity, although the hematologic viscosity rises.Citation18 In consequence, breathing becomes more difficult, and the cardiac workload increases.Citation18
In COPD patients, by far the most important determinant of hypoxemia is ventilatory/respiratory mismatch (V/Q mismatch).Citation11,Citation18 V/Q mismatch is the consequence of hypoxic pulmonary vasoconstriction that develops in areas with reduced ventilation (eg, emphysema).Citation11 In the COPDGene study, female sex, higher BMI and reduced FEV1 were associated with the development of chronic hypoxemia in COPD patients.Citation19
As demonstrated in our study, chronic hypoxemia is a major risk factor for mortality in COPD patients. Several other studies showed similar results.Citation20–Citation22 The severity of chronic hypoxemia is strengthened by the fact that long-term oxygen treatment (LTOT) may not decrease mortality in mild-to-moderate hypoxemic COPD patients.Citation23,Citation24 In the CMRD study, almost 11% of the included COPD individuals (78 out of 725) were treated with LTOT. The evidence for indication of LTOT is traditionally based on the results of three studies conducted in the 1970s.Citation25–Citation27 Recent research confirms that LTOT in stable COPD patients with moderate desaturation (ie, with mild-to-moderate chronic respiratory failure) does not provide any substantial benefit in relation to mortality, time to first hospitalization or any other followed endpoint.Citation24 However, for COPD patients with severe chronic hypoxemia, LTOT significantly reduces long-term mortality and remains one of the most important treatment options.Citation20 In a systematic review of randomized trials, no mortality benefit was observed if hypoxemia was present because of cause other than COPD or cardiogenic pulmonary edema.Citation28 Our results showed positive correlation between basal SpO2, minimal SpO2 and hypoxemia. The relationships between PaO2 and SpO2 were assessed in a Spanish study published in 2015.Citation29 The authors demonstrated that in patients with acute exacerbation of COPD (AE-COPD), SpO2 had a high correlation coefficient with PaO2 (0.89), and the optimal cutoff value for the detection of hypoxemia was SpO2 90%.Citation29
In our study, hypercapnia >7 kPa was predictive of poor outcome in Kaplan–Meier survival analyses and in univariate analyses. However, in multivariate analyses, PaCO2 failed to be an independent risk factor. These findings are in accordance with previous research. The prognostic value of carbon dioxide in the blood and hypercapnia were much weaker than that of hypoxemia in relation to mortality. Jones et al reported PaCO2 to be a significant predictor of long-term mortality in COPD patients.Citation30 Foucher et al reported 30%–40% two-year mortality of COPD patients with chronic hypercapnia.Citation31 Chailleux et al found hypercapnia associated with higher mortality in COPD patients receiving LTOT at the 3-year follow-up.Citation32 Ahmadi et al referred to the U-shaped association between capnemia and mortality, with values >7.0 and <5.0 kPa at increased risk of death.Citation27 However, Aida et al found no association between capnemia and mortality.Citation33
Research data supporting the prognostic value of capnemia are more consistent for acute hypercapnia.Citation12,Citation34–Citation36 Lun et al reported association between hypercapnia and respiratory acidosis during AE-COPD with higher risk of future life-threatening events and mortality.Citation12 Acute hypercapnia during AE-COPD has been found as a significant prognostic factor of long-term mortality in a number of studies.Citation34–Citation36
In COPD patients with chronic respiratory failure, acute respiratory failure is the most common cause of death, followed by cardiovascular causes, respiratory infection and cancer.Citation3,Citation37,Citation38 Acute respiratory failure is frequently associated with exacerbations (and vice versa).Citation38 In-hospital mortality of patients with AE-COPD and acute respiratory failure was only 2.5% in a cohort examined by Patil et al,Citation39 but 20.3% in a study by Breen et al.Citation40 In the same study, postdischarge mortality at 3 years was 63.5%.Citation40 In-hospital mortality of mechanically ventilated patients with acute respiratory failure ranges between 21% and 82%, according to the results of various studies.Citation41 The association between acute/chronic respiratory failure and mortality applies despite the discovery of several prognosis-modifying treatments and strategies for COPD in the last decades, including ICS,Citation42 their combination with long-acting bronchodilatorsCitation43 or noninvasive ventilation.Citation44 Considering the data obtained from the ECLAIR study, extracorporeal carbon dioxide removal for acute hypercapnic respiratory failure has been found to be neither an effective nor a safe procedure.Citation45
Our study has several limitations. The first one is the preselection bias caused by inclusion of patients with post-bronchodilator FEV1 ≤60% only. Seventy-two percent of the study population were men, which might introduce another bias (gender). In the study cohort, only a minimum (ca. 10% in total) of group A and group C patients were present. In consequence, the number of deaths for these groups was so low that it did not allow us to perform mortality analyses. Another important limitation is related to relatively lower availability of ABG (54% of patients) and 6-MWT data (76% of patients) from the CMRD study cohort. The primary aim of the CMRD study was to observe the rate of all-cause mortality in a real-life COPD population. Monitoring of respiratory parameters (ABG and SpO2 during 6-MWT) was considered an additional and a nonmandatory component only. This might bias the composition of the current study cohort because more expressed impairment of lung function and more frequent hospitalization because of COPD exacerbation (before enrolment) might slightly increase the patient’s chance of having ABG analysis. In contrast, better lung functions were associated with a gently higher frequency of 6-MWT being performed. According to our ex-post analysis, the differences between these groups were minimal (Table S4). Moreover, of the 725 enrolled subjects, SpO2 was measured during a mandatory physical exam and the results strongly correlated with SpO2 assessed during a 6-MWT (Table S5A and B).
Despite these limitations, we believe that we demonstrated the importance and the prognostic role of respiratory parameters, particularly of PaO2 <7.3 kPa in COPD category B patients (GOLD 2017 Update).
Conclusion
Our results show that certain respiratory parameters are associated with increased risk of death among patients in different COPD categories. Of the tested parameters, severe hypoxemia (PaO2 <7.3 kPa) was identified as the strongest risk factor for long-term, all-cause mortality in the complete cohort as well as in group B (using the GOLD 2017 Update). The importance of this finding is underlined by the fact that group B seems to be the largest group of COPD individuals in real practice.Citation2 In emergency cases, SpO2 may be used to determine the presence of hypoxemia. Undoubtedly, for exact PaO2 measures, arterial blood gasometry should be performed.
Another important observation is that COPD category D (GOLD 2017 Update) now seems to be a well-defined group with the highest rate of long-term mortality and a minimum of risk-modifying signs and factors.
Acknowledgments
We thank the physicians of participating centers of the Czech multicenter research database of severe COPD, namely, Tomas Dvorak – Pulmonary Department, Hospital Mlada Boleslav, Petr Safranek – Pulmonary Department, University Hospital, Plzen, Ondrej Sobotik – Pulmonary Department, University Hospital Motol, Prague, Maria Majerciakova – Pulmonary Department, Hospital of St Svorad, Nitra, Slovakia, Jaroslav Lnenicka – Pulmonary Department, Masaryk Hospital, Usti nad Labem, Pavlina Musilova – Pulmonary Department, Jihlava Hospital, Barbora Novotna – Pulmonary Department, Bulovka Hospital, Prague, Zuzana Liptakova – Pulmonary Department, Ceske Budejovice Hospital, Eva Kocova, Michal Kopecky, Sarka Pracharova, Libor Nevoranek and Lukas Varhanik – University Hospital Hradec Kralove, Katerina Neumannova – Palacky University, Olomouc and Milada Sipkova – Pulmonary Department, Liberec Hospital. Special thanks to Ondrej Zindr – Karlovy Vary for English corrections. This research was funded by Ministry of Health of the Czech Republic (15/14/NAP, 5/15/NAP, and UHHK, 00179906), moreover by consortium of several pharmaceutical companies (Angelini CZ, AstraZeneca CZ, Boehringer Ingelheim CZ, Cipla CZ, CSL Behring CZ, GSK CZ, Novartis CZ and Sandoz CZ).
Supplementary materials
Table S1 Frequency of desaturation (n=552)
Table S2 (A) Prediction of all-cause mortality – multivariate analysis containing PaCO2 (kPa) – categories (<5; 5–7 – reference; >7). (B) Prediction of all-cause mortality – multivariate analysis containing desaturation. (C) Prediction of mortality by parameters of blood gases – ideal cutoff values. (D) Prediction of mortality – multivariate analysis containing PaO2 (kPa) ≤7.1.
Table S3 Relationship between comorbidities and all-cause mortality (A); relationship between comorbidity and PaO2 (B); relationship between comorbidity and PaCO2 (C)
Table S4 Comparison of parameters between groups according to valid data (n=725)
Table S5 SpO2 according to physical examination (n=725) (A), correlation between SpO2 (physical examination*) and SpO2 (6-MWTTable Footnote°) (n=552) (B)
Disclosure
M Plutinsky has received payments on COPD lectures from Boehringer Ingelheim. P Popelkova has received consulting/lectures payment from AstraZeneca, Boehringer Ingelheim and Novartis regarding the COPD field within past 36 months. J Zatloukal has received payment related to COPD clinical trials from AstraZeneca, GSK and Novartis within past 36 months, and received consulting/lectures payment from AstraZeneca, Novartis, Angelini and Berlin-Chemie regarding the COPD field within past 36 months. E Volakova has received COPD research funding from GSK within past 36 months, and received consulting/lectures payment from Boehringer Ingelheim and Berlin-Chemie regarding the COPD field within past 36 months. L Heribanová has received COPD research funding from AstraZeneca within past 36 months. V Koblizek has received COPD research funding from Boehringer Ingelheim, and Novartis within past 36 months, and received consulting/lectures payment from Angelini, AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, GSK, Mundipharma, Novartis and Sandoz regarding the COPD field within past 36 months. K Brat, K Hejduk, M Svoboda and M Fecaninova have not received any payments within past 36 months. The authors report no other conflicts of interest in this work.
References
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD)2017 Available from: http://goldcopd.orgAccessed May 30, 2017
- TudoricNKoblizekVMiravitllesMGOLD 2017 on the way to a phenotypic approach? Analysis from the Phenotypes of COPD in Central and Eastern Europe (POPE) CohortEur Respir J2017494160251828446560
- SinDDAnthonisenNRSorianoJBAgustiAGMortality in COPD: role of comorbiditiesEur Respir J20062861245125717138679
- de TorresJPCasanovaCMarínJMPrognostic evaluation of COPD patients: GOLD 2011 versus BODE and the COPD comorbidity index COTEThorax201469979980424969641
- CelliBRCoteCGMarinJMThe body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary diseaseN Engl J Med2004350101005101214999112
- SteerJGibsonGJBourkeSCPredicting outcomes following hospitalization for acute exacerbations of COPDQJM20101031181782920660633
- SundhJJansonCLisspersKMontgomerySStällbergBClinical COPD Questionnaire score (CCQ) and mortalityInt J Chron Obstruct Pulmon Dis2012783384223277739
- Global Strategy for Diagnosis, Management, and Prevention of COPD2016 Available from: http://goldcopd.orgAccessed May 30, 2017
- AgustiAEdwardsLDCelli B; for ECLIPSE InvestigatorsCharacteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohortEur Respir J201342363664623766334
- LangePMarottJLVestboJPrediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general populationAm J Respir Crit Care Med20121861097598122997207
- SarkarMNiranjanNBanyalPKMechanisms of hypoxemiaLung India2017341476028144061
- LunCTTsuiMSChengSLDifferences in baseline factors and survival between normocapnia, compensated respiratory acidosis and decompensated respiratory acidosis in COPD exacerbation: a pilot studyRespirology201621112813626603971
- NovotnaBKoblizekVZatloukalJCzech multicenter research database of severe COPDInt J Chron Obstruct Pulmon Dis201491265127425419124
- AgustiAHurdSJonesPFAQs about the GOLD 2011 assessment proposal of COPD: a comparative analysis of four different cohortsEur Respir J20134251391140123645406
- KoblizekVMilenkovicBBarczykAPhenotypes of COPD patients with a smoking history in Central and Eastern Europe: the POPE StudyEur Respir J2017495160144628495687
- SlenterRHSprootenRTKotzDWesselingGWoutersEFRohdeGGPredictors of 1-year mortality at hospital admission for acute exacerbations of chronic obstructive pulmonary diseaseRespiration2013851152623037178
- PanosRJEschenbacherWExertional desaturation in patients with chronic obstructive pulmonary diseaseCOPD20096647848719938972
- CorradoARendaTBertiniSLong-term oxygen therapy in COPD: evidences and open questions of current indicationsMonaldi Arch Chest Dis2010731344320499792
- KimDKJacobsonFLWashkoGRClinical and radiographic correlates of hypoxemia and oxygen therapy in the COPDGene studyRespir Med201110581211122121396809
- KimVBendittJOWiseRASharafkhanehAOxygen therapy in chronic obstructive pulmonary diseaseProc Am Thorac Soc20085451351818453364
- KentBDMitchellPDMcNicholasWTHypoxemia in patients with COPD: cause, effects, and disease progressionInt J Chron Obstruct Pulmon Dis2011619920821660297
- AanerudMSaureEWBenetMSerial measurements of arterial oxygen tension are associated with mortality in COPDCOPD201512328729425230156
- GóreckaDGorzelakKSliwińskiPTobiaszMZielińskiJEffect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemiaThorax19975286746799337824
- AlbertRKAuDHBlackfordALfor Long-Term Oxygen Treatment Trial Research GroupA randomized trial of long-term oxygen for COPD with moderate desaturationN Engl J Med2016375171617162727783918
- Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial GroupAnn Intern Med19809333913986776858
- Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working PartyLancet1981182226816866110912
- AhmadiZBornefalk-HermanssonAFranklinKAMidgrenBEkströmMPHypo- and hypercapnia predict mortality in oxygen-dependent chronic obstructive pulmonary disease: a population-based prospective studyRespir Res2014153024625018
- KeenanSPSinuffTCookDJHillNSDoes noninvasive positive pressure ventilation improve outcome in acute hypoxemic respiratory failure? A systematic reviewCrit Care Med200432122516252315599160
- Garcia-GutierrezSUnzurrunzagaAArosteguiIfor IRYSS-COPD groupThe use of pulse oximetry to determine hypoxemia in acute exacerbations of COPDCOPD201512661362025774875
- JonesNLBurrowsBFletcherCMSerial studies of 100 patients with chronic airway obstruction in London and ChicagoThorax19672243273356035796
- FoucherPBaudouinNMeratiMRelative survival analysis of 252 patients with COPD receiving long-term oxygen therapyChest19981136158015879631797
- ChailleuxEFaurouxBBinetFDautzenbergBPoluJMPredictors of survival in patients receiving domiciliary oxygen therapy or mechanical ventilation. A 10-year analysis of ANTADIR ObservatoryChest199610937417498617085
- AidaAMiyamotoKNishimuraMAibaMKiraSKawakamiYPrognostic value of hypercapnia in patients with chronic respiratory failure during long-term oxygen therapyAm J Respir Crit Care Med199815811881939655728
- Ai-PingCLeeKHLimTKIn-hospital and 5-year mortality of patients treated in the ICU for acute exacerbation of COPD: a retrospective studyChest2005128251852416100133
- GalliJAKrahnkeJSJames MamaryAShenoyKZhaoHCrinerGJHome non-invasive ventilation use following acute hypercapnic respiratory failure in COPDRespir Med2014108572272824702885
- ChuCMChanVLLinAWWongIWLeungWSLaiCKReadmission rates and life threatening events in COPD survivors treated with non-invasive ventilation for acute hypercapnic respiratory failureThorax200459121020102515563699
- ZielinskiJMacNeeWWedzichaJCauses of death in patients with COPD and chronic respiratory failureMonaldi Arch Chest Dis199752143479151520
- VidalSGonzálezNBarrioIfor Investigación en Resultados y Servicios Sanitarios (IRYSS) COPD GroupPredictors of hospital admission in exacerbations of chronic obstructive pulmonary diseaseInt J Tuberc Lung Dis201317121632163724200281
- PatilSPKrishnanJALechtzinNDietteGBIn-hospital mortality following acute exacerbation of chronic obstructive pulmonary diseaseArch Int Med2003163101180118612767954
- BreenDChurchesTHawkerFTorzilloPJAcute respiratory failure secondary to chronic obstructive pulmonary disease treated in the intensive care unit: a long term follow up studyThorax2002571293311809986
- NevinsMLEpsteinSKPredictors of outcome for patients with COPD requiring invasive mechanical ventilationChest200111961840184911399713
- SinDDWuLAndersonJAInhaled corticosteroids and mortality in chronic obstructive pulmonary diseaseThorax2005601299299716227327
- KliberALyndLDSinDDThe effects of long-acting bronchodilators on total mortality in patients with stable chronic obstructive pulmonary diseaseRespir Res2010115620459831
- DretzkeJBlissettDDaveCThe cost-effectiveness of domiciliary non-invasive ventilation in patients with end-stage chronic obstructive pulmonary disease: a systematic review and economic evaluationHealth Technol Assess201519811246
- BrauneSSiewekeABrettnerFThe feasibility and safety of extracorporeal carbon dioxide removal to avoid intubation in patients with COPD unresponsive to noninvasive ventilation for acute hypercapnic respiratory failure (ECLAIR study): multicentre case-control studyIntensive Care Med20164291437144427456703
