Abstract
Background
Inhaled medication is central to the treatment of COPD. Various types of inhaler devices, which directly deliver medication to the lung, have been developed. However, patients often exhibit incorrect techniques of inhaler usage. Effectiveness of therapy may be affected by the ease of device usage, size, convenience of use, durability, clarity of instructions and device preferences of patients. This study compares the satisfaction and preference, as well as error occurrence, with the use of Genuair®, Ellipta™ and Breezhaler™ by healthy subjects in Hong Kong.
Subjects and methods
One hundred and thirty healthy Hong Kong Chinese subjects aged ≥40 years without a previous diagnosis of COPD and asthma and with no experience of using dry powder inhalers (DPIs) were recruited. Subjects learned to use the three DPIs by initially reading the instructions and then observing a demonstration with verbal explanation. The number of errors committed was evaluated. Subjects also completed a questionnaire to indicate their satisfaction and preference.
Results
The satisfaction score of comfort for Breezhaler was significantly higher than that for Ellipta (p≤0.05), while the satisfaction score on confidence to have inhaled the entire dose was highest for Genuair compared with Ellipta (p≤0.0001) or Breezhaler (p≤0.05). The overall satisfaction score was significantly higher for Genuair than Ellipta (p≤0.05) or Breezhaler (p≤0.01). After reading the instructions, the highest number of subjects committing one or more critical errors was with Breezhaler (97) followed by Genuair (70) and then Ellipta (33). Demonstration reduced the number of critical errors made by subjects for each DPI to one third or lower.
Conclusion
Breezhaler seemed to be more comfortable and easy to carry, but users made less critical errors when using Ellipta after reading the instructions only. Genuair provided the clearest indication of correct dose preparation and inhalation.
Introduction
Inhaled medications, including short-acting and long-acting bronchodilators as well as inhaled corticosteroids, are central to the management of COPD. Various types of inhalers have been developed to enable direct delivery of inhaled medications to the lung, thus minimizing unwanted systemic effects and allowing smaller doses to be used with faster onset of drug action;Citation1–Citation4 for instance, the pressurized metered-dose inhalers, soft-mist inhalers, dry powder inhalers (DPIs) and nebulizers. DPIs and soft-mist inhalers represent significant improvements from pressurized metered-dose inhalers, as they do not contain propellant gases and importantly are breath-actuated, circumventing the need for coordination of inhaler actuation and inspiration by the patient.Citation3,Citation5,Citation6 However, only 40%–60% of COPD patients adhered to their prescribed therapyCitation7 and, depending on the types of inhalers, between 4% and 94% of patients exhibited incorrect techniques of inhaler device usage.Citation8 Although patients claimed that their techniques of inhaler use is adequate, as many as 94% still committed at least one error when they were asked to demonstrate the correct techniques,Citation9 showing the need for patient education and critical assessments on inhaler device usage by health care professionals. Moreover, the risk of COPD increases by 5-fold for people aged over 65 years as compared to those under 40 years of age.Citation10 Elderly patients are more likely to have reduced dexterity and cognitive function, which limit their ability to correctly operate inhalers. Inadequate adherence to prescribed regimens and failure to use the correct technique result in reduced effectiveness of therapy.Citation11,Citation12 Factors affecting patient satisfaction and preference of inhalers include ease of use, size, convenience, durability and clarity of instructions.Citation13,Citation14
The three most recently launched DPIs for COPD were studied – Genuair® (AstraZeneca, Cambridge, UK), Ellipta™ (GlaxoSmithKline, Brentford, UK) and Breezhaler™ (Novartis, Basel, Switzerland). Genuair is a multidose, medium-resistance DPI for the delivery of aclidinium bromide, a long-acting muscarinic agonist (LAMA), or aclidinium bromide in combination with formoterol fumarate dihydrate, a long-acting beta agonist (LABA).Citation14,Citation15 It provides both visual and acoustic feedback to patients to indicate correct inhalation of the dose.Citation16 Ellipta is also a multidose DPI for the delivery of umeclidinium bromide (LAMA) with vilanterol (LABA), or fluticasone furoate, an inhaled corticosteroid, and vilanterol (LABA), or umeclidinium bromide (LAMA) alone.Citation17 Breezhaler is a single-dose capsule inhaler for the delivery of glycopyrronium bromide (LAMA) with indacaterol (LABA), or glycopyrronium bromide (LAMA) alone, or indacaterol (LABA) alone. Inhalation causes the capsule to vibrate in the Breezhaler device and such vibrations act as a form of acoustic feedback for the release of medication from the device.
The objectives of this study were to compare the satisfaction and preference, as well as error occurrence with use of these three DPI devices by healthy subjects in Hong Kong.
Subjects and methods
Subjects and study design
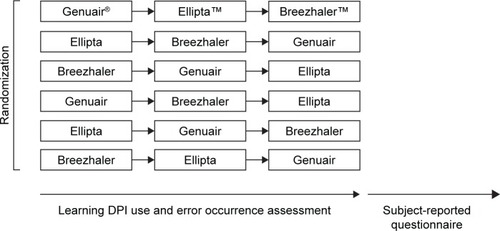
This was a single-centred, randomized, closed-labeled study. One hundred and thirty healthy Hong Kong Chinese subjects aged ≥40 years who had no previous diagnosis of COPD or asthma and no experience of using DPIs were recruited to the study, thus ruling out any bias on inhaler operation. Subjects who had any condition which impaired their ability to operate the inhaler, who had any history of chronic respiratory diseases, and who were unable to read the instructions, answer subject-reported questionnaires or give written informed consent were excluded from the study. Subjects were randomized with respect to the sequence of learning and the use of the three placebo DPI devices, namely, Genuair, Ellipta and Breezhaler, such that each subject undertook one of the sequences depicted in .
Figure 1 A schematic diagram showing randomization and sequence of learning to use the different DPI devices.
Placebo devices of Genuair and Ellipta did not contain any active medication or powder, and in the case of the Breezhaler, empty capsules were used in place of medication-containing capsules. The brand names of the inhalers were not disclosed to the subjects. Subjects were first asked to read a set of written instructions for one of the DPIs and then demonstrate the use of the DPI to an assessor. The demonstration was evaluated by the assessor who noted the errors committed based on a checklist (Table S1). Subjects were then given a physical demonstration by the assessor on how to use the DPI with a pre-recorded verbal explanation. Subjects were asked to demonstrate the use of the DPI for a second time, while the assessor observed and noted any error committed. The two learning stages were repeated for the remaining two inhalers. The errors were classified into critical and non-critical errors (Table S1). Critical errors were defined as errors which prevented the subjects from inhaling any of the medications contained in the DPI. Non-critical errors were defined as errors which caused suboptimal administration of the drug or use of the DPI, although the subject could still inhale part of the dose. One main assessor and two assistants were employed to evaluate the errors. The main assessor demonstrated the borderline actions between the correct actions and errors to the assistant. All of them assessed several subjects together at the beginning of the project to ensure the standard was agreed. Then the three assessors evaluated the errors individually. After the subjects had demonstrated the use of all three DPIs, they completed a questionnaire to indicate their level of satisfaction and preference for various attributes of the three inhalers, using a Likert-type scale with a score from 1 to 7. Subjects were also asked, using a 100-point scale for overall satisfaction, to indicate how willing they would be to use each DPI if they were diagnosed with COPD.
The study protocol was approved by the Research Ethics Subcommittee of the University of Hong Kong School of Professional and Continuing Education. Written consent was provided by each subject prior to the commencement of the study procedure.
Statistical analyses
Age of subjects and satisfactory scores were presented as mean ± standard error of the mean. The occurrence of one or more critical errors during the use of each DPI was analyzed by McNemar’s test. Briefly, the three DPIs were compared in a pair-wise manner, that is, Genuair vs Ellipta, Ellipta vs Breezhaler and Genuair vs Breezhaler. In each comparison between two DPIs A vs B, subjects were classified into four groups: 1) committing critical errors when using both A and B, 2) committing critical errors when using A only (the number of which is designated a), 3) committing critical errors when using B only (designated b) and 4) committing no errors when using A or B. The chi-square statistics was calculated by the formula (a−b)2/(a+b).
Satisfaction scores for each attribute and overall satisfaction scores for each DPI were analyzed for statistical significance by one-way analysis of variance followed by post hoc Tukey’s test. For comparisons of scores for each attribute between male and female subjects and between subjects <60 and ≥60 years of age, two-way analysis of variance followed by Sidak’s multiple comparisons test was used. Minimally important difference was analyzed by comparing the differences in satisfactory score for each attribute between DPIs with the SDs of the scores, with the differences >0.2 SD and <0.5 SD being a small effect and ≥0.5 SD and <0.79 SD being a medium effect.Citation18 Distribution of DPI preference was analyzed by the procedure described in Sharpe’s studyCitation19 for deviation from uniform distribution by the chi-square goodness-of-fit tests. In order to determine the DPI causing the deviation, we computed the standardized residuals, R. When the absolute value of the standardized residual exceeds 2, this shows lack of fit in the cells, with p≤0.05.Citation20
Results
Subjects
Data on satisfaction, preference and error occurrence for the three DPIs were collected from a total of 130 healthy Chinese subjects (aged 41–84 years, mean age=59.82±0.88 years) consisting of 61 males (aged 42–84 years, mean age=60.98±0.93 years) and 69 females (aged 41–79 years, mean age=58.78±0.82 years).
Satisfaction
Subjects were asked to evaluate and indicate their satisfaction on various attributes of the three DPIs using a seven-point Likert-type scale (). In terms of comfort of the DPIs, the satisfaction score for Breezhaler was significantly higher than that for Ellipta (5.13±0.13 vs 4.70±1.65, p=0.047). However, the satisfaction score for Breezhaler in terms of ease of dose preparation was lowest among the DPIs, being significantly lower than those of both Ellipta (4.75±0.15 vs 5.24±0.15, p=0.0463) and Genuair (4.75±0.15 vs 5.39±0.13, p=0.0038).
Figure 2 Satisfaction scores on different attributes of the three DPIs using a seven-point Likert-type scale.
Abbreviations: DPIs, dry powder inhalers; SEM, standard error of the mean.
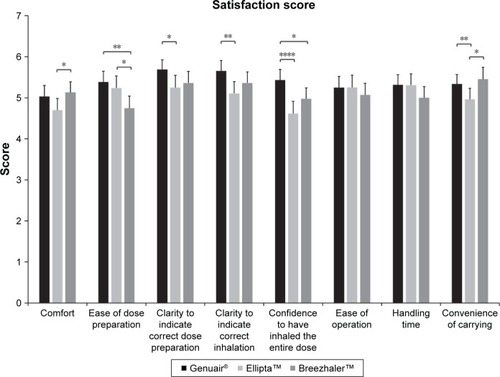
When being asked about the clarity of the DPIs to indicate correct dose preparation, subjects indicated significantly higher satisfaction for Genuair than Ellipta (5.69±0.12 vs 5.25±0.15, p=0.0120). In terms of clarity of DPIs to indicate correct inhalation, subjects had greater satisfaction with Genuair compared with Ellipta (5.65±0.13 vs 5.11±0.15, p=0.0014). The satisfaction score on confidence to have inhaled the entire dose when using the DPIs was highest for Genuair compared with Ellipta (5.43±0.13 vs 4.62±0.15, p≤0.0001) and Breezhaler (5.43±0.13 vs 4.98±0.13, p=0.0328).
No significant difference was observed in the satisfaction score for ease of operation and handling time among the three DPIs (). Finally, subjects considered Breezhaler (5.45±0.15 vs 4.96±0.14, p=0.0304) and Genuair (5.34±0.12 vs 4.96±0.14, p=0.0087) to be more convenient to carry than Ellipta. Similar trends were seen in the subanalysis by gender and age (younger than 60 years of age vs 60 years of age and above) and these data can be found in Figures S1 and S2. An analysis that directly compares the effects of gender (male vs female) and age (<60 vs ≥60 years of age) on the satisfactory scores of the different attributes is provided in Figures S3 and S4, respectively.
The satisfaction score data were further analyzed to determine whether the effect sizes exceed the threshold for clinical relevance by considering the minimally important difference using a distribution-based method.Citation18,Citation21 Conventional benchmarks were used, wherein an effect size >0.2 SD and <0.5 SD is a small effect and an effect size ≥0.5 SD and <0.79 SD is a medium effect. After analyzing the differences between the satisfaction scores of each DPI for each attribute ( and S2), we found that all effects which reached statistical significance were small effects, with the exception of clarity to indicate correct inhalation, with Genuair being more superior than Ellipta and approaching a medium effect.
Table 1 Differences of satisfactory scores between each DPI for each attribute
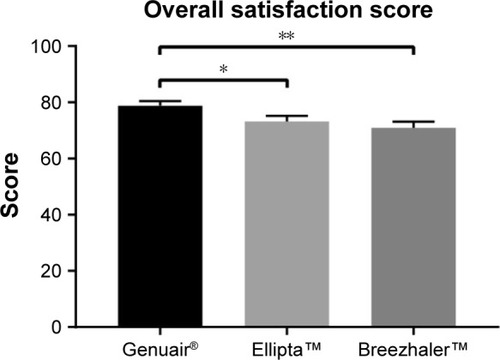
The overall satisfaction score, used as a measure to indicate the willingness of subjects to use each of the DPIs if they were diagnosed with COPD, was significantly higher for Genuair than for both Ellipta (78.79±1.70 vs 73.23±1.94, p=0.0132) and Breezhaler (78.79±1.70 vs 70.97±2.15, p=0.0063; ).
Preferences
Subjects were asked to indicate their preferred inhaler for each attribute evaluated (). The distribution of preference for each attribute was analyzed for significant deviations from uniform distribution (ie, 33.3% for each DPI) using chi-square tests.Citation17
Table 2 Preference of subjects for different attributes of the three DPIs
Genuair was the preferred DPI in terms of clarity to indicate correct dose preparation (38.5%, R=2.137), clarity to indicate correct inhalation (37.7%, R=2.299) and confidence to have inhaled the entire dose (42.3%, R=2.824). In terms of confidence to have inhaled the entire dose, the standardized residual for Ellipta was −2.716, indicating a significant lack of preference (16.2%). Breezhaler was the preferred DPI for convenience of carrying, as preferred by 42.3% of subjects (R=2.372), while Ellipta was less preferred by subjects for this attribute (16.2%, R=−3.004). For the attributes of comfort, ease of dose preparation, ease of operation and handling time, the distributions were not found to deviate significantly.
Error occurrence during use of DPIs
After reading the instructions, the highest number of subjects committing one or more critical errors was with Breezhaler (96 [73.8%]) followed by Genuair (70 [53.8%]) and then Ellipta (33 [25.4%]), as shown in . The likelihood for subjects to commit critical errors when using Breezhaler was higher than those when using either Genuair (p≤0.0001, chi-square statistic=15.36) or Ellipta (p≤0.0001, chi-square statistic=55.90). Similarly, subjects were more likely to commit one or more critical errors when using Genuair than Ellipta (p≤0.0001, chi-square statistic=27.94; ).
Figure 4 (A) Number of subjects who committed one or more critical errors during the two learning stages. (B) McNemar’s tests for the number of subjects who committed one or more critical errors during the two learning stages.
Abbreviation: DPIs, dry powder inhalers.
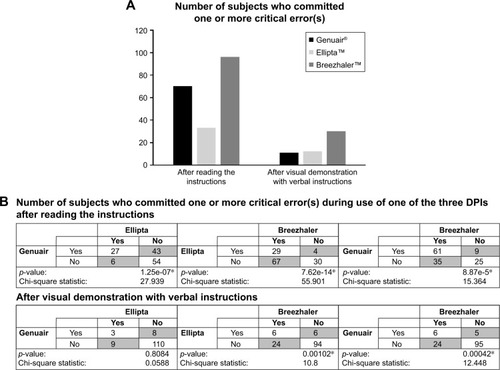
For all three DPIs, the number of subjects who committed critical errors reduced after visual demonstration with verbal instructions. The highest number of subjects committing one or more critical errors, after the demonstration, was with Breezhaler (30 [23.1%]) followed by Ellipta (12 [9.2%]) and then Genuair (11 [8.5%]), as shown in . The likelihood for subjects to commit critical errors when using Breezhaler was still higher than for those using either Genuair (p≤0.001, chi-square statistic=12.45) or Ellipta (p≤0.01, chi-square statistic=10.80). There was no significant difference between Genuair and Ellipta (p=0.8084, chi-square statistic=0.0588; ).
The number and percentage of subjects who committed each non-critical error is depicted in . The total number of non-critical errors committed during use of each DPI is presented in Table S3.
Table 3 Number and percentage of subjects who committed non-critical errors
Discussion
The correct and preferred use of DPIs is essential to deliver the relevant drugs appropriately to the patient. Besides the efficacy of bronchodilators and corticosteroids, patient preference and the skills required in using inhaler devices can affect the effectiveness of the treatment. It is important that an inhaler device is easy to use, so that the correct dosage is administered. In this study, 130 healthy subjects were recruited and the numbers of male (n=61) and female (n=69) subjects were evenly distributed. The subjects were aged 41–84 years with the average age 59.82±0.88 years. As the prevalence of COPD is higher in people aged 60 years or above compared to those aged 40–60 years,Citation22,Citation23 the age distribution of our subjects also covered a cross section of the high and low prevalence groups. Besides disease conditions such as Huntington’s chorea, stroke and so on, which cause dysfunction of brain,Citation24,Citation25 degeneration of brain structure with aging is highly related to reduced dexterity and cognitive function.Citation26,Citation27 Thus, ease of use is very important for elderly patients. The study did not include the education level of the subjects. Although education background might affect the ability to understand instructions, Blasi et al reported no difference in the time required to perform a correct inhalation of Genuair between different education levels.Citation28
In our study, subjects rated Breezhaler with the highest score for “comfort” (). Interestingly, the score given by males did not show any significant difference among the three DPIs, and the high score of Breezhaler for “comfort” was mainly contributed by females (Figure S2). This could be related to the shape and size of Breezhaler. Compared to the other two DPIs, Breezhaler is smaller and the medicine is not built into the device, which could also explain the higher score for “convenience of carrying”. However, the design seems to also affect dose preparation, as the user required extra steps to put a capsule into Breezhaler. The subjects in our study gave a significantly lower score for Breezhaler for “ease of dose preparation”.
Both Ellipta and Genuair are equipped with acoustic feedback mechanism to indicate that a dose has been correctly prepared. Genuair also has a visual feedback mechanism in the form of a red/green window to indicate empty and loaded dose. These visual and acoustic feedback mechanisms also indicate correct inhalation of a dose when enough inhalation flow is generated in Genuair. The higher scores for Genuair in “clarity to indicate correct dose preparation” and “clarity to indicate correct inhalation” () reflected that subjects were more satisfied with the device’s feedback mechanism. The subjects also rated a significantly higher score for Genuair in “confidence to have inhaled the entire dose”. Rajan and Gogtay suggested that confidence was important to new users of DPI, as this would lead to better adherence to therapy.Citation29 The clearer indications of dose preparation and correct inhalation together with higher confidence could explain the significantly higher overall satisfaction score for Genuair. A previous study in Germany, Spain and the UK compared the satisfaction of COPD patients on Genuair and Breezhaler.Citation30 The results also showed that a higher overall satisfaction score was found in Genuair than Breezhaler.
The satisfaction scores of different attributes were analyzed by gender and age (Figures S1 and S2). The pattern of the scores for the three DPIs was similar between males and females. When analyzing the satisfaction scores according to age groups, the patterns were also very similar. However, in terms of “ease of operation” and “handling time”, the younger group (aged 40–60) reported a significantly higher score for Ellipta than Breezhaler, while the older group (aged 60 and above) gave a higher score to Breezhaler. The satisfaction scores of different attributes were further compared by male vs female and the younger group vs older group (Figures S3 and S4). The only significant result was found in “ease of operation”, where the older group gave a significantly lower score to Ellipta. These results suggested that the handling procedures of Ellipta may be difficult for the older group.
The errors during the use of DPIs were also evaluated based on the list of errors (Table S1). The handling procedures and complexity of the three DPIs are different, and thus, the error list is designed based on the instruction manual of each DPI. After reading the instructions only, subjects were less likely to commit critical errors when using Ellipta than Genuair or Breezhaler (). The data suggest that Ellipta may be easier to learn by reading the instructions alone. Regardless of reading the instructions only or after observation of the visual demonstration with verbal instructions, the number of subjects committing critical errors was higher with Breezhaler. These results may indicate that the design of Breezhaler is more complex, especially for new users. Interestingly, subjects also gave a lower satisfaction score for Breezhaler on “ease of operation” and “handling time” (). A previous study in Japan also showed that the volunteers made more errors when using Breezhaler compared to Ellipta.Citation17 It is also worth noting that visual demonstration with verbal instructions reduced the number of subjects making critical errors for each DPI to one third or lower compared to reading the instructions only, with the effect being the greatest for Genuair. This finding suggests that visual demonstration with verbal instructions is crucial to new users of DPIs. In a study conducted by Svedsater et al, subjects also reported that with briefing and demonstration, they could understand how to use DPIs even without reading the instructions.Citation31
This study excluded subjects who had been diagnosed with COPD or asthma and had experience of using DPIs. Thus, the results would represent newly diagnosed COPD patients who are device naïve. In the study of the usability of Genuair, Blasi et al reported that there was no difference in the time required to perform a correct inhalation when considering the different previous experience of inhaler devices.Citation28 However, patients’ experience of using DPI may affect their satisfaction. The results could be used as reference for future validation studies in COPD patients with previous experience in using DPIs. A dose counter is found in Genuair and Ellipta to indicate the number of doses left in the device. The satisfaction to this function was not tested, as the subjects demonstrated the use of each DPI for only two times.
Conclusion
The results of our study showed that each DPI has its own user advantages: Breezhaler seemed to be more comfortable to use and easy to carry. Device-naive users made less critical errors when using Ellipta after reading the instructions only. Genuair gave significantly better indications of correct dose preparation and dose inhalation. Overall, healthy volunteers had the greatest satisfaction scores when using Genuair. This is important, as satisfaction might improve patient compliance to inhaler therapy. Instead of learning to use a DPI by reading the instructions only, visual demonstration with verbal instructions given by health care professionals is crucial as they can reduce the critical errors during inhaler use. These results cannot be applied at the individual level, so good practice should be followed.
Acknowledgments
We would like to thank Mr Kwok Yan Ng, University of Hong Kong, School of Biological Sciences for his help in recruiting participants. We appreciate the support from Dr Hiu Fai Law with the statistical analysis of the data.
Supplementary materials
Figure S1 Satisfaction scores on different attributes of the three DPIs as evaluated by male and female subjects.
Notes: Mean values are plotted and the error bars represent the SEM. *p≤0.05; **p≤0.01.
Abbreviations: DPIs, dry powder inhalers; SEM, standard error of the mean.
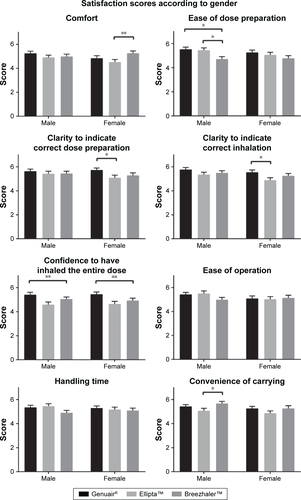
Figure S2 Satisfaction scores on different attributes of the three DPIs as evaluated by subjects below 60 years of age or above or equal to 60 years of age.
Notes: Mean values are plotted and the error bars represent the SEM. *p≤0.05; **p≤0.01; ***p≤0.001.
Abbreviations: DPIs, dry powder inhalers; SEM, standard error of the mean.
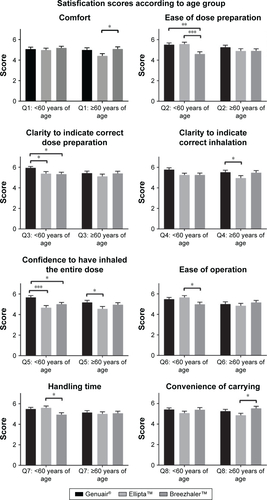
Figure S3 Satisfaction scores on different attributes of the three DPIs by male and female subjects.
Notes: Mean values are plotted and the error bars represent the SEM. No significance was detected.
Abbreviations: DPIs, dry powder inhalers; SEM, standard error of the mean.
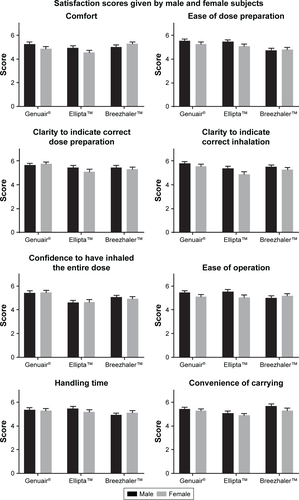
Figure S4 Satisfaction scores on different attributes of the three DPIs by subjects <60 and ≥60 years of age.
Notes: Mean values are plotted and the error bars represent the SEM. *p≤0.05.
Abbreviations: DPIs, dry powder inhalers; SEM, standard error of the mean.
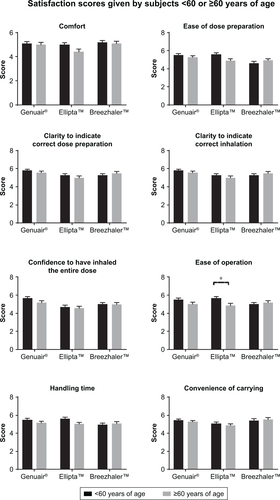
Table S1 List of critical and non-critical errors for each dry powder inhaler
Table S2 0.2 SD and 0.5 SD of satisfactory scores of the three DPIs
Table S3 Total number of non-critical errors committed during use of the three DPIs
Disclosure
The authors report no conflicts of interest in this work.
References
- BarnesPJIntroduction: how can we improve asthma management?Curr Med Res Opin200521Suppl 4S1S3
- CrossSAsthma inhalation delivery systems: the patient’s viewpointJ Aerosol Med200114Suppl 1S3S711424891
- MagnussenHNovolizer: how does it fit into inhalation therapy?Curr Med Res Opin200521Suppl 4S39S46 discussion S4716138944
- MelaniASInhalatory therapy training: a priority challenge for the physicianActa Biomed200778323324518330086
- Dal NegroRWPoveroMAcceptability and preference of three inhalation devices assessed by the Handling Questionnaire in asthma and COPD patientsMultidiscip Respir Med201511726865979
- VirchowJCGuidelines versus clinical practice-which therapy and which device?Respir Med200498Suppl BS28S3415481286
- RestrepoRDAlvarezMTWittnebelLDMedication adherence issues in patients treated for COPDInt J Chron Obstruct Pulmon Dis20083337138418990964
- LavoriniFMagnanADubusJCEffect of incorrect use of dry powder inhalers on management of patients with asthma and COPDRespir Med2008102459360418083019
- SouzaMLMeneghiniACFerrazEViannaEOBorgesMCKnowledge of and technique for using inhalation devices among asthma patients and COPD patientsJ Bras Pneumol2009359824831 Portuguese19820807
- RaherisonCGirodetPOEpidemiology of COPDEur Respir Rev20091811421322120956146
- ChrystynHSmallMMilliganGHigginsVGilEGEstruchJImpact of patients’ satisfaction with their inhalers on treatment compliance and health status in COPDRespir Med2014108235836524209768
- LareauSCYawnBPImproving adherence with inhaler therapy in COPDInt J Chron Obstruct Pulmon Dis2010540140621191434
- HodderRPriceDPatient preferences for inhaler devices in chronic obstructive pulmonary disease: experience with Respimat Soft Mist inhalerInt J Chron Obstruct Pulmon Dis2009438139019888356
- van der PalenJGinkoTKrokerAPreference, satisfaction and errors with two dry powder inhalers in patients with COPDExpert Opin Drug Deliv20131081023103123745954
- ChrystynHNiederlaenderCThe Genuair® inhaler: a novel, multidose dry powder inhalerInt J Clin Pract201266330931722340451
- MagnussenHWatzHZimmermannIPeak inspiratory flow through the Genuair inhaler in patients with moderate or severe COPDRespir Med2009103121832183719651504
- KomaseYAsakoAKobayashiASharmaREase-of-use preference for the ELLIPTA® dry powder inhaler over a commonly used single-dose capsule dry powder inhaler by inhalation device-naïve Japanese volunteers aged 40 years or olderInt J Chron Obstruct Pulmon Dis201491365137525525354
- KozmaCMSlatonTLMonzBUHodderRReesePRDevelopment and validation of a patient satisfaction and preference questionnaire for inhalation devicesTreat Respir Med200541415215725049
- SharpeDYour chi-square test is statistically significant: now what?Pract Assess Res Eval2015208110
- AgrestiAAn Introduction to Categorical Data AnalysisHoboken, New Jersey, USAWiley2007
- GuyattGHOsobaDWuAWWyrwichKWNormanGRClinical Significance Consensus Meeting GroupMethods to explain the clinical significance of health status measuresMayo Clin Proc200277437138311936935
- MenezesAMPerez-PadillaRJardimJRPLATINO TeamChronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence studyLancet200536695001875188116310554
- Global Strategy for Diagnosis, Management, and Prevention of COPD — 2016Global Initiative for Chronic Obstructive Lung Disease – GOLD Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/Accessed May 26, 2017
- MontoyaAPriceBHMenearMLepageMBrain imaging and cognitive dysfunctions in Huntington’s diseaseJ Psychiatry Neurosci2006311212916496032
- SunderlandABowersMPSlumanSMWilcockDJArdronMEImpaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficitStroke199930594995510229726
- ParkDCReuter-LorenzPThe adaptive brain: aging and neurocognitive scaffoldingAnnu Rev Psychol20096017319619035823
- SeidlerRDBernardJABurutoluTBMotor control and aging: links to age-related brain structural, functional, and biochemical effectsNeurosci Biobehav Rev201034572173319850077
- BlasiFCanonicaGWCentanniSGenuair® usability test: results of a national public survey of the elderlyCOPD201613336737126645660
- RajanSKGogtayJAEase-of-use, preference, confidence, and satisfaction with Revolizer(®), a novel dry powder inhaler, in an Indian populationLung India201431436637425378845
- PascualSFeimerJDe SoyzaAPreference, satisfaction and critical errors with Genuair and Breezhaler inhalers in patients with COPD: a randomised, cross-over, multicentre studyNPJ Prim Care Respir Med2015251501825927321
- SvedsaterHDalePGarrillKWalkerRWoepseMWQualitative assessment of attributes and ease of use of the ELLIPTA™ dry powder inhaler for delivery of maintenance therapy for asthma and COPDBMC Pulm Med2013137224314123