Abstract
Background
Sex differences in chronic thromboembolic pulmonary hypertension (CTEPH) have been revealed in few studies. Although right heart catheterization (RHC) is the gold standard for clinical diagnosis and assessment of prognosis in pulmonary hypertension (PH), cardiopulmonary exercise testing (CPET) has been a more widely used assessment of functional capacity, disease severity, prognosis, and treatment response in PH. We hypothesized that the “sex-specific” CPET indices could estimate the severity of inoperable CTEPH.
Methods
Data were retrieved for 33 male (age, mean ± standard deviation [SD] =62.5±13.4 years) and 40 female (age, mean ± SD =56.3±11.8 years) patients with stable CTEPH who underwent both RHC and CPET at Shanghai Pulmonary Hospital from February 2010 to February 2016. Univariate and forward/backward multiple stepwise regression analysis was performed to assess the predictive value of CPET indices to hemodynamic parameters. Event-free survival was estimated using the Kaplan–Meier method and analyzed with the log-rank test. Cox proportional hazards models were performed to determine the independent event-free survival predictors.
Results
Numerous CPET parameters were different between male and female patients with CTEPH and the control group. There were no significant differences in both clinical variables and RHC parameters between male and female patients with CTEPH. O2 pulse, workload, minute ventilation (VE), and end-tidal partial pressure of O2 (PETO2) at anaerobic threshold, as well as peak O2 pulse, workload, VE, and nadir VE/CO2 were significantly higher in male patients than in female patients (P<0.05). Only oxygen uptake efficiency plateau (OUEP) showed a significantly higher difference in female than male patients (P<0.05). In addition, several CPET indices correlated with hemodynamic parameters, especially pulmonary vascular resistance (PVR), which was distinctly different between the sexes. Nadir VE/CO2 was an independent predictor of PVR in male patients with CTEPH, whereas OUEP was an independent predictor of PVR in female patients with CTEPH.
Conclusion
Even after confounding for age and body mass index, different CPET measurements of gas exchange efficiency correlated with PVR differently between male and female patients. This potentially could be used to estimate the severity of CTEPH.
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare, debilitating, and life-threatening complication of acute pulmonary embolism. It is associated with significant morbidity and high mortality, resulting from persistent obstruction of pulmonary arteries, progressive vascular remodeling, and elevated mean pulmonary arterial pressure (mPAP).Citation1,Citation2 Recent registries suggest an incidence of about 5 per million inhabitants per year who have been affected with CTEPH.Citation3 The preferred treatment for CTEPH is surgical pulmonary endarterectomy, whereas inoperable patients are treated with drugs and endovascular interventions.Citation4 Although some studies have assessed the differences in overall survival rate and sex-specific differences of clinical phenotype in surgical and nonsurgical CTEPH cohorts,Citation5–Citation7 there is still a paucity of information regarding sex-specific differences of cardiopulmonary function in Chinese patients with CTEPH.
Cardiopulmonary exercise testing (CPET) has been widely used in the assessment of cardiopulmonary functionCitation8 and has also been reported to be useful in the assessment of pulmonary hypertension (PH).Citation9 CPET with gas exchange measurements is potentially useful in the noninvasive grading of the severity of exercise limitation, quantifying the hypoperfusion of the lungs and systemic circulation, and assessing responses to therapy.Citation8,Citation10 In addition, CPET allows reproducible assessment of functional capacity and treatment efficacy in patients with PH.Citation8 The usefulness of CPET is based on its ability to uncover anomalies in the oxygen consumption cascade and point toward the underlying physiological abnormalities associated with the hypoperfusion of the pulmonary vascular bed in PH.Citation10,Citation11 Furthermore, significant differences in CPET parameters have been observed between healthy male and female subjects.Citation12,Citation13 We reported earlier that in patients with idiopathic pulmonary arterial hypertension (IPAH), sex-specific CPET parameters could potentially predict different hemodynamic indices and survival between the two sexes. In addition, we found that sex-specific differences in CPET indices appeared earlier than that of hemodynamic parameters in a small sample of patients with IPAH.Citation14,Citation15 Therefore, we hypothesized that similar differences exist in patients with CTEPH and that potential sex-specific differences exist when making prognostic assessment by performing CPET.
Therefore, our primary aim in this study was to investigate the “sex-specific” CPET indices in relation to hemodynamic parameters and to attempt to determine if sex-specific difference was associated with clinical outcomes in inoperable patients with CTEPH.
Materials and methods
Population study
Seventy-three patients (33 males and 40 females) with incident CTEPH who were more than 18 years of age were evaluated at Shanghai Pulmonary Hospital, Shanghai, from February 2010 to February 2016. The diagnosis of CTEPH was established according to the most recent European Society of Cardiology and the European Respiratory Society guidelines for the diagnosis and treatment of PH.Citation16 Precapillary PH was defined based on the right heart catheterization (RHC) (mean pulmonary artery pressure ≥25 mmHg and mean pulmonary arterial wedge pressure ≤15 mmHg) in the presence of mismatched perfusion defects on lung scans and specific diagnostic signs for CTEPH seen by multi-detector computed tomography (CT), magnetic resonance imaging, or conventional pulmonary cine-angiography, such as ring-like stenosis, webs/slits, and chronic total occlusions (pouch lesions or tapered lesions), even after at least 3 months of effective anticoagulation, in order to discriminate it from “subacute” pulmonary embolism. Patients with PAH or PH due to lung diseases or left heart disease were excluded. We also excluded patients with acute or chronic illnesses that might influence hormonal metabolism (ie, acute or chronic infections, chronic autoimmune diseases, and previously established primary endocrine disorders) and patients receiving any treatment with hormones (thyroid hormones, anabolic steroids, and corticosteroids) or drugs that markedly inhibit hormone production, either at the time of the study or in the past.Citation14,Citation15 Participants with CTEPH, PH, and other underlying cardiopulmonary diseases were excluded by specialists prior to being included in the control group.
The study protocol was reviewed and approved by the Ethics Committee of Shanghai Pulmonary Hospital. Written informed consent was obtained from each patient for inclusion into the study and prior to the performance of any study-related procedures.
Assessment of patients
Demographic variables such as sex, age, body mass index (BMI), 6-minute walk distance (6MWD), N-terminal pro-brain natriuretic peptide (NT-proBNP), World Health Organization functional classification (WHO FC), and hemodynamic parameters were obtained at baseline. RHC was performed as described in a previous study.Citation17 The 6MWD test was performed according to the guidelines of the American Thoracic Society.Citation18
Each patient performed a physician-supervised, progressively increasing work rate CPET to maximum tolerance on an electromagnetically braked cycle ergometer. The protocol comprised of 3 min of rest, 3 min of unloaded cycling at 55–65 revolutions per minute (rpm), followed by a progressively increasing work rate of 5–15 watts (W)/ min for patients with PH and 20–25 W/min for the normal subjects to the maximum tolerance, and 4 min of recovery.Citation19 Pulse oximetry, heart rate (HR), 12-lead electrocardiogram (ECG), and automated cuff blood pressure measurements were monitored and recorded. Minute ventilation (VE, body temperature and pressure-saturated), oxygen uptake (VO2, standard temperature and pressure, dry), carbon dioxide uptake (VCO2, standard temperature and pressure, dry), and other exercise variables were computer-calculated breath by breath, interpolated second by second, and averaged over 10-second intervals.Citation20 The ratio of VO2 and HR (O2 pulse) was determined as previously described.Citation21 Cardiovascular and exercise capacity were expressed as HR, O2 pulse, workload, and VO2, respectively. Ventilation and gas exchange efficiency was expressed as VE, end-tidal partial pressure of O2 (PETO2), end-tidal partial pressure of CO2 (PETCO2), oxygen uptake efficiency plateau (OUEP), and nadir minute ventilation/carbon dioxide output (VE/VCO2).Citation22
Outcomes
In this study, the primary outcome was clinical worsening, including cardiopulmonary death, re-hospitalization, or initiation of new active therapy due to worsening CTEPH, with the criteria for worsening being defined after an earlier study.Citation15 Event-free survival was estimated from the date of diagnosis to February 20, 2017. Patients lost to follow-up were censored as alive on the last day of contact.
Statistical analysis
Results are expressed as mean with standard deviation (SD) or medians (and interquartile range) for continuous variables and absolute number for categorical variables. Comparisons were performed using independent-sample t-test or Mann– Whitney U test for continuous variables and chi-square test for categorical variables. Correlations were assessed using rho coefficient of Spearman. Event-free survival was estimated using the Kaplan–Meier method and analyzed with log-rank test. Cox proportional hazards models were performed to determine the predictors of independent event-free survival. Univariate and forward/backward multiple stepwise regression analysis was performed with hemodynamic variables as the dependent outcome with 95% confidence intervals to determine the strength of the association between hemodynamic and CPET variables. Standardization of the coefficient was usually performed to find out which of the independent variables had a greater effect on the dependent variable in a multiple regression analysis. R2 is defined as the square of the coefficient of multiple correlations; it provides a measure of how well the observed outcomes are replicated by the model, based on the proportion of total variation of outcomes explained by the model. Age, BMI, and WHO FC were the most important influencing factors on CPET; therefore, 95% confidence intervals were adjusted by age, BMI, and WHO FC into the multiple regression models for the different subgroups based on sex.Citation23 P-values less than 0.05 were considered significant. Data were analyzed using SPSS (Statistic Package for Social Science, Chicago, IL) version 19.0 and GraphPad Prism (San Diego, CA, USA) version 6.0 softwares.
Results
Characteristics of the studies
A total of 73 patients with CTEPH (33 males and 40 females) matched inclusion criteria. Their mean duration of follow-up was 30±18 months. Seventeen men and 10 women had an event: 4 men and 2 women died; 7 men and 4 women required re-hospitalization due to clinical worsening. Four men and 4 women required additional PH-active medication or switched from oral PH-active therapy to parenteral therapy. No patient was lost to follow-up, giving us a 100% follow-up rate. presents the demographic and hemodynamic data. There were no differences found with respect to age, BMI, 6MWD, and WHO FC either between male and female patients, or between event and event-free subgroups of male and female patients. NT-proBNP was higher in the event subgroup of female patients with CTEPH, with significant statistical difference compared with the event-free subgroup of female patients.
Table 1 Baseline characteristics in CTEPH and control groups
Although the mean pulmonary capillary wedge pressure (mPAWP) was significantly lower in the event subgroup of female patients, compared with the event subgroup of male patients and event-free subgroup of female patients, there were no statistically significant differences in other hemodynamic parameters between male and female groups. Moreover, the event subgroup of female patients had higher mPAP and pulmonary vascular resistance (PVR) than that of the event-free subgroup of female patients.
Target medication included oral sildenafil, oral tadalafil and oral vadenafil, oral ambrisentan and oral bosentan, oral beraprost, inhaled iloprost, and intravenous iloprost. Patients of both sexes used phosphodiesterase type 5 inhibitors (12 men and 13 women), endothelial receptor antagonists (1 man and 2 women), prostacyclin analogs (3 men and 5 women), or combination medicine (11 men and 16 women). Six men and 4 women took nonspecific medication. There were no apparent differences either in male and female or inter-subgroup patients for medication use ( and ).
Table 2 Comparison of baseline characteristics between event and event-free subgroups in the two sexes
Sex differences of CPET indices in patients with CTEPH
and illustrate CPET assessments. There were many sex-specific differences observed in the case of CPET parameters in both control group and in patients with CTEPH. We performed a comparison between male and female patients with CTEPH and the healthy group, separately. Almost all CPET parameters showed significant differences between each sex, except HR at anaerobic threshold (AT) and peak VE in male patients, and HR, VE at AT, and peak VE in female patients. Meanwhile, in the CTEPH cohort, male patients had significantly higher O2 pulse, workload, VE and PETO2 at AT, peak O2 pulse, workload, and VE, along with nadir VE/VCO2 compared with female patients. Higher OUEP value turned out to be statistically different in female patients than in that of male patients with CTEPH. However, in the control group, O2 pulse, VO2, VE at AT and peak O2 pulse, workload, VO2, and VE were higher in male subjects.
Figure 1 (A–O). Comparison of CPET parameters in CTEPH and control groups.
Note: *P<0.05 and **P<0.01.
Abbreviations: AT, anaerobic threshold; CPET, cardiopulmonary exercise testing; CTEPH, chronic thromboembolic pulmonary hypertension; HR, heart rate; OUEP, oxygen uptake efficiency plateau; PETCO2, end-tidal partial pressure of CO2; PETO2, end-tidal partial pressure of O2; VCO2, carbon dioxide output; VE, minute ventilation; VE/VCO2, minute ventilation/carbon dioxide output; VO2, oxygen uptake.
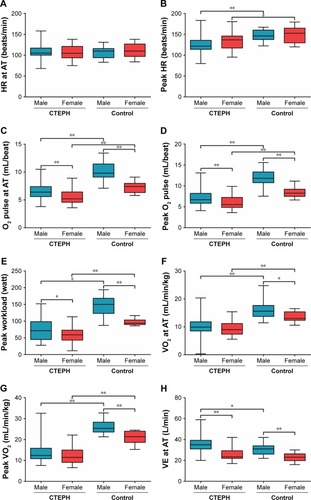
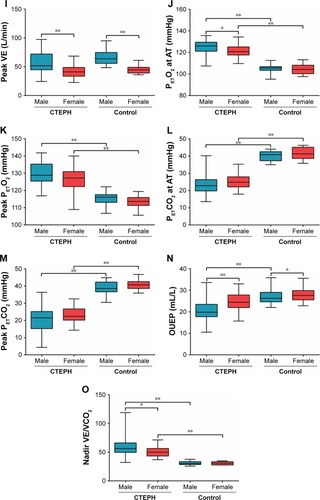
Figure 2 (A–P). Comparison of CPET parameters in event and event-free subgroups by sex.
Abbreviations: AT, anaerobic threshold; CPET, cardiopulmonary exercise testing; CTEPH, chronic thromboembolic pulmonary hypertension; HR, heart rate; OUEP, oxygen uptake efficiency plateau; PETCO2, end-tidal partial pressure of CO2; PETO2, end-tidal partial pressure of O2; VCO2, carbon dioxide output; VE, minute ventilation; VE/VCO2, minute ventilation/carbon dioxide output; VO2, oxygen uptake.
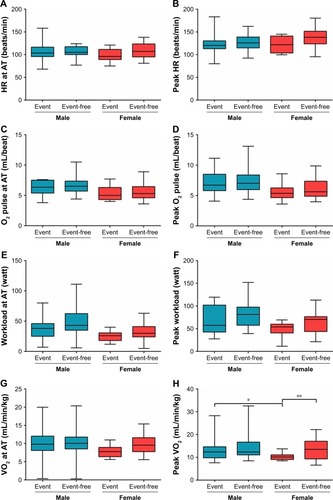
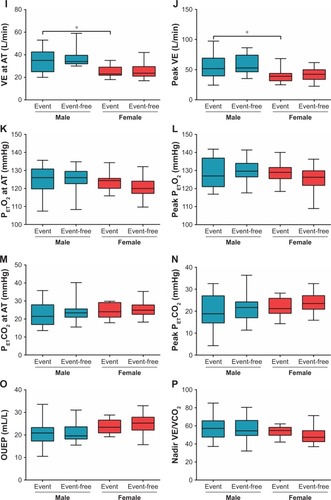
In the male cohort, there were no differences between event and event-free subgroups, whereas event-free female patients with CTEPH had significantly higher peak VO2. In the event subgroup, female patients showed lower peak VO2, VE at AT, and peak VE, with the difference being statistically significant.
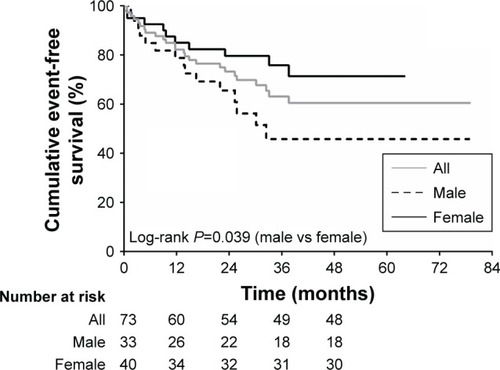
Sex-specific difference in survival assessment
There were 25 events during a mean follow-up period of 30±18 months. The overall event-free survival rate was found to be 82%, 73%, 63%, for the 1st year; 79%, 64%, 44% during the 2nd year, and 85%, 80%, 76% for the 3rd year, respectively, for all CTEPH cohort, male and female cohorts subgroups. The overall event-free survival rates were statistically different between the two sexes; log-ranked P-value was found to be 0.039 (). However, no sex-specific independent predictors for event-free survival were determined after performing Cox proportional hazards models.
Correlation between PVR and CPET parameters in patients with CTEPH
illustrates correlation between PVR and CPET parameters. VO2 at AT, peak O2 pulse, workload, VO2, PETCO2, and OUEP showed negative correlation with PVR in male patients with CTEPH, whereas peak PETO2 and nadir VE/VCO2 correlated positively. In the female cohort, VO2 at AT, peak O2 pulse, VO2, and OUEP correlated negatively with the PVR, whereas peak PETO2 and nadir VE/VCO2 had positive correlation.
Table 3 Correlation of CPET parameters with PVR in CTEPH patients by sex
Independent determinants predicting PVR elevation
Significantly correlated CPET parameters were entered in a stepwise multiple regression analyses to determine the strength of each index predicting PVR elevation. As shown in , nadir VE/VCO2 was the independent predictor of PVR accounting for 60.7% (R2=0.607) of the variation in male patients with CTEPH. In the females, OUEP was the sole independent predictor of PVR, separately accounting for 22.4% (R2=0.224) of the variation.
Table 4 Determinants of PVR in CTEPH patients by sex
Discussion
There were some significant findings in this study. To the best of our knowledge, this is the first study to reveal “sex-specific” CPET indices as predictors of markedly impaired hemodynamic parameters in inoperable patients with CTEPH. We selected several different CPET indices between the two sexes and assessed their correlation with hemodynamic parameters. Nadir VE/VCO2 and OUEP were separately, sole independent predictors of PVR, which is an important parameter taken into account during the management of patients with CTEPH.Citation24,Citation25
Although patients with CTEPH are a heterogeneous group with respect to their hemodynamic status and surgical accessibility of pulmonary thromboemboli, resulting in demarcation as operable or inoperable patients, they also present with many similarities at diagnosis, suggesting a common underlying disease process.Citation26 In line with previous registries from the US, Canada, Europe, Japan, and Mexico,Citation5,Citation26–Citation30 CTEPH was almost equally frequent in men and women in their 6th decade of life, approximately 63 years, which is comparable to our cohort. The slight female predominance observed in this study is also consistent with the findings from the international registry.Citation25 No differences were observed as regards BMI, 6MWD, WHO FC, and specific medications amongst either subgroups; NT-proBNP was higher in event compared with event-free female patients.
RHC was routinely performed throughout the study duration and pulmonary hemodynamics were found to be comparable to previous reports, with only the cardiac index being modestly elevated (average =2.8 L/min/m2).Citation26,Citation27,Citation29,Citation31 Interestingly, PVR in both event male and female subgroups was higher than average PVR of CTEPH cohort in our study while in event-free subgroups of both sexes, it was found to be lower than borderline patients suggesting that elevation of PVR is a crucial factor in indicating that patients with CTEPH are having an event.
Several CPET parameters evaluated differed significantly between patients with CTEPH and controls. Although there were many sex-specific differences of CPET parameters in patients with CTEPH, these differences were similarly observed in controls.Citation12,Citation13 This means that the general trend of heart and lung function impairment in male and female patients with CTEPH is consistent. Furthermore, we aimed to investigate how these differences in CPET indices relate to hemodynamics and impact clinical outcomes. CPET’s increasing credibility is reflected by the fact that major guidelines for the treatment and management of PH have endorsed its use.Citation16 Evidence from the last three decades has already established that there are differences in exercise capacities between the two sexes.Citation32–Citation34 Furthermore, work efficiency in humans is relatively fixed for a given task. While race, body build, and genetic predispositions can all influence exercise capacity, how a differently reduced exercise capacity between the two sexes reflects impaired hemodynamics is still unclear.Citation34 This is the first study to report that several CPET parameters could predict PVR in CTEPH. Sex-specific differences in CPET parameters have received far less attention than hemodynamics in CTEPH. We hope that this study will serve its purpose of trying to discover a reason as to why the difference in exercise capacities affects hemodynamics in a particular disease spectrum, in this case CTEPH.
On comparing between the two sexes, the event subgroup female patients had statistically significant lower peak VO2, VE at AT, and peak VE than that of male patients. In addition to cardiovascular and exercise functions, ventilation and gas exchange efficiency also contribute to the differences between the two sexes, that is, in event/event-free subgroups, proving that patients with CTEPH might have vasculopathy at the precapillary level beyond the obstructive thrombotic lesions.Citation35
This study also hints at sex-specific differences in the survival rate in the case of CTEPH. The overall event-free survival rate at 1, 2, and 3 years was found to be 82%, 73%, and 63% in the CTEPH cohort, which is slightly lower than a 2012 Spanish study and in a recent German study.Citation5,Citation28 The decrease in event-free survival might be due to the definition of the “event”, not only cardiopulmonary death, but also re-hospitalization, addition of another active therapy or a switch from oral PH-active therapy to parenteral for clinical worsening. The event-free survival rate at 1, 2, and 3 years was found to be 79%, 64%, and 44% in male subgroup, comparing to 85%, 80%, and 76% in female subgroup, respectively, over a follow-up period averaging 30 months from study enrollment. Since we did not find any sex-specific determinants to estimate event-free survival in our study, we decided to investigate whether they could predict hemodynamics.
Multiple regression analyses indicated that nadir VE/ VCO2 and OUEP could be independent predictors of PVR elevation in the two sexes, respectively. Previous reports have suggested that pulmonary arteriopathy induces vascular obstruction and/or stenosis, and this may play a principal role in the elevation of PVR in patients with CTEPH.Citation36–Citation38 Interestingly, both nadir VE/VCO2 ratio and OUEP are ventilatory CPET parameters. It has been proven that in distal CTEPH, dead-space ventilation correlated with exercise capacity and was associated with survival,Citation39 resulting in a clinical syndrome characterized by progressive obstructive pulmonary vasculopathy and leading to increased PVR in CTEPH.Citation40,Citation41 Previous data suggested that nadir VE/VCO2 is associated with survival in other pulmonary diseases, that is, idiopathic pulmonary fibrosis (IPF),Citation42 whereas male cohort in this study showed the same trend of the association. However, OUEP has been confirmed to be an advantageous, noninvasive parameter dependent only on age, sex, height, and cardiovascular health,Citation43 which we feel needs to be explored further to determine its value in relation to PVR in female patients with CTEPH.
Taken together, our findings show that sex-specific differences exist not only in cardiopulmonary function but also in event-free survival rate between the two sexes of inoperable patients with CTEPH. In particular, OUEP and nadir VE/VCO2, which as indices of ventilatory and gas exchange efficiency, could predict hemodynamic parameters especially the PVR as an independent determinant. Therefore, we suggest that CPET be routinely performed and included in the operability and clinical assessment for patients with CTEPH to achieve more precise treatment goals in the future.
The major limitation of this study is that the patient sample size was not big enough. Our original idea was to investigate whether sex-specific CPET parameters were related to clinical outcome in inoperable patients with CTEPH. Even though we found sex-specific relations between CPET indices and PVR, which is a very important hemodynamic variable to predict survival of cardiopulmonary diseases, we still failed to find out which specific indices could predict the survival directly, perhaps due to the limited sample size.
In conclusion, our study for the first time reported sex-specific CPET indices can be used to predict hemodynamic parameters in inoperable patients with CTEPH. Even after confounding for age, BMI and pulmonary function, diverse CPET measurement of gas exchange efficiency correlated with PVR differently in male and female patients, which potentially could aid in estimating the severity of CTEPH.
Author contributions
Tian-Xiang Chen, Jin-Ming Liu, and Ping Yuan designed the study; Tian-Xiang Chen, Bigyan Pudasaini, and Ping Yuan contributed to data acquisition; Tian-Xiang Chen, Jian Guo, Rong Jiang, and Ping Yuan contributed to statistical analysis; Jin-Ming Liu contributed to study supervision; Wen-Hui Wu, Ping Yuan, and Jin-Ming Liu contributed to the acquisition of funding. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Prof Rui Zhang, Jing He, and all the other study investigators, fellows, nurses, and research coordinators who participated in this study. This work was supported by the National Health and Family Planning Commission of the People’s Republic of China (W2015RNA09B and 2015BAI12B10), the Program of Shanghai Natural Science Foundation (16ZR1429000), the Program of National Natural Science Foundation of China (81500040 and 81600032) and the YangFan Program of Shanghai Science and Technology Committee (15YF1409700). All the work in this study was completed in the Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University, Shanghai, People’s Republic of China.
Disclosure
The authors report no conflicts of interest in this work.
References
- HoeperMMMadaniMMNakanishiNMeyerBCebotariSRubinLJChronic thromboembolic pulmonary hypertensionLancet Respir Med20142757358224898750
- DiversCPlattDWangELinJLingohr-SmithMMathaiSCA Review of clinical trial endpoints of patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension and how they relate to patient outcomes in the United StatesJ Manag Care Spec Pharm20172319210428025931
- DelcroixMKerrKFedulloPChronic thromboembolic pulmonary hypertension. Epidemiology and risk factorsAnn Am Thorac Soc201613Suppl 3S201S20627571001
- HoeperMMGhofraniHAGrünigEKloseHOlschewskiHRosenkranzSPulmonary hypertensionDtsch Arztebl Int20171145738428241922
- Escribano-SubiasPBlancoILópez-MeseguerMREHAP investigatorsSurvival in pulmonary hypertension in Spain: insights from the Spanish registryEur Respir J201240359660322362843
- RådegranGKjellströmBEkmehagBStefanSöderberg jon behalf of SveFPH and SPAHRCharacteristics and survival of adult Swedish PAH and CTEPH patients 2000–2014Scand Cardiovasc J201650424325027146648
- ShigetaATanabeNShimizuHGender differences in chronic thromboembolic pulmonary hypertension in JapanCirc J200872122069207418931448
- WenselROpitzCFAnkerSDAssessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testingCirculation2002106331932412119247
- ZhaiZMurphyKTigheHDifferences in ventilatory inefficiency between pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertensionChest201114051284129121527511
- OudizRJBarstRJHansenJECardiopulmonary exercise testing and six-minute walk correlations in pulmonary arterial hypertensionAm J Cardiol200697112312616377296
- PinkstaffSOBurgerCDDaughertyJBondSArenaRCardiopulmonary exercise testing in patients with pulmonary hypertension: clinical recommendations based on a review of the evidenceExpert Rev Respir Med201610327929526789612
- HeldMGrünMHollRCardiopulmonary exercise testing to detect chronic thromboembolic pulmonary hypertension in patients with normal echocardiographyRespiration201487537938724732343
- IwaseTNagayaNAndoMAcute and chronic effects of surgical thromboendarterectomy on exercise capacity and ventilatory efficiency in patients with chronic thromboembolic pulmonary hypertensionHeart200186218819211454839
- YuanPChenTXPudasainiBSex-specific cardiopulmonary exercise testing indices related to hemodynamics in idiopathic pulmonary arterial hypertensionTher Adv Respir Dis201711313514528043202
- YuanPNiHJChenTXSex-specific cardiopulmonary exercise testing parameters as predictors in patients with idiopathic pulmonary arterial hypertensionHypertens Res2017401086887528566737
- GalièNHumbertMVachieryJL2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT)Eur Respir J201546490397526318161
- JingZCYuZXShenJYEfficacy and Safety of Vardenafil in the Treatment of Pulmonary Arterial Hypertension (EVALUATION) Study GroupVardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled studyAm J Respir Crit Care Med2011183121723172921471085
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function LaboratoriesATS statement: guidelines for the six-minute walk testAm J Respir Crit Care Med2002166111111712091180
- SueDYWassermanKImpact of integrative cardiopulmonary exercise testing on clinical decision makingChest19919949819922009806
- MagalangUJGrantBJDetermination of gas exchange threshold by nonparametric regressionAm J Respir Crit Care Med19951511981067812580
- ShiXGuoJGongSOxygen uptake is more efficient in idiopathic pulmonary arterial hypertension than in chronic thromboembolic pulmonary hypertensionRespirology2016211149156
- SunXGHansenJEOudizRJWassermanKExercise pathophysiology in patients with primary pulmonary hypertensionCirculation2001104442943511468205
- DournesGLaurentFCosteFComputed tomographic measurement of airway remodeling and emphysema in advanced chronic obstructive pulmonary disease. Correlation with pulmonary hypertensionAm J Respir Crit Care Med20151911637025393421
- JujoTSakaoSIshibashi-UedaHEvaluation of the microcir-culation in chronic thromboembolic pulmonary hypertension patients: the impact of pulmonary arterial remodeling on postoperative and follow-up pulmonary arterial pressure and vascular resistancePLoS One2015108e013316726252755
- KasaiHMatsumuraASugiuraTNoninvasive assessment of pulmonary vascular resistance by echocardiography in chronic thromboembolic pulmonary hypertensionRespir Investig2015535210216
- Pepke-ZabaJDelcroixMLangIChronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registryCirculation2011124181973198121969018
- MadaniMMAugerWRPretoriusVPulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patientsAnn Thorac Surg201294197103 discussion 10322626752
- GallHFelixJFSchneckFKThe Giessen Pulmonary Hypertension Registry: survival in pulmonary hypertension subgroupsJ Heart Lung Transplant201736995796728302503
- TanabeNSugiuraTTatsumiKRecent progress in the diagnosis and management of chronic thromboembolic pulmonary hypertensionRespir Investig2013513134146
- Al-NaamaniNEspitiaHGVelazquez-MorenoHChronic thromboembolic pulmonary hypertension: experience from a single center in MexicoLung2016194231532326748498
- LangIMSimonneauGPepke-ZabaJWFactors associated with diagnosis and operability of chronic thromboembolic pulmonary hypertension. A case-control studyThromb Haemost20131101839123677493
- AstrandPOHuman physical fitness with special reference to sex and agePhysiol Rev195636330733513359126
- AstrandIAstrandPOHallbäckIKilbomAReduction in maximal oxygen uptake with ageJ Appl Physiol19733556496544770349
- BruceRAKusumiFHosmerDMaximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular diseaseAm Heart J19738545465624632004
- HirashikiAAdachiSNakanoYCardiopulmonary exercise testing to evaluate the exercise capacity of patients with inoperable chronic thromboembolic pulmonary hypertension: an endothelin receptor antagonist improves the peak PETCO2Life Sci2014118239740324641953
- HoeperMMMayerESimonneauGRubinLJChronic thromboembolic pulmonary hypertensionCirculation2006113162011202016636189
- SacksRSRemillardCVAgangeNAugerWRThistlethwaitePAYuanJXMolecular biology of chronic thromboembolic pulmonary hypertensionSemin Thorac Cardiovasc Surg200618326527617185190
- DelcroixMVonk NoordegraafAFadelELangISimonneauGNaeijeRVascular and right ventricular remodelling in chronic thromboembolic pulmonary hypertensionEur Respir J201341122423222903956
- GodinasLSattlerCLauEMDead-space ventilation is linked to exercise capacity and survival in distal chronic thromboembolic pulmonary hypertensionJ Heart Lung Transplant201736111234124228666570
- RubinLJPrimary pulmonary hypertensionN Engl J Med199733621111178988890
- HumbertMSitbonOSimonneauGTreatment of pulmonary arterial hypertensionN Engl J Med2004351141425143615459304
- VainshelboimBOliveiraJFoxBDKramerMRThe prognostic role of ventilatory inefficiency and exercise capacity in idiopathic pulmonary fibrosisRespir Care20166181100110927165419
- SunXGHansenJEStringerWWOxygen uptake efficiency plateau: physiology and reference valuesEur J Appl Physiol2012112391992821695524