Abstract
Early identification of people at risk of developing COPD is crucial for implementing preventive strategies. We aimed to systematically review and assess the performance of all published models that predicted development of COPD. A search was conducted to identify studies that developed a prediction model for COPD development. The Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies was followed when extracting data and appraising the selected studies. Of the 4,481 records identified, 30 articles were selected for full-text review, and only four of these were eligible to be included in the review. The only consistent predictor across all four models was a measure of smoking. Sex and age were used in most models; however, other factors varied widely. Two of the models had good ability to discriminate between people who were correctly or incorrectly classified as at risk of developing COPD. Overall none of the models were particularly useful in accurately predicting future risk of COPD, nor were they good at ruling out future risk of COPD. Further studies are needed to develop new prediction models and robustly validate them in external cohorts.
Introduction
COPD is a progressive debilitating lung condition with major impact on both morbidity and early mortality. Of global concern, COPD is projected to rank seventh in worldwide disease burden and as the third leading cause of death by 2030.Citation1 The burden of COPD is projected to rise in Africa and Asia due to the rising prevalence of smoking in these regions and high levels of air pollution.Citation2
COPD carries a very poor prognosis with ~30% of those hospitalized for an exacerbation dying within 2 yearsCitation3 and an in-hospital case fatality rate of ~15%.Citation4 These high fatality rates may be directly related to under-diagnosis of early-stage COPD in the community, as those presenting to tertiary care generally have advanced disease.Citation5,Citation6 There is an urgent need to implement strategies to better identify those at increased risk of COPD or those who have early disease.
There are several well-known risk factors for COPD including tobacco smoking;Citation7 occupational exposures to gases, dusts, and fumes;Citation8 and genetic factors, such as alpha-1-antitrypsin deficiency.Citation9 Smoking is strongly associated with COPD, however there is known individual variation in smoking effects on the lung with up to 30% of smokers never developing COPD.Citation10 There is increasing evidence that early life factors are related to reduced growth and early decline in lung function, both of which are associated with a greater risk of developing COPD at an earlier age.Citation11
Current clinical practice guidelines in the US, UK, and Australia do not recommend screening asymptomatic adults for COPD using spirometry.Citation12 From the primary prevention perspective, predicting those at increased risk of developing COPD can allow for implementation of interventions which may not only prevent COPD developing, but may also help preserve lung function and quality of life in those who do go on to develop COPD. Risk prediction tools that incorporate the best available evidence in order to stratify patients based on their individual risk profiles could facilitate early targeted interventions and management. These tools would also facilitate early detection, accurate diagnosis, and determination of prognosis.
There have been several reviews of predictive models for determining COPD prognosisCitation12,Citation13 but no systematic reviews have investigated the potential of models for prediction of development of COPD. We aimed to systematically synthesize the evidence from studies that have investigated prediction models for subsequent development of COPD in those without a prior diagnosis.
Methods
Search strategy and selection criteria
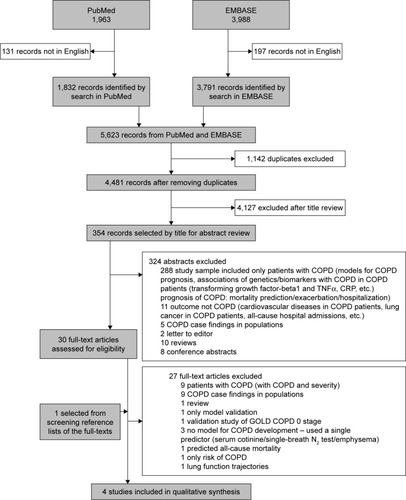
EMBASE and Medline (PubMed) databases were systematically searched from inception to November 30, 2016 (Supplementary material). Reference lists of articles selected for full-text reading (30 articles) were manually searched for additional eligible articles. This review is reported in accordance with the recommendations set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statementCitation14 and was prospectively registered in PROSPERO systematic review registry (registration number 42017064447). Details of inclusion and exclusion criteria are given in Supplementary material.
Data extraction and critical appraisal
Two independent reviewers (GB and CS) ran the same search strategy in PubMed and EMBASE, independently screened titles and abstracts, assessed full-texts of eligible articles, and extracted data following the Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies (CHARMS).Citation15,Citation16 Development of the research questions for the systematic review was based on the CHARMS checklist (). For the selected studies, data were extracted for study design, derivation cohort, predictors, outcomes, and performance of prediction models in the model derivation and validation cohorts. Methods of risk of bias and applicability assessments are given in Supplementary material.
Table 1 Systematic review questions developed using the CHARMS checklist
Results
Overview of the studies included in the review
Our search identified 4,481 non-duplicate records, from which 30 were selected for full-text review (). Only four of these were selected for inclusion, data extraction, and synthesis. The four models developed by these studies differed on many characteristics including the derivation cohort (age, nationality, at-risk), predictors used, statistical methods, and COPD definitionsCitation17–Citation20 ().
Table 2 Study characteristics of the selected prediction models
Study type and population
All the model development studies included in this review concerned the development of original prediction models with the objective of developing a risk prediction tool for the development of COPD (). Of the four studies, one was from a general population cohort,Citation18 two were from electronic medical records databases (one from primary care dataCitation20 and one from hospital record data of asthma patients),Citation19 and one was a hospital-based case–control study with participants selected from the respiratory medicine department.Citation17 One study did not report any specific method for the selection of the initial model predictors,Citation18 two studies used predictors previously reported in the literature,Citation17,Citation20 and the remaining study used a Bayesian network approach to identify predictors.Citation19
Predictors included in all models
All four studies included smoking as a predictor ().Citation17–Citation20 The studies by Guo et al,Citation17 Kotz et al,Citation20 and Himes et alCitation19 all defined smoking as a binary variable; either ever or never smoker. Higgins et alCitation18 reported smoking in three ways: 1) any cigarette smoking (non-smoker, current smoker, ex-smoker), 2) cigarettes per day (categories of none; 1–19; 20+), and 3) change in cigarette smoking between the baseline and follow-up study.
Table 3 Potential risk factors for COPD considered for inclusion in the COPD risk prediction models
Predictors included in some models
One model included asthma as a predictorCitation20 and one study developed its model using a group of asthma patients.Citation19 Three studies included age as a predictor.Citation18–Citation20 One used lung function (% predicted FEV1)Citation18 and two studies used comorbidities (ie, “acute bronchitis and bronchiolitis”, “pneumonia, organism unspecified”, “shortness of breath”, “respiratory distress or insufficiency”, “diabetes mellitus”, “acute upper respiratory infection”, “viral and chlamydial infections”, and “heart failure”) or having respiratory infections as predictors (respiratory infections in childhood).Citation17,Citation19 Included in the final model of Guo et alCitation17 were five single nucleotide polymorphisms (SNPs) (rs2070600, rs10947233, rs1800629, rs2241712, and rs1205) chosen out of a possible 76 identified in a preliminary genome wide association study (GWAS). Two studies developed models separately for men and women.Citation18,Citation20
Outcome definitions
The outcome definition of COPD varied between the different studies (Supplementary material). Two studies used spirometry to define COPD: one used the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criterion of post-bronchodilator FEV1/FVC ratio <0.70 as a cut-offCitation17 and one defined COPD as an FEV1 <65% of the predicted value in combination with an FEV1/FVC ratio <80%.Citation18 Two studies used disease classifications given in the electronic database systems which were based on clinical diagnosis with no further details provided.Citation19,Citation20
Critical appraisal
Risk of bias and applicability concerns in the selected studies were assessed using the CHARMS checklist ( and the details of CHARMS checklist [Supplementary material]).Citation15
Table 4 Assessment of the risk of bias and applicability concerns based on the CHARMS checklist for the selected COPD risk prediction model studies
Of the four model development studies, three used long-term follow-up or long-term data collected in electronic databases, while one used cross-sectionally collected data. Although this latter study rated good quality based on reporting model development and validation, it lacked the ability to predict the risk of COPD in a temporal fashion.Citation17 Three of the four model development studies had a low risk of bias in the selection of participants and one had moderate risk of bias due to the use of a case–control study design, which generally leads to incorrect estimates of the model intercept or baseline hazard. For the selection of predictors, two studies had low risk and two had moderate risk of bias. One study rated moderate risk because 109 variables were considered leading to an event-per-variable ratio of <10,Citation19 potentially leading to model over-fitting. The other moderate risk study defined all variables as binary, potentially leading to the selection of spurious predictors and over-fitting. This study also did not provide detail on measurement and collection of predictor data.Citation17
Outcome assessment in three of four studies had low risk of biasCitation18–Citation20 as the outcomes were measured after predictor measurement. One had moderate risk of bias due to the synchronous measurement of outcome and predictors.Citation17 Risk of attrition was high in three studies that used a cohort design or electronic databases.Citation18–Citation20 Two of the studies did not describe attrition and one study had more than 20% attrition. In the latter study, when baseline characteristics were compared between those followed-up and those who were not, lower smoking rates and better lung function were found in those followed-up.Citation18 All the studies had moderate risk of bias in relation to the analysis. No study accounted for over-fitting (ie, data for the model is well described but not applicable for new individuals) and optimism (ie, caused by over-fitting and leading to an over-estimate of the model’s ability to predict the outcome in new individuals) by shrinkage or other methods; one study did not report on handling of missing data,Citation18 and only one study assessed calibration and discrimination.Citation20
Development and presentation of the prediction model
At the model development stage, two studies selected predictors based on evidence from the literature and availability of predictors.Citation17,Citation20 Another study a priori selected anthropometric predictors and smoking, and included all predictors of comorbidities recorded by the electronic database.Citation19 One study did not report on the criteria used to select predictors included in model development (, , and Supplementary material).Citation18 In model building, two studies used stepwise multiple regression models,Citation17,Citation18 one used Cox regression,Citation20 and another used Bayesian networks.Citation19
Table 5 Risk factors included in the final COPD risk prediction models
Two of the studies that used electronic databases internally validated their models.Citation19,Citation20 The case–control study model was externally validated.Citation17 The long-term cohort study validated the model using a small sample of hospital clinic patients ().Citation18 Three studies used complete case analysis, whereas the handling of missing data was not reported by the fourth.Citation17–Citation20 To use COPD risk prediction tools in clinical practice, thresholds are needed to either rule in or rule out COPD. However, only oneCitation17 study reported clear cut-off points for determining COPD in their models (Supplementary material). One study provided a score chart with regression coefficients,Citation20 and one reported a predictive network based on Bayesian statistics (Supplementary material).Citation19
Performance of the prediction model
The performance of the models was reported in several different ways including a narrative description,Citation18 graphical depictions,Citation19,Citation20 and quantitative performance estimates.Citation17,Citation19,Citation20 Two studies reported calibration using the Hosmer–Lemeshow test; one did not report the p-valueCitation18 and the other reported p=0.86, that is, no significant deviation between the observed and predicted events of COPD.Citation17 Two studies reported area under the receiver operating characteristic curve (ROC AUC); the discriminatory ability of the test to correctly classify those with and without COPD. One study reported an overall ROC AUC of 0.83Citation19 and another study reported the ROC AUC for females as 0.85 and for males ROC AUC as 0.83.Citation20
Of the four studies only one reported sensitivity, specificity, false positive, and false negative rates for a selected cut-off.Citation17 This model reported good sensitivity (83%) and specificity (85%). For two studies, we were able to calculate the sensitivity and specificity.Citation19 No study reported positive or negative predictive values. We calculated positive and negative likelihood ratios using the calculated or provided sensitivity and specificity for three studies.Citation17,Citation19,Citation20 The positive likelihood ratio ranged from 1.85 to 5.53 and negative likelihood ratio ranged from 0.04 to 0.22 ( and Supplementary material).
Table 6 Performance of the COPD risk prediction tools based on derivation cohort
Applicability
Three of the four studies had a moderate risk of applicability issues related to selection of participants.Citation17–Citation19 Two were in specific population groups which limited their models’ wider applicability; one in a Chinese population including SNPs which were likely to be specific for Asian populationsCitation17 and, another in asthmatics.Citation19 The third study was performed 30 years ago and so has lower applicability to current COPD risk.Citation18 One study had applicability issues related to selection of predictors. The classification of predictors as binary variables can lead to the selection of spurious predictors in the model and reduce applicability to new patients.Citation17
All the studies had high or moderate applicability concerns in relation to the analysis. No study used methods to address the possibility of model overfitting. This may limit their applicability to be used in new patients.Citation17–Citation20 Only one study assessed both calibration and discrimination.Citation20 Two studies reported calibration via a Hosmer–Lemeshow testCitation17,Citation18 but neither of these studies reported any assessment of discrimination.
Discussion
From a systematic search of the existing literature, we identified only four models that aimed to predict an individual’s future risk of COPD. The models differed significantly in predictors used, outcome definitions, and populations from which they were developed. Overall the models performed well using a limited number of predictors; age, sex, smoking, and lung function. However, few were validated in external populations and only one included novel risk factors such as genetic markers.
Three models were developed in Western countries and the fourth in China. China now consumes over a third of the world’s tobaccoCitation21 and Chinese men have a rapidly increasing death rate from tobacco-related causes.Citation22 This huge burden of smoking-related disease is likely to also impact COPD prevalence in China and with similar smoking trends in other Asian countries is a burgeoning epidemic. Regional variance in smoking legislation between Asian and Western countries will also affect generalizability of COPD risk prediction models.
All studies included sex in their final models and two studies derived separate models for females and males.Citation18,Citation20 One model identified that smoking in women led to a greater increase in risk of COPD than it did for men.Citation20 Evidence suggests that women are more susceptible to the risks of smoking and occupational exposures.Citation23,Citation24 They receive a greater dose of toxin for the same amount of inhaled smoke because of their smaller airway size.Citation25 Another possible explanation is the role of hormonal factors during the transitional and postmenopausal periods which are associated with a more rapid decline in lung function and may increase the risk of COPD in older women.
Surprisingly only one model included lung function parameters. There is mounting evidence that low lung function predicts subsequent risk of COPD. We have shown that childhood lung function predicts COPD by middle age.Citation26 A study of three longitudinal cohorts found that, of those with COPD at the end of the follow-up period, 50% already had a low FEV1 at the age of 40 years.Citation27 A study of lung function trajectories from the Tuscon cohortCitation28 found that those with the persistently low trajectory reached a maximal level of lung function that was 10% lower than those in the normal trajectory.Citation28 These data provide strong support for the use of lung function measures in prediction of subsequent COPD risk and also provide support for the examination of other early life factors as important potentially modifiable determinants of COPD risk.Citation29,Citation30
Smoking is an important risk factor for COPD and was included in all models. Smoking is known to increase the risk of COPD and in clinical guidelines is a defining criterion for the diagnosis of COPD.Citation31 However, up to 45% of people with COPD are non-smokers and only 30% of smokers will develop the disease.Citation10 Most studies included smoking as a binary measure of ever or never smoking. However, Higgins et alCitation18 included a measure of change in smoking status, a useful predictor for clinical practice as it gives clinicians estimates based on the patient’s current smoking status but also an estimate of risk associated with making changes to those characteristics. Apart from the likely increased precision of a model nuanced for smoking amount and duration, the inclusion of such predictors can enable clinicians to more realistically tailor risk management advice to the individual.
The inclusion of a measure of socioeconomic status that is transferable between different populations is difficult. Measures of socioeconomic status reflect a combination of factors including lifestyle, occupational, environmental, and demographic characteristics. Only one model included a measure of socioeconomic status, defined by a regional specific measure of deprivation. Alternative options include measures of educational level, income level, or number of people living in the home.
The model from the Chinese population considered air pollution exposure as a potential predictor, although this was not included in the final model. There is strong evidence that air pollution is associated with increased risk of COPD.Citation32,Citation33 Other environmental factors such as occupational exposure,Citation32 were not considered by any study. Given the strong evidence supporting the role of exposure to dusts, gases, and fumes in the risk of COPD,Citation8 this is a risk factor that should be considered.
Beyond smoking, few models considered other lifestyle factors. One considered alcohol consumption, but it was not included in the final model. Only one model considered childhood factors including childhood respiratory infections and low birth weight. Early life factors have important potential as future risk predictors. There is increasing evidence that low birth weight has an impact on asthma risk in middle lifeCitation34 and other early life factors and derived indicesCitation30 have been shown to impact on risk of COPD into adulthood.
Basic models, including only a few selected predictors, may be easier to introduce into clinical practice, but they do not make the best use of all relevant clinical knowledge to predict COPD. However, the use of an extended model with a large number of clinical tests may be burdensome for patients, clinicians, and health resources, so a balance between the two is needed.
The main sources of bias related to the statistical analyses performed and how they were presented in the manuscripts. There was often insufficient information to fully assess the development and validation of the models. Objective measures of bias and performance did not rate well in any of the studies. We could not assess the performance of the prediction models quantitatively, because the characteristics of the derivation cohorts, the prediction models themselves, and reporting of model performance varied widely between the studies. Two models had good ability to discriminate between people who were correctly or incorrectly classified as having COPD (concordance statistic 0.830–0.845).Citation19,Citation20
The clinical usefulness of a model requires the determination of positive and negative likelihood ratios in order to determine the ability to rule in COPD versus ruling it out. The positive and negative likelihood ratios were presented in one study and calculable in two. The models had a small to moderately increased ability to detect future COPD risk (positive likelihood ratios ranged between 1.85 and 5.53), and a small to moderate chance of failing to identify someone at risk of future COPD (negative likelihood ratios ranged from 0.04 to 0.22). Overall these results suggest the models developed to date have poor discriminatory ability to predict future COPD risk.
The weak predictive ability of the models in this study highlights the need for future research and the development of more comprehensive models. The inclusion of early life and childhood factors could improve the discriminatory ability of the models. Model development strategies, including the comprehensive validation of all existing models and any newly developed models in a single external study population, are needed. This would allow for direct model comparison of predictors across all cohorts. Future COPD risk prediction models could incorporate new and better predictors including genetic risk prediction scores. Risk prediction model studies in cancer have shown that the addition of susceptibility SNPs can improve the discriminatory power of established risk prediction models by more than 20%.Citation33 One study in this review used selected SNPs identified from a GWAS study of COPD, however the authors did not compare the discriminatory ability of the model with and without the inclusion of the SNPs and so it was not possible to determine how they improved its overall performamce.
In conclusion, our review identified only four models for the prediction of future COPD risk. The models included the most important known predictors of smoking, sex, and age, but pre-existing lung function measurements and other important predictors were not routinely included. Overall none of the models were particularly accurate at predicting future risk of COPD, nor were they good at ruling out future COPD risk. With the emergence of new evidence that low lung function can start in early life and that early life asthma can predict not only low lung function levels into adulthood, but also an increased risk of COPD, future COPD risk prediction models will need to incorporate some of these important early life risk factors.
Author contributions
MC Matheson is the guarantor of the paper. MC Matheson, SC Dharmage, CJ Lodge, G Bowatte, and CV Senaratna: conception, design, literature search, data interpretation and writing the manuscript.
JL Perret, AJ Lowe, GL Hall, PD Sly, N de Klerk, L Keogh, NT Waidyatillake, CF McDonald, D Jarvis, and MJ Abramson: conception, design, and writing the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This project was funded by European Union’s Horizon 2020 research and innovation programme (Ageing Lungs in European Cohorts [ALEC] Study under grant agreement no 633212), and Australian National Health and Medical Research Council European Union collaboration grant ID1101313.
Disclosure
The authors report no conflicts of interest in this work.
References
- MathersCDLoncarDProjections of global mortality and burden of disease from 2002 to 2030PLoS Med2006311e44217132052
- Chan-YeungMAit-KhaledNWhiteNIpMSTanWCThe burden and impact of COPD in Asia and AfricaInt J Tuberc Lung Dis20048121414974740
- SinganayagamASchembriSChalmersJDPredictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary diseaseAnn Am Thorac Soc2013102818923607835
- HoogendoornMHoogenveenRTRuttenvan MolkenMPVestboJFeenstraTLCase fatality of COPD exacerbations: a meta-analysis and statistical modelling approachEur Respir J201137350851520595157
- AbramsonMJSchattnerRLSulaimanNDDel ColleEAAroniRThienFAccuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods studyPrim Care Respir J201221216717322234387
- ToelleBGXuanWBirdTERespiratory symptoms and illness in older Australians: the Burden of Obstructive Lung Disease (BOLD) studyMed J Aust2013198314414823418694
- SametJMThe surgeon generals’ reports and respiratory diseases. From 1964 to 2014Ann Am Thorac Soc201411214114824575983
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- StollerJKAlpha-1 antitrypsin deficiency: an underrecognized, treatable cause of COPDCleve Clin J Med201683750751427399863
- SalviSSBarnesPJChronic obstructive pulmonary disease in nonsmokersLancet2009374969173374319716966
- McGeachieMJYatesKPZhouXPatterns of growth and decline in lung function in persistent childhood asthmaN Engl J Med2016374191842185227168434
- Guirguis-BlakeJMSengerCAWebberEMMularskiRAWhitlockEPScreening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task ForceJAMA2016315131378139327046366
- HanMKSteenrodAWBacciEDIdentifying patients with undiagnosed COPD in primary care settings: insight from screening tools and epidemiologic studiesChronic Obstr Pulm Dis20152210312126236776
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementAnn Intern Med2009151426426919622511
- MoonsKGde GrootJABouwmeesterWCritical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklistPLoS Med20141110e100174425314315
- SmitHAPinartMAntóJMChildhood asthma prediction models: a systematic reviewLancet Respir Med201531297398426597131
- GuoYIQianYGongYIPanCShiGWanHA predictive model for the development of chronic obstructive pulmonary diseaseBiomed Rep20153685386326623030
- HigginsMWKellerJBBeckerMAn index of risk for obstructive airways diseaseAm Rev Respir Dis198212521441517065515
- HimesBEDaiYKohaneISWeissSTRamoniMFPrediction of chronic obstructive pulmonary disease (COPD) in asthma patients using electronic medical recordsJ Am Med Inform Assoc200916337137919261943
- KotzDSimpsonCRViechtbauerWvan SchayckOCSheikhADevelopment and validation of a model to predict the 10-year risk of general practitioner-recorded COPDNPJ Prim Care Respir Med2014241401124841327
- YangGMarketing ‘less harmful, low-tar’ cigarettes is a key strategy of the industry to counter tobacco control in ChinaTob Control201423216717223349230
- ChenZPetoRZhouMContrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studiesLancet2015386100021447145626466050
- PettyTThe Rising Epidemic of COPD in Women: Why women are more susceptible; how treatment should differWomen’s Health in Primary Care1999212
- SinDDCohenSBDayACoxsonHParéPDUnderstanding the biological differences in susceptibility to chronic obstructive pulmonary disease between men and womenProc Am Thorac Soc20074867167418073400
- ChapmanKRChronic obstructive pulmonary disease: are women more susceptible than men?Clin Chest Med200425233134115099893
- BuiDSBurgessJALoweAJChildhood lung function predicts adult COPD and asthma-COPD overlap syndrome (ACOS)Am J Respir Crit Care Med20171961394628146643
- LangePCelliBAgustiALung-function trajectories leading to chronic obstructive pulmonary diseaseN Engl J Med2015373211112226154786
- BerryCEBillheimerDJenkinsICA distinct low lung function trajectory from childhood to the fourth decade of lifeAm J Respir Crit Care Med2016194560761227585385
- PostmaDSWeissSTvan den BergeMKerstjensHAKoppelmanGHRevisiting the Dutch hypothesisJ Allergy Clin Immunol2015136352152926343936
- SvanesCSunyerJPlanaEEarly life origins of chronic obstructive pulmonary diseaseThorax2010651142019729360
- Global Initiative for AsthmaAsthma COPD and Asthma – COPD Overlap Syndrome (ACOS)2014 Available from: http://goldcopd.org/asthma-copd-asthma-copd-overlap-syndrome/Accessed October 31, 2017
- CuiLGallagherLGRayRMUnexpected excessive chronic obstructive pulmonary disease mortality among female silk textile workers in Shanghai, ChinaOccup Environ Med2011681288388721486992
- DiteGSMacInnisRJBickerstaffeABreast cancer risk prediction using clinical models and 77 independent risk-associated SNPs for women aged under 50 years: Australian breast cancer family registryCancer Epidemiol Biomarkers Prev201625235936526677205
- MathesonMCD OlhaberriagueALBurgessJAPreterm birth and low birth weight continue to increase the risk of asthma from age 7 to 43J Asthma201754661662327791430