 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
This study aimed to investigate the impact of cigarette smoke exposure upon CD40–CD40L ligation between bone marrow-derived dendritic cells (BMDCs)and CD4+T cells, and to examine the effects of cigarette smoke exposure upon differentiation of CD4+T cells toward Th17 cells through blockade of CD40-CD40L pathway in mice.
Methods
The study was processed in vivo and in vitro. In vivo, Th17 cells, CD40, interleukin (IL)-17A, and IL-27 in the lung tissues were quantified and compared between mice with and without cigarette smoke exposure. In vitro, Th17 cells, IL-17A, and IL-27 yielded by multiple cell cultivations in which BMDCs from mice with or without cigarette smoke exposure were fostered with CD4+ T cells from healthy mice spleens in the presence of antagonistic CD40 antibody and/or cigarette smoke extract (CSE) were quantified and compared. The flow cytometry was used to detect expressions of Th17 cells and CD40, and the liquid chip was used to detect levels of IL-17A and IL-27.
Results
Both in vivo exposed to cigarette smoke and in vitro to CSE, CD40 expressions noticeably escalated on the surfaces of BMDCs. The presence of Th17 cells, IL-17A, and IL-27 in the lung tissues prominently increased in mice exposed to cigarette smoke. The in vitro culture of CD4+ T cells and BMDCs significantly enhanced the differentiation of CD4+ T cells toward Th17 cells and secretions of IL-17A and IL-27 in the case that BMDCs were produced from mice exposed to cigarette smoke or the culture occurred in the presence of CSE. Usage of antagonistic CD40 antibody evidently reduced the number of Th17 cells, IL-17A, and IL-27 that increased due to cigarette smoke exposure.
Conclusion
The CD40–CD40L ligation is associated with the quantities of Th17 cells and relevant cytokines in the context of cigarette smoke exposure. Reducing the number of Th17 cells via the usage of antagonistic CD40 antibody can be an inspiration for pursuing a novel therapeutic target for immune inflammation in COPD.
Introduction
COPD is a globally common lung disease with high morbidity and mortality, low life quality, and heavy disease burden.Citation1 Profound immune inflammation has been well documented as one of the crucial pathophysiologic mechanisms associated with occurrence and development of COPD. Patients with COPD manifested typical signs of immune inflammation, including noticeable infiltration of inflammation cells in airway and in lung tissue, obviously increased number of myeloid dendritic cells (mDCs) in lavage fluid of brochoalveolus and airway,Citation2–Citation4 and excessive presence of Th1 cells, Th17 cells, and CD8+ T cells.Citation5–Citation7 Th17 cells actively engage in the immune inflammation occurring in COPD, secreting CCL2, recruiting macrophage, releasing metalloprotease,Citation8 and enhancing toxicity of CD8+ T cells through secreting interleukin (IL)-21.Citation9 Besides, the presence of IL-17 mRNA significantly increased in the lung tissues of smokers and COPD patients, suggesting potential involvement of IL-17A in the development of COPD,Citation10 which is mainly secreted by Th17 cells and positively correlated with the number of Th17 cells. However, further mechanism behind the engagement of Th17 cells in the pathogenesis of COPD remains unclear. Among COPD patients with heavy cigarette smoke exposure showed noticeably high expressions of CD40 costimulatory molecules on the surfaces of mDC1 and mDC2.Citation11 The CD40–CD40L cross-talk combining CD40 on the surface of dendritic cell (DC) and CD40L on the surface of activated T cells provides the crucial costimulatory signal for the initiation and regulation of specific immunity.Citation12 CD40–CD40L promotes the activation of DC and increases the secretion of IL-27Citation13 that is yielded by DC and can enhance the proliferation and differentiation of CD8+ T cells.Citation14 In addition to as the main bearer of CD40, DC functions as a powerful antigen-presenting cell (APC) required for optimal activation of naive T cell. It induces proliferation and differentiation of CD4+ T cells and engages in the maintenance of effective immune defense and tolerance. According to the aforementioned literature over roles of CD40–CD40L pathway and DC in human immune response, potential connections amid CD40–CD40L pathway, DC, and physiological activities of Th17 cells as well as relevant cytokines have been strongly suggested and also served as the fundamental hypothesis of our study.
We conducted an animal study containing an in vivo experiment and an in vitro experiment to investigate the hypothetical effects of CD40–CD40L and DC over the differentiation of CD4+ T cells toward Th17 cell. First, a mouse model of cigarette smoke exposure was performed to imitate lung immune inflammation in vivoCitation15,Citation16 and quantities of CD40 and Th17 cells along with relevant cytokines in the mice lungs were evaluated. Second, in vitro experiment, CSE was used to continue the environment of cigarette smoke exposure and bone marrow-derived dendritic cells (BMDCs) of mice with or without cigarette smoke exposure were cultured with CD4+ T cells of healthy mice in the presence of antagonistic CD40 antibody. Then, Th17 and relevant cytokines yielded from the culture of BMDCs and CD4+ T cells were examined.
Methods
Ethics
The Laboratory Animal Ethics Committee of Guangxi Medical University approved all the in vivo and in vitro experiments of this study. “Regulation on the Administration of Experimental Animals (2017 edition)” and “Guideline on the Good Treatment of Experimental Animals (2006 edition)”, both issued by the Ministry of Science and Technology of our country, had been followed to deal with all animals involved in this study.
Animals
The research subjects used in this study were 90 male Balb/c mice, aged 6–8 weeks, weight 20–22 g, specific pathogen free, and purchased from the Animal Experiment Center of Guangxi Medical University (Nanning, China).
Cigarette smoke exposure
Of the 90 healthy Balb/c mice, 60 mice were randomly assigned into two groups by random number table, such as the control group (A group) and the cigarette smoke exposure group (B group) (n=30). Two identical glass boxes marked as “No 1” and “No 2”, respectively, were self-made in the size of 90×55×40 cm, and two holes at the diameter of 2 cm were made on each one for ventilation. The A group was put inside No 1 glass box and allowed to move freely inside for 30 minutes 1 day, 5 days per week up to 4 weeks. The B group was placed into No 2 box and exposed whole bodies to cigarette smoke produced by 10 Marlboro cigarettes (unfiltered cigarette: 12 mg of tar, 0.9 mg of nicotine, and 12 mg of carbon monoxide; Longyan Tobacco Co, Ltd, Nanning, China) on fire for 30 minutes a day, 5 days per week up to 4 weeks. Mice tolerated cigarette smoke exposure without evidence of toxicity (carboxyhemoglobin levels 10% and no weight loss). The rest 30 healthy Balb/c mice were used to attain spleen T lymphocytes.
Preparation of lung mononuclear cells and production of lung homogenate
Mice of both the A group and the B group underwent cervical dislocation and, then, were positioned with the back on the operating board and sterilized three times with iodophor. Both lungs were perfused through right ventricle by 10 mL of phosphate buffer solution (PBS) containing 0.6 M ethylene diamine tetraacetic acid (EDTA) in order to fend off any remaining circulating mononuclear cells. The left lower lung and the right upper lung were removed from the mice chest cavity, rinsed with PBS at 4°C, diced into pieces in 1–2 mm of diameter, and digested in Roswell Park Memorial Institute (RPMI) 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) including 5% of fetal calf serum and 330 U/mL of collagenase IV in a constant temperature oscillator for 30 minutes. After digestion, lung tissues were filtered through a 200 mesh stainless steel filter and centrifuged at low temperature and 1,000 rpm for 5 minutes. Residual red blood cells from lungs were lysed with 15 mL of red blood cell (RBC) lysis solution. The cell suspension was resuspended twice with 2 mL of ice-cold PBS and centrifuged at low temperature for 5 minutes at 1,000 rpm to attain lung mononuclear cells. Th17 cells and CD40 expressed on the surfaces of lung mononuclear cells were quantified by the flow cytometry.
The left upper lung was fetched out of the mice chest, washed with ice-cold PBS, weighed on a scale, immersed in ice-cold PBS, and diced into pieces. Smashed lung tissues were placed into glass homogenate tubes and grinded in a homogenizer. A total of 10% of lung homogenate were centrifuged at low temperature for 10 minutes at 3,000 rpm and restored in a refrigerator at −80°C. Levels of IL-17A and IL-27 were quantified in the lung homogenate by the liquid chip.
Preparation of BMDCs
The thigh bones and tibias of mice from both the A group and the B group were taken out. The bone marrow cell suspension was collected by repeatedly rinsing the bone marrow cavities, centrifuged at 1,500 rpm for 10 minutes, then overlying mouse monocyte lymphocyte separation in twice volume, and centrifuged at 2,000 rpm for 20 minutes. The myeloid mononuclear cell belt was sucked out, washed with PBS twice, and suspended in the RPMI 1640 complete medium containing 150 mL/L of fetal calf serum (FCS), 40 ng/mL of recombinant mouse granulocyte-macrophage colony-stimulating factor (rmGM-CSF) (PeproTech, Rocky Hill, CT, USA), and 10 ng/mL of recombinant mouse interleukin 4 (rmIL-4) (PeproTech). The myeloid mononuclear cell suspension was adjusted to 106/L, distributed in six-pore plates, and fostered in a 50 mL/L CO2 incubator at 37°C for 6 days. At the second, third, and fifth day of the fostering, fresh RPMI 1640 complete medium renewed half of the old medium of each pore in order to keep the optimal concentration including 150 mL/L of fetal calf serum, 40 ng/mL of rmGM-CSF, and 10 ng/mL of rmIL-4. Adherent cells and semiadherent cells were collected at the sixth day of the fostering and cultured with fluorescein isothiocyanate (FITC)-labeled anti-mouse CD11c Monoclonal Antibody (R&D Systems, Inc., Minneapolis, MN, USA). The positive incidence of CD11c+ cells detected by the flow cytometry exceeded 70%.
Cigarette smoke extract (CSE) production
CSE was produced through the process reported by Li et al.Citation17 A total of 15 burning cigarettes (unfiltered cigarette: 12 mg of tar, 0.9 mg of nicotine, and 12 mg of carbon monoxide) were put inside a glass 50 mL syringe that was connected by a rubber tube with a plastic 50 mL syringe containing 10 mL of PBS. CSE was collected by repeated suctions of the plastic 50 mL syringe, purified through a sterile strainer, and measured for the concentration by ultraviolet spectrophotometry.
The in vitro cultures of CD4+ T cells and BMDCs
Spleens were fetched out from the rest 30 healthy mice, treated with RPMI 1640 medium, diced into pieces, and filtered by 200 mesh stainless steel filter. The spleen cells’ suspension was added with PBS, centrifuged at 1,500 rpm for 10 minutes, overlying mouse monocyte lymphocyte separation in twice volume, and centrifuged at 2,000 rpm for 20 minutes. The spleen mononuclear cells were sucked out of centrifuge tubes, rinsed with PBS twice, and centrifuged at 1,000 rpm for 5 minutes. The mononuclear cells were purified by CD4+ T-negative immunomagnetic beads (Thermo Fisher Scientific) and examined for purity by the flow cytometry. The CD4+ T cells were put into 96-well plates, 200 µL unit in each well, and fostered with BMDCs in 24-well plates in the following seven groups: 1) the CD4+ T group; 2) the CD4+ T + CSE group in which CD4+ T cells were cultured in the presence of 10 mL/L CSE; 3) the CD4+ T + BMDC group in which CD4+ T cells were cultured with BMDCs of healthy mice; 4) the CD4+ T + smoke BMDC group in which CD4+ T cells were cultured with BMDCs of mice exposed to the cigarette smoke for 4 weeks; 5) the CD4+ T + BMDC + CSE group in which CD4+ T cells were cultured with BMDCs of healthy mice along with 10 mL/L CSE; 6) the CD4+ T + smoke BMDC + CD40 antibody group in which CD4+ T cells were cultured with BMDCs of mice exposed to the cigarette smoke for 4 weeks along with 10 µg/mL of antagonistic CD40 antibodies (BD Pharmingen, San Diego, CA, USA); and 7) the CD4+ T + BMDC + CSE + CD40 antibody group in which CD4+ T cells were cultured with BMDCs of healthy mice in the presence of 10 µg/mL of antagonistic CD40 antibodiesCitation18 and 10 mL/L CSE (). Each well was inoculated 106/mL of cells and fostered in serum-free mediums for 24 hours.
Figure 1 The in vitro cultures of CD4+ T cells and BMDCs.
Abbreviations: BMDCs, bone marrow-derived dendritic cells; CSE, cigarette smoke extract.
A range of concentrations of CSE, including 5, 10, 15, and 20 mL/L, were used to sift out the optimal one, to which BMDCs were exposed and showed more active proliferation measured by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay than to other three concentrations. All absorbance values of BMDCs detected at 490 nm at 12, 24, 48, and 72 hours culminated with 10 mL/L CSE in comparison with other three concentrations of CSE (). Hence, the 10 mL/L CSE was applied to the cultures of CD4+ T cells and BMDCs in vitro. Before these in vitro cultures started, parts of BMDCs produced from the A group were cultured with 10 mL/L CSE in 24-well plates for 24 h. Then, the flow cytometry was used to evaluate CD40 expression on the surfaces of BMDCs that were sampled from ones prepared from the A group, from the B group, and from the A group and cultured with CSE. Th17 cells produced by cultivations of CD4+ T cells and BMDCs were quantified by the flow cytometry, and IL-17A and IL-27 were quantified by the liquid chip.
Table 1 BMDCs proliferation exposed to varied concentrations of CSE(A490 nm, )
Flow cytometry
Cells were stained with FITC-labeled CD11c+ mAb for the detection of expression of CD11c+, with PE-labeled CD40 mAb (R&D Systems, Inc.) for the quantification of CD40, with peridinin chlorophyll protein (PercP)-labeled antirat CD4 mouse mAb (R&D System, Inc.) for purification of CD4+ T cells, and with PE-labeled antirat IL-17 mAb (R&D System, Inc.) for Th17 cells count according to each manufacturer’s instruction. The stained cells were analyzed on a FACSCalibur Four Color Fluorescence Flow Cytometer (BD Pharmingen).
Liquid chip
The concentrations of IL-17A and IL-27 in mice lung homogenate and in the above seven groups of cultivations of CD4+ T cells and BMDCs were measured by Luminex liquid phase chips (ProcartaPlex Mouse-Il-27 and IL-17A-Simplex 96; eBioscience, Dummer, Germany) through a Luminex 200 standard high-throughput detection technology platform (LianKe Bio, Hangzhou, China).
Statistical analysis
The SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. All data were expressed as . The independent sample t-test was used to examine the difference between two groups. Repeated measures analysis of variance with post hoc Bonferroni procedure were used for comparisons of multiple groups over normally distributed samples. In the case of sample with heteroscedasticity, nonparametric Kruskall–Wallis test and Mann–Whitney test were used for measuring differences. The differences were considered to be statistically significant when P≤0.05.
Results
In vivo: Th17 cell, IL-17A, IL-27, and CD40 on the surface of lung mononuclear cell
Mice exposed to cigarette smoke for 4 weeks indicated significantly higher quantity of CD4+IL-17+ T cells (2.81±0.58) () and levels of IL-17A (484.00±74.08) and IL-27 (25.30±3.74) in the lung than that of the controls (Th17 cell: 1.12±0.36, P<0.05, IL-17A: 220.31±83.09 and IL-27: 7.22±1.18, P<0.01). As well, CD40 expressions on surfaces of lung mononuclear cells of mice with cigarette smoke exposure noticeably increased (43.67±7.03) in comparison with that of those exempt from smoke exposure (15.86±3.84, P<0.01; ).
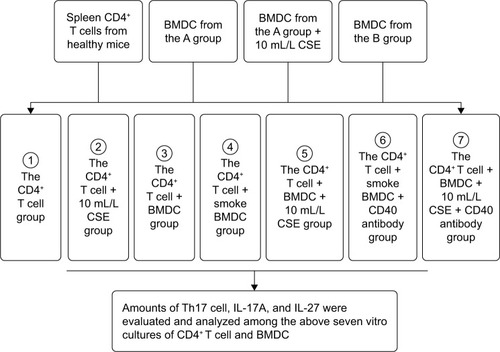
Figure 2 The number of CD4+IL-17+ T lymphocytes in lungs of the A groupa and the B groupb.
Notes: aThe A group in which mice were exempt from cigarette smoke exposure. bThe B group in which mice were exposed to cigarette smoke for 4 weeks. (A) Lymphocytes were marked with a circle in lung mononuclear cells by the flow cytometry. (B) The number of CD4+IL-17+ T lymphocytes in lungs of the A group. (C) The number of CD4+IL-17+ T lymphocytes in lungs of the B group.
Abbreviations: FSC, forward scatter; PE, phycoerythrin.
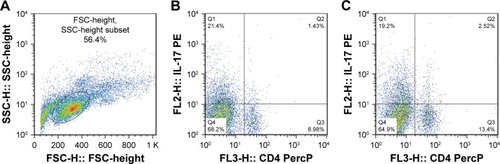
Figure 3 CD40 on surfaces of lung mononuclear cells of the A groupa and the B groupb.
Abbreviation: PE, phycoerythrin.
In vitro: CD40 on the surface of BMDCs
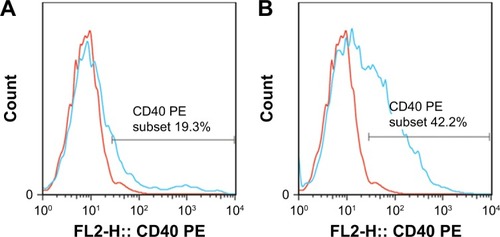
CD40 expression obviously escalated on the surfaces of BMDCs that were sampled from ones prepared from the B group (51.61±2.23, P<0.01), and from the A group and fostered with 10 mL/L CSE(44.93±5.71,P<0.01), when compared to that from the A group without exposed to CSE(10.39±2.81). (see and ).
Table 2 CD40 expression on the surfaces of BMDCs
Figure 4 CD 40 expression on surfaces of BMDCs.
Abbreviations: BMDCs, bone marrow-derived dendritic cells; CSE, cigarette smoke extract; PE, phycoerythrin.
In vitro: Th17 cells, IL-17A, and IL-27
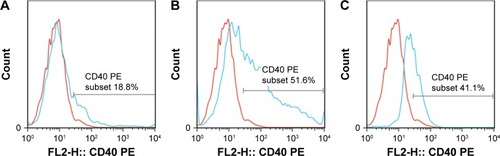
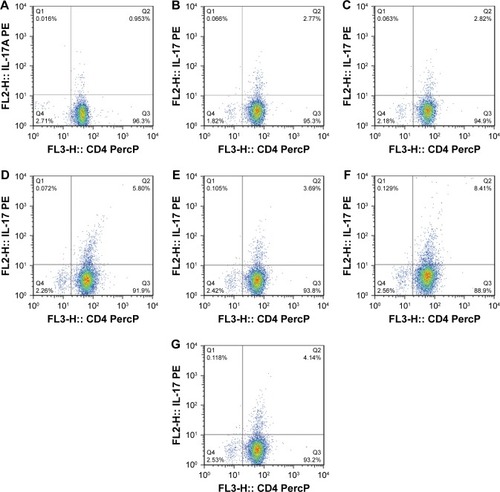
All the CD4+ T + CSE group (Th17: 3.85±0.73, IL-17A: 93.83±14.71), the CD4+ T + BMDC group (Th17: 3.91±0.42, IL-17A: 109.29±16.09), the CD4+ T + smoke BMDC group (Th17: 5.35±1.03, IL-17A: 313.13±49.35), and the CD4+ T + BMDC + CSE group (Th17: 7.78±0.76, IL-17A: 329.36±45.18) exhibited prominent increases in a comparison with the CD4+ T group (Th17:1.39±0.48, IL-17A: 38.42±9.41) with respect to the number of Th17 cells () and IL-17A (). Among these groups, the CD4+ T + BMDC + CSE group achieved the most positive impact upon the number of Th17 cells and IL-17A. Besides no values of IL-27 showed in either the CD4+ T group or the CD4+ T + CSE group, values of IL-27 () indicated a similar trend as that of Th17 and IL-17A among the CD4+ T + BMDC group, the CD4+ T + smoke BMDC group, and CD4+ T + BMDC + CSE group. The administration of antagonistic CD40 antibody substantially reversed the rising tendency on the above three indicators in the case of CSE or BMDCs from cigarette smoke-exposed mice, resulting in obviously decreased presence of Th17 cells, IL-17A, and IL-27 as revealed either in the CD4+ T + smoke BMDC + CD40 antibody group (Th17: 3.27±0.80; IL-17A 174.24±35.21; IL-27 14.37±4.95) or in the CD4+ T + BMDC + CSE + CD40 antibody group (Th17: 3.88±0.41; IL-17A 141.18±29.27; IL-27 13.75±5.11) ( and and ).
Table 3 Th17 cell expressions in the in vitro cultures of CD4+ T cells and BMDCs
Table 4 Concentrations of IL-17A and IL-27 in the in vitro cultures of CD4+ T cells and BMDCs
Figure 5 CD4+IL-17+ T lymphocytes in the seven in vitro cultures of CD4+ T cells and BMDCs.
Abbreviations: BMDCs, bone marrow-derived dendritic cells; CSE, cigarette smoke extract; PE, phycoerythrin.
Discussion
Among the studies focusing on the role of CD4+IL-17+ T cells in the pathogenesis and development of COPD, this study is one of the first attempts to explore the impacts that DCs and CD40–CD40L costimulatory pathway generated on CD4+IL-17+ T cells under the circumstance of cigarette smoke exposure. As demonstrated in the “Results” section, regardless of in vivo or in vitro, cigarette smoke exposure always enhanced the differentiation of CD4+ T toward CD4+IL-17+ T cells, significantly expanding the expression of CD4+IL-17+ T cells. Our previous study similarly indicated the excessive presence of CD4+IL-17+ T cells in the lungs of enrolled COPD patients and smokers and further demonstrated that IL-17 cells positively correlated with the size of alveolar space or thickness of pulmonary artery wall or the number of lung CD4+ and CD8+ cells while negatively related to FEV1%pred of patients.Citation10 CD4+IL-17+ T cells, also called Th17 cells and generally identified as one subpopulation of helper T cell, are characterized with promoting the expression of transcription factor ROR-γt and yielding a variety of cytokines mediating immune response.Citation19 Hence, Th17 cells are indispensable to immune response targeting antigens and immune toleration in normal situations. Activated IL-17 triggers recruitments of neutrophils and macrophages, induces releases of CXCL8 and CXCL10 from bronchial epithelial cells and macrophages, and, in consequence, promotes airway chemotaxis of neutrophils and mononuclear cells.Citation20–Citation23 IL-17 is mainly secreted by Th17 cells and IL-27 produced by activated DCs. However, there is evidence showing increased expression of IL-17A/F in the cultivation of lung tissue cells and CSE in healthy population and COPD patientsCitation24 and elevated level of IL-27 in sputum and blood plasma of COPD patients,Citation25 which is consistent with our study conducted on mice that indicated significantly elevated levels of IL-17A and IL-27 in vivo or in vitro after cigarette smoke exposure or CSE stimulation.
DC is the most powerful APC in human body and acts as a bridge connecting innate immunity and acquired immunity. DCs not only capture and present antigens but also activate native T lymphocytes. Cigarette smoke exposure induces proliferation and maturation of DCs in the lung tissues and drives DCs immigrating to the lung lymphoid tissue. Toll-like receptor located in the DCs functions as a pattern recognition receptor, selectively recognizing pathogen, activating downstream signal pathway, strengthening secretions of cytokines from activated immune cells, upregulating major histocompatibility complex (MHC) molecules and costimulatory molecules, and promoting maturation of DC and activation of T cells.Citation26–Citation28 COPD patients with smoking habit demonstrated increased expression levels of CD40 on mDC1 and mDC2. Plasma-soluble CD40L that constitutes another half part of the CD40–CD40L ligation significantly increased in COPD patients. The activation and synergy of CD40/CD40L signal contributed to the development of the emphysema caused by sensitized Fas-mediated apoptosis of alveolar epithelial cells.Citation29 Otherwise, COPD is a lung alien with systemic inflammation that involves not only the lung but also other organs.Citation30 Accordingly in our study, the BMDCs produced from mice exposed to cigarette smoke for 4 weeks exhibited obviously increased expressions of CD40, which echoed with the BMDCs from healthy mice cultured in vitro with CSE and the lung mononuclear cells from mice exposed to cigarette smoke for 4 weeks.
It is well documented that CD40–CD40L costimulatory pathway is required to optimal activation of naive T lymphocytes and proliferation of T lymphocytes,Citation31 which also activates CD80 and CD86 and elevates their count. During the process of T-lymphocyte activation, CD86 combined with CD28 provides synergetic signals necessary for the early activation of T-cell clone and CD80 and CD28 maintain effective proliferation of T-cell clone.Citation32 Our study revealed that the CD40–CD40L costimulatory pathway was clearly associated with the quantities of Th17, IL-17A, and IL-27. As indicated in the “Results” section, BMDCs with high expression of CD40 as a result of cigarette smoke exposure or CSE exposure substantially enhanced the differentiation of CD4+ T cells toward Th17 cells and the secretions of IL-17A and IL-27, giving rise to elevated expression of Th17 cells and levels of IL-17A and IL-27 after being cultured with CD4+ T cells, while the administration of antagonistic CD40 antibody that effectively blocked CD40–CD40L broke the augment effects over Th17 cells, IL-17A, and IL-27 due to cigarette smoke exposure or CSE exposure.
CD40–CD40L promotes the activation of DCs and the secretion of IL-27, activating STAT1 through IL-27-27R axis, inducing expressions of T-bet and eomesodermin (EOMES), and further promoting the expression of CD8+ T cells and secretions of perforin and granzyme B.Citation33 IL-27 was reported to generate a positive impact upon differentiation of T lymphocytes toward Th1 cellsCitation34 but a restrictive impact upon differentiation toward Th17 cells, although either of the two opposed impacts were suggested mild in the context of inflammatory response. Hence, IL-27 scarcely inhibits mature Th17 in the process of inflammation.Citation35 In our study, increased level of IL-27 did not deliver specific depression in the presence of Th17 and IL-17. The CD40–CD40L pathway blocked by the antagonistic CD40 antibody reduced levels of IL-27and IL-17A in our study, so blockade of CD40–CD40L ligation might mitigate heated inflammatory responses caused by exposure to cigarette smoke or to CSE. Iezzi et alCitation36 reported that DCs with low expression of CD40 reduced productions of related cytokines and restrained differentiations toward Th17. Martin et alCitation37 found that DCs with deficient presence of CD40 suppressed RelB of nuclear factor-κB (NF-κB) family, ceasing the initiation of immune response and curbing the unfoldment of ongoing immune response. The aforementioned two studies revealed the indispensable role that CD40 has played in the action of antigen presented by DCs.
Conclusion
DCs and CD40–CD40L costimulatory pathway are associated with the development of immune inflammation caused by cigarette smoke exposure. The blockade of CD40–CD40L pathway with antagonistic CD40 antibody effectively restrains differentiations of T lymphocytes toward Th17 cells and, in consequence, substantially cuts down excessive presence of Th17 cell, IL-17A, and IL-27 due to cigarette smoke exposure. Depressing the CD40–CD40L ligation between DCs and T cells can be an inspiration for pursuing a new therapeutic target for immune inflammation in COPD.
Author contributions
XZ made substantial contributions to concept and design of this study, data interpretation, and critical modifications of some important intellectual contents. YL and YS equally made substantial contributions over drafting this article, data acquisition, and data interpretation. LK, GZ, and LZ were significantly involved in data acquisition and data analysis. JZ managed some important modifications over the content. JL substantially contributed to data analysis. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This study was funded by grants from National Natural Science Foundation of China (grant number 81660007), Youth Foundation of Guangxi Medical University (grant number GXMUYSF-201614), and Talents Highland of Emergency and Medical Rescue of Guangxi Province in China (grant number GXJZ201514). YL and YS are the co-first authors.
Disclosure
The authors report no conflicts of interest in this work.
References
- FanerRCruzTAgustiAImmune response in chronic obstructive pulmonary diseaseExpert Rev Clin Immunol20139982183324070046
- LommatzschMBratkeKKnappeTAcute effects of tobacco smoke on human airway dendritic cells in vivoEur Respir J20103551130113619741025
- VassalloRWaltersPRLamontJKottomTJYiESLimperAHCigarette smoke promotes dendritic cell accumulation in COPD; a Lung Tissue Research Consortium studyRespir Res2010261145
- Van PottelbergeGRBrackeKRDemedtsIKSelective accumulation of langerhans-type dendritic cells in small airways of patients with COPDRespir Res2010221135
- EppertBLWorthamBWFluryJLBorchersMTFunctional characterization of T cell populations in a mouse model of chronic obstructive pulmonary diseaseJ Immunol201319031331134023264660
- LiXNPanXQiuDImbalances of Th17 and Treg cells and their respective cytokines in COPD patients by disease stageInt J Clin Exp Med20147125324532925664038
- LiHLiuQJiangYZhangYZhangYXiaoWDisruption of th17/treg balance in the sputum of patients with chronic obstructive pulmonary diseaseAm J Med Sci2015349539239725782336
- ChenKPociaskDAMcAleerJPIL-17RA is reacquired for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smokePLoS One201165e2033321647421
- DuanMCHuangYZhongXNTangHJTh17 cell enhances CD8 T-cell cytotoxicity via IL-21 production in emphysema miceMediators Inflamm2012201289805323319833
- ZhangJChuSZhongXLaoQHeZLiangYIncreased expression of CD4+IL-17+ cells in the lung tissue of patients with stable chronic obstructive pulmonary disease and smokersInt Immunopharmacol2013151586623127823
- FreemanCMMartinezFJHanMKLung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2009180121179118819729666
- KawabeTNakaTYoshidaKThe immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formationImmunity1994131671787534202
- PflanzSTimansJCCheungJIL-27, a heterodimeric cytokine composed of EBI3 and P28 protein, induces proliferation of naive CD4+ T cellsImmunity200216677979012121660
- de GrootRvan BeelenAJBakdashGTaanman-KueterEWde JongECKapsenbergMLViral dsRNA-activated human dendritic cells produce IL-27, which selectively promotes cytotoxicity in naive CD8+ T cellsJ Leukoc Biol201292360561022701040
- WangHPengWWengYImbalance of Th17/Treg cells in mice with chronic cigarette smoke exposureInt Immunopharmacol201214450451223044435
- KuangLJDengTTWangQDendritic cells induce Tc1 cell differentiation via the CD40/CD40L pathway in mice after exposure to cigarette smokeAm J Physiol Lung Cell Mol Physiol20163113L581L58927448664
- LiMZhongXHeZEffect of erythromycin on cigarette-induced histone deacetylase protein expression and nuclear factor-κB activity in human macrophages in vitroInt Immunopharmacol201212464365022265969
- KarimiMHEbadiPPourfathollahAAMoazzeniSMTolerance induction by CD40 blocking through specific antibody in dendritic cellsIran J Allergy Asthma Immunol20109314114720952803
- ParkHLiZYangXOA distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17Nat Immunol20056111133114116200068
- VanaudenaerdeBMWuytsWADupontLJVan RaemdonckDEDemedtsMMVerledenGMInterleukin-17 stimulates release of interleukin-8 by human airway smooth muscle cells in vitro: a potential role for interleukin-17 and airway smooth muscle cells in bronchiolitis obliterans syndromeJ Heart Lung Transplant200322111280128314585390
- WuytsWAVanaudenaerdeBMDupontLJVan RaemdonckDEDemedtsMGVerledenGMInterleukin-17-induced interleukin-8 release in human airway smooth muscle cells: role for mitogen-activated kinases and nuclear factor-kappa BJ Heart Lung Transplant200524787588115982617
- LindénAHoshinoHLaanMAirway neutrophils and interleukin-17Eur Respir J200015597397710853869
- YePRodriguezFHKanalySRequirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defenseJ Exp Med2001194451952711514607
- ChangYAl-AlwanLAlshakfaSUpregulation of IL-17A/F from human lung tissue explants with tobacco smoke exposure: implications for COPDRespir Res201415114525427574
- CaoJZhangLLiDIL-27 is elevated in patients with COPD and patients with pulmonary TB and induces human bronchial epithelial cells to produce CXCL10Chest2012141112113021778255
- ParkerLCPrinceLRSabroeITranslational mini-review series on Toll-like receptors: networks regulated by Toll-like receptors mediate innate and adaptive immunityClin Exp Immunol2007147219920717223959
- VermaelenKPauwelsRPulmonary dendritic cellsAm J Respir Crit Care Med2005172553055115879415
- ColonnaMTrinchieriGLiuYJPlasmacytoid dendritic cells in immunityNat Immunol20045121219122615549123
- ShigetaATadaYWangJYCD40 amplifies Fas-mediated apoptosis: a mechanism contributing to emphysemaAm J Physiol Lung Cell Mol Physiol20123032L141L15122610351
- AgustíAEdwardsLDRennardSIEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) InvestigatorsPersistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotypePLoS One201275e3748322624038
- SunYPengDLecandaJIn vivo gene transfer of CD40 ligand into colon cancer cells induces local production of cytokines and chemokines, tumor eradication and protective antitumor immunityGene Ther20007171467147611001366
- SlavikJMHutchcroftJEBiererBECD80 and CD86 are not equivalent in their ability to induce the tyrosine phosphorylation of CD28J Biol Chem19992745311631249915850
- MorishimaNMizoguchiIOkumuraMA pivotal role for interleukin-27 in CD8+ T cell functions and generation of cytotoxic T lymphocytesJ Biomed Biotechnol2010201060548320454646
- OwakiTAsakawaMMorishimaNA role for IL-27 in early regulation of Th1 differentiationJ Immunol200517542191220016081786
- El-behiMCiricBYuSZhangGXFitzgeraldDCRostamiADifferential effect of IL-27 on developing versus committed Th17 cellsJ Immunol200918384957496719786534
- IezziGSondereggerIAmpenbergerFSchmitzNMarslandBJKopfMCD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cellsProc Natl Acad Sci U S A2009106387688119136631
- MartinEO’SullivanBLowPThomasRAntigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory T cells secreting interleukin-10Immunity200318115516712530984
