Abstract
Background
Pulmonary vascular disease, especially pulmonary hypertension, is an important complication of COPD. Bronchiectasis is considered not only a comorbidity of COPD, but also a risk factor for vascular diseases. The main pulmonary artery to aorta diameter ratio (PA:A ratio) has been found to be a reliable indicator of pulmonary vascular disease. It is hypothesized that the co-existence of COPD and bronchiectasis may be associated with relative pulmonary artery enlargement (PA:A ratio >1).
Methods
This retrospective study enrolled COPD patients from 2012 through 2016. Demographic and clinical data were collected. Bhalla score was used to determine the severity of bronchiectasis. Patient characteristics were analyzed in two ways: the high (PA:A >1) and low (PA:A ≤1) ratio groups; and COPD with and without bronchiectasis groups. Logistic regression analysis was used to assess risk factors for high PA:A ratios.
Results
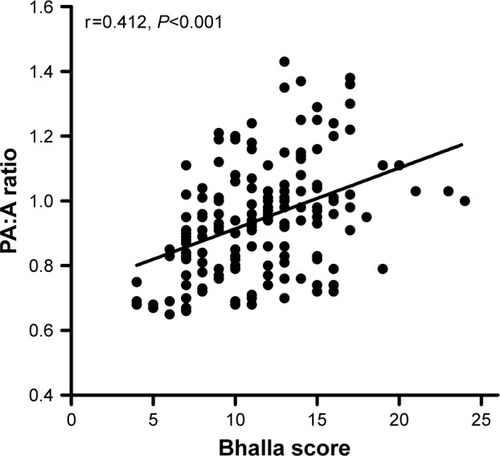
In this study, 480 COPD patients were included, of whom 168 had radiographic bronchiectasis. Patients with pulmonary artery enlargement presented with poorer nutrition (albumin, 35.6±5.1 vs 38.3±4.9, P<0.001), lower oxygen partial pressure (74.4±34.5 vs 81.3±25.4, P<0.001), more severe airflow obstruction (FEV1.0, 0.9±0.5 vs 1.1±0.6, P=0.004), and a higher frequency of bronchiectasis (60% vs 28.8%, P<0.001) than patients in the low ratio group. Patients with both COPD and bronchiectasis had higher levels of systemic inflammation (erythrocyte sedimentation rate, P<0.001 and fibrinogen, P=0.006) and PA:A ratios (P<0.001). A higher PA:A ratio was significantly closely correlated with a higher Bhalla score (r=0.412, P<0.001). Patients with both COPD and bronchiectasis with high ratios presented higher levels of NT-proBNP (P<0.001) and systolic pulmonary artery pressure (P<0.001). Multiple logistic analyses have indicated that bronchiectasis is an independent risk factor for high PA:A ratios in COPD patients (OR =3.707; 95% CI =1.888–7.278; P<0.001).
Conclusion
Bronchiectasis in COPD has been demonstrated to be independently associated with relative pulmonary artery enlargement.
Introduction
Pulmonary vascular disease is a common comorbidity of end-stage COPD and a common cause of poor prognosis. Approximately 50% of patients with severe COPD develop pulmonary hypertension (PH),Citation1 while out-of-proportion PH was also noted in patients with mild airflow obstruction.Citation2 PH has been found to be associated with increased risk of exacerbation and death of COPD.Citation3,Citation4 It also places a huge economic burden on patients.Citation5 This indicates that insight into the pulmonary vascular disease of COPD patients is of major importance.
With the extensive use of high-resolution computed tomography (HRCT), the presence of bronchiectasis, a previously unrecognized comorbidity, has been identified.Citation6 The prevalence of bronchiectasis ranged from 4% to 57% in COPD patients.Citation7,Citation8 Patients with both COPD and bronchiectasis experience more frequent exacerbations and have a worse health status.Citation9 The occurrence of bronchiecta-sis was based on the destruction of the airway wall, which also combined with repair and remodeling of the vascular bed. Recently, there has been increased interest in the relationship between bronchiectasis and vascular diseases.Citation10–Citation12 Bronchiectasis was recognized as a risk factor for vascular diseases,Citation10 and PH was demonstrated to be a prognostic indicator of bronchiectasis patients.Citation12 Possible mechanisms underlying the process include hypoxia, systemic inflammation, and arterial stiffness.Citation10 In theory, there may be a close crosstalk between bronchiectasis and pulmonary vascular disease. Given this, we here assumed that the co-existence of COPD and bronchiectasis may be a risk factor for pulmonary vascular disease.
The main pulmonary artery to aorta diameter ratio (PA:A ratio) has been shown to be a reliable index of pulmonary vascular diseaseCitation4 and a predictor of frequent exacerbations of COPD.Citation4,Citation13 Patients with PA:A ratios of more than 1 were recognized as having relative pulmonary artery enlargement. They were characterized with impaired physical activityCitation14 and PH.Citation15 However, it remains unknown which characteristic of COPD patients is the risk factor for relative pulmonary artery enlargement.
In the present study, we hypothesized that COPD- bronchiectasis co-existence patients would be the risk population for relative pulmonary artery enlargement.
Methods
Study population
Patients who attended Qilu Hospital of Shandong University from April 2012 to December 2016 were retrospectively reviewed. COPD was diagnosed based on Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines,Citation16 with respiratory symptoms, physical signs, and persistent airflow limitation (post-BD FEV1/FVC <0.70). Patients with HRCT scans and pulmonary function tests of stable state within 12 months were included. Patients with other respiratory diseases influencing the incidence of pulmonary vascular enlargement, such as interstitial lung disease and pulmonary thromboembolism, were excluded from the study. Other exclusion criteria included asthma, thoracic pleural disease, community-acquired pneumonia, or pulmonary masses >3 cm on a chest CT. All of the demographic and clinical characteristics were collected, including age, gender, smoking history, history of illness, comorbidities, peripheral blood tests (PH value, PaO2, PaCO2, white blood cells, neutro-phils, eosinophils, albumin (ALB), erythrocyte sedimentation rate (ESR), fibrinogen, and N-terminal pro-B-type natriuretic peptide), HRCT scans, systolic pulmonary arterial pressure of echocardiography, and pulmonary function test data.
The study was approved by the Ethics Committee of our hospital, Qilu Hospital of Shandong University (approval number: 2015091). The data were anonymous, and retrospective, the Ethics Committee of Qilu Hospital of Shandong University agreed that written informed consent from participants was not required.
Pulmonary function testing (PFT)
A computerized spirometer (MasterScreen, Jaeger, Hoechberg, Germany) was used for PFT, according to recommendations issued by the American Thoracic Society and European Respiratory Society (ATS/ERS).Citation17 Basic information (age, height, and weight) and spirometry parameters (FVC, FEV1.0, and FEV1.0/FVC) were collected.
HRCT scan
Chest HRCT scans were performed on a 64-slice spiral CT scanner (SOMATOM Definition AS, Sie-mens Healthcare, Erlangen, Germany) at full inspiration. Tube voltage was 120 kV and tube current varied between 20 and 500 mA by automatic regulation. All scans were acquired using the standard parameters: 0.5 s rotation time and 512×512 pixels. Consecutive images with a 1 mm slice thickness were reconstructed.
Diagnosis and assessment of bronchiectasis
The presence of bronchiectasis was confirmed by an experienced respiratory radiologist and a respiratory physician, according to criteria published by Naidich et al:Citation20 (1) lack of tapering of bronchi, (2) dilation of bronchi when the internal diameter was larger than that of the adjacent pulmonary artery, or (3) visualization of the peripheral bronchi within 1 cm of the costal pleural surface or adjacent mediastinal pleural surface.Citation18–Citation20 The severity of bronchiectasis was evaluated using Bhalla score,Citation21 which analyzed nine radiological characteristics of the lesions. Lingula was considered an independent lobe. Bronchiectasis in a single pulmonary segment was not included because it could appear in healthy individuals.Citation8 Bronchiectasis occurring before age 40 was also excluded from the group.
PA:A ratio measurements
The PA:A ratio was measured using previously widely used methods.Citation4,Citation22–Citation24 Briefly, pulmonary artery (PA) and aorta (A) diameters were measured at the same level of pulmonary artery bifurcation. The PA diameter was perpendicular to the long axis of the pulmonary artery at the tangent of PA and A, with two perpendicular lines averaged for the A diameter by use of a CT scale. In order to collect accurate data, two blinded reviewers worked independently. As in previous reports,Citation4,Citation23 PA:A ratios of more than 1 were considered high.
Statistical analysis
Statistical analyses were performed using SPSS version 19.0 (IBM Corporation, Armonk, NY, USA). Data are presented as mean ± SD for continuous variables and as a percentage for categorical variables. The Student’s t-test was used for normally distributed values and Mann–Whitney U-test was used for non-normally distributed values. Quantitative data from three or more groups were compared using Kruskal–Wallis test followed by Bonferroni correction. Considering categorical variables, the chi-square test was used to determine statistical differences. The Pearson’s r coefficient was used to evaluate the linear correlation between severity of bronchiectasis and PA:A ratios. Multivariate logistic regression analysis was used to identify predictive values for PA:A ratios >1. Variables found to be statistically significant in bivariate analysis and those of clinical interest were included in the multivariate analysis. P<0.05 was considered statistically significant.
Results
Baseline characteristics of the study population
A total of 480 COPD patients were included in this study. Mean age was 69.5±9.6 years, and approximately 76% of the patients were male. Of all the subjects, 70.6% were smokers or ex-smokers. Mean FEV1, FVC, and FEV1/FVC were 1.1 L, 2.2 L, and 50.0%, respectively. In this study population, there were 168 (35%) patients with radiographic bronchiectasis. Other clinical characteristics are shown in .
Table 1 Characteristics of COPD patients
Comparisons of clinical features between patients with and without relative pulmonary artery enlargement
As previously reported,Citation4,Citation23,Citation25 a PA:A ratio >1 was designated as relative pulmonary artery enlargement. Out of the 480 patients with measurable CT ratios, 95 were in the high ratio group, and the others were in the low ratio one. Patients with relative pulmonary artery enlargement were more likely to be younger (P=0.003) and female (P<0.001) and had fewer pack-years of smoking (P=0.016). These patients also presented with lower levels of PaO2 (P<0.001), higher levels of PaCO2 (P<0.001), and worse nutrition (ALB, 35.6±5.1 vs 38.3±4.9, P<0.001). Considering the pulmonary function test, patients with a PA:A ratio of more than 1 had worse pulmonary function parameters, including FVC (P=0.003), FEV1.0 (P=0.004), and FEV1% predicted (P=0.008). As for underlying diseases, the high PA:A ratio group had more patients with bronchiectasis (60% vs 28.8%, P<0.001) and diabetes mellitus (23.2% vs 8.6%, P<0.001). With respect to CT measurement data, the PA (P<0.001) and PA:A ratio (P<0.001) were absolutely larger in the high ratio group, regardless of the slightly shorter A diameter ().
Table 2 Characteristics of COPD patients with high and low PA:A ratios
Clinical characteristics of the study population with bronchiectasis
Of all the subjects in our study, 168 had the diagnosis of bronchiectasis, and the other 312 did not. Clinical characteristics are shown in . Compared with patients without bronchiectasis, patients with both conditions were less likely to be current or former smokers (P=0.026) and under a worse nutritional status (ALB, P<0.001). These patients also presented with higher peripheral levels of ESR (P<0.001) and fibrinogen (P=0.006), which suggested greater systemic inflammation. CT signs of pulmonary vascular changes were significantly different. PA (32.5±5.7 vs 30.0±5.6, P<0.001) and PA:A ratios (0.95±0.17 vs 0.83±0.15, P<0.001) were greater in patients with bron-chiectasis, despite the slightly shorter aorta diameter observed in this group. Besides that, the proportion of sex, BMI (body mass index), and impairment of pulmonary function did not show prominent differences between two groups.
Table 3 Characteristics of COPD patients with and without bronchiectasis
The Balla score was used to quantify the severity of bronchiectasis in this study. As shown in , correlation analysis showed that PA:A ratios increased as the Balla score increased (r=0.412, P<0.001). The NT-proBNP and pulmonary artery pressure of echocardiography were more severe in COPD-bronchiectasis co-existence patients who had high ratios than those who had lower ratios ().
Table 4 Comparison of NT-proBNP and pulmonary artery pressure between COPD patients with and without bronchiectasis
Logistic regression analysis of the risk factors of the PA:A ratio
Seven variables (age, sex, ALB, PaO2, FEV1% predicted, bronchiectasis, and diabetes mellitus) were included in the logistic regression analysis (). Multivariate logistic regression analysis showed that bronchiectasis (OR =3.707; 95% CI =1.888–7.278; P<0.001) and diabetes mellitus (OR =3.721; 95% CI =1.490–9.289; P=0.005) correlated with a PA:A ratio of more than 1 in COPD patients. COPD patients with better nutrition (higher level of ALB) were more likely to have low PA:A ratios.
Table 5 Logistic regression analysis for predictors of high PA:A ratio in COPD patients
Discussion
To the best of our knowledge, this is the first report to establish a relationship between bronchiectasis and the marker of pulmonary vascular disease in COPD patients. In this study, COPD patients with concomitant radiographic bronchiectasis were demonstrated to be at increased risk of relative pulmonary artery enlargement, regardless of the severity of spirometry impairment.
PA:A ratio, the ratio of the diameter of the pulmonary artery to that of the aorta, is a remarkable marker indicating pulmonary vascular disease, especially PH.Citation15,Citation22,Citation23 Unlike right heart catheterization and echocardiography, the PA:A ratio is recorded as a reproducible and reliable measurement.Citation4,Citation26 A PA:A ratio of more than 1 is considered an independent predictor of mortality in COPD patients,Citation27 and these patients tend to have worse airflow obstruction and suffer exacerbation more frequently.Citation4,Citation13 It has been reported that about 5%–50% of COPD patients have PH.Citation28 In addition, the prevalence of cardiovascular disease in COPD patients was 20%–22%, and 13%–28% deaths were cardiac-related.Citation5 The significance of focusing on the PA:A ratio might lie in the ability to identify patients at risk of pulmonary vascular disease or cardiac-related exacerbations.Citation4
PA and A diameters may also correlate with age, causing a decrease in PA:A ratio with aging. In our study, patients with relative pulmonary artery enlargement tended to be younger than patients without (66.8±9.5 vs 70.1±9.5, P=0.003). However, when we focused on the influence that bronchiectasis had on the PA:A ratio in COPD patients, age showed no significant difference between the two groups (P=0.188). Bronchiectasis was still the risk factor for high PA:A ratios in COPD patients after adjusting for confounding factors, including age.
This is the first study to explore whether bronchiectasis in COPD patients is a risk factor for high PA:A ratios. Although the co-existence of COPD and bronchiectasis has recently been recognized as an overlap syndrome, it is still not clear how bronchiectasis participates in the development of COPD progress and vice versa. Recently, researchers have reported that the severity of bronchiectasis was independently associated with the development of vascular diseases.Citation10,Citation12 Thus, it is intriguing to illustrate the impact that bronchiectasis had on pulmonary vascular disease in COPD patients.
The mechanism underlying pulmonary vascular disease in patients with both COPD and bronchiectasis has not been elucidated clearly. Hypoxia has been recognized as an important cause.Citation29 COPD patients often require increasing oxygen when conditions deteriorate, and bronchiectasis might exacerbate this situation. The poorer pulmonary ventilation and gas exchange in overlap patients render them more susceptible to PH. Inevitably, patients with overlap syndrome suffer not only from impairment of the parenchyma, but also destruction of the airway wall, both of which can destroy the vascular bed and induce hemodynamic alterations.Citation30,Citation31
Nowadays, endothelial dysfunction has been recog-nized as a major mechanism underlying chronic pulmonary and vascular disease.Citation28 COPD patients with concomitant bronchiectasis have been reported to have higher levels of inflammation in the airway and peripheral blood,Citation32 which might influence the blood vessel endothelium. In this study, COPD and bronchiectasis co-existence patients had higher levels of peripheral ESR and fibrinogen, which reflected the higher level of systemic inflammation. This might destroy the microenvironment and function of endothelial cells, resulting in imbalanced release of vasoactive mediators.Citation28 This is in accordance with the newly proposed concept of “sick lung circulation”.Citation33,Citation34 Information from the sick lung is transported to the myocardium via blood circulation, which, thus, brings about physical dysfunction of the heart, such as abating systolic function. Right heart dysfunction, in turn, causes congestion of pulmonary circulation, and PH may eventually develop. Studies examining the information from the affected lungs of patients with both COPD and bronchiectasis, including levels of cytokine, microRNA, and microparticles, should be encouraged.Citation33
Despite regular assessment of pulmonary function and airway inflammation, clinicians should pay attention to the impact that bronchiectasis has on vascular disease in COPD patients. More prospective studies are needed to define the significance of high PA:A ratios in patients with COPD and bronchiectasis.
Limitations
The present study has some limitations. First, because right heart catheterization is not a regular test for COPD patients, it is not possible to confirm that patients with high PA:A ratios actually have PH. However, regardless of whether they have PH, COPD patients with PA:A ratios of more than 1 tend to experience poor outcomes.Citation4 This makes it important to focus on patients at risk for relative pulmonary artery enlargement. Second, as a retrospective study, information about bacterial infection, long-term prognosis, and history of exacerbation of COPD-bronchiectasis co-existence patients with high PA:A ratios was absent. Future prospective studies focusing on these aspects and a large population of patients with both stable and exacerbated conditions are needed. Third, these results showed that diabetes mellitus might also influence the PA:A ratio, which was not the main focus of the current study. Future studies focusing on the impact that diabetes mellitus has on pulmonary vascular disease in COPD patients are also needed.
Conclusion
COPD and bronchiectasis co-existence subjects are considered at risk for relative pulmonary artery enlargement (PA:A ratio >1). Future comprehensive assessment should pay close attention to the prevalence of and mechanisms underlying pulmonary vascular disease in patients with COPD and concomitant bronchiectasis.
Acknowledgments
This work was supported by the National Natural Science Foundation of the People’s Republic of China (No 81370148).
Disclosure
The authors report no conflicts of interest in this work.
References
- ThabutGDauriatGSternJBPulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantationChest200512751531153615888824
- ChaouatANaeijeRWeitzenblumEPulmonary hypertension in COPDEur Respir J20083251371138518978137
- HurdmanJCondliffeRElliotCAASPIRE registry: assessing the Spectrum of Pulmonary hypertension Identified at a REferral centreEur Respir J201239494595521885399
- WellsJMWashkoGRHanMKPulmonary arterial enlargement and acute exacerbations of COPDN Engl J Med20123671091392122938715
- DecramerMJanssensWChronic obstructive pulmonary disease and comorbiditiesLancet Respir Med201311738324321806
- O’BrienCGuestPJHillSLStockleyRAPhysiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary careThorax200055863564210899238
- AgustiACalverleyPMCelliBCharacterisation of COPD heterogeneity in the ECLIPSE cohortRespir Res20101112220831787
- Martínez-GarcíaMASoler-CataluñaJJDonat SanzYFactors associated with bronchiectasis in patients with COPDChest201114051130113721546440
- GatheralTKumarNSansomBCOPD-related bronchiectasis; independent impact on disease course and outcomesCOPD201411660561424983298
- EvansIEBediPQuinnTMHillATBronchiectasis severity is an independent risk factor for vascular disease in a bronchiectasis cohortChest2017151238338827720881
- ÖcalSPortakalOÖcalADemirAUTopeliAÇoplüLFactors associated with pulmonary hypertension and long-term survival in bronchiectasis subjectsRespir Med201611910911427692130
- DevarajAWellsAUMeisterMGLoebingerMRWilsonRHansellDMPulmonary hypertension in patients with bronchiectasis: prognostic significance of CT signsAJR Am J Roentgenol201119661300130421606292
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease2017 Available from: http://goldcopd.orgAccessed January 13, 2017
- ChungKSKimYSKimSKFunctional and prognostic implications of the main pulmonary artery diameter to aorta diameter ratio from chest computed tomography in Korean COPD patientsPLoS One2016115e015458427152915
- WellsJMDransfieldMTPathophysiology and clinical implications of pulmonary arterial enlargement in COPDInt J Chron Obstruct Pulmon Dis2013850952124235822
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease2012 Available from: http://wwwgoldcopd.org/Accessed June 20, 2012
- LaszloGStandardisation of lung function testing: helpful guidance from the ATS/ERS Task ForceThorax200661974474616936234
- Martínez-GarcíaMAde la Rosa CarrilloDSoler-CataluñaJJPrognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187882383123392438
- MaoBLuHWLiMHThe existence of bronchiectasis predicts worse prognosis in patients with COPDSci Rep201551096126077673
- NaidichDPMcCauleyDIKhouriNFStitikFPSiegelmanSSComputed tomography of bronchiectasisJ Comput Assist Tomogr1982634374447096687
- BhallaMTurciosNAponteVCystic fibrosis: scoring system with thin-section CTRadiology199117937837882027992
- NgCSWellsAUPadleySPA CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameterJ Thorac Imaging199914427027810524808
- IyerASWellsJMVishinSBhattSPWilleKMDransfieldMTCT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPDChest2014145482483224114440
- Ortaç ErsoyEDurusu TanrioverMÖcalSGulsun AkpinarMTopeliAMeasurement of pulmonary artery to aorta ratio in computed tomography is correlated with pulmonary artery pressure in critically ill chronic obstructive pulmonary disease patientsJ Crit Care201633424626936041
- WellsJMMorrisonJBBhattSPNathHDransfieldMTPulmonary artery enlargement is associated with cardiac injury during severe exacerbations of COPDChest201614951197120426501747
- DevarajAWellsAUMeisterMGCorteTJWortSJHansellDMDetection of pulmonary hypertension with multidetector CT and echocardiography alone and in combinationRadiology2010254260961620093532
- ShinSKingCSBrownAWPulmonary artery size as a predictor of pulmonary hypertension and outcomes in patients with chronic obstructive pulmonary diseaseRespir Med2014108111626163225225149
- BlancoIPiccariLBarberàJAPulmonary vasculature in COPD: the silent componentRespirology201621698499427028849
- BarberàJAMechanisms of development of chronic obstructive pulmonary disease-associated pulmonary hypertensionPulm Circ20133116016423662194
- AlzeerAHAl-MobeirekAFAl-OtairHAElzamzamyUAJoherjyIAShaffiASRight and left ventricular function and pulmonary artery pressure in patients with bronchiectasisChest2008133246847318071019
- DournesGLaurentFCosteFComputed tomographic measurement of airway remodeling and emphysema in advanced chronic obstructive pulmonary disease. Correlation with pulmonary hypertensionAm J Respir Crit Care Med20151911637025393421
- NiYShiGYuYHaoJChenTSongHClinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysisInt J Chron Obstruct Pulmon Dis2015101465147526251586
- VoelkelNFGomez-ArroyoJMizunoSCOPD/emphysema: the vascular storyPulm Circ20111332032622140621
- VoelkelNFNatarajanRDrakeJIBogaardHJRight ventricle in pulmonary hypertensionCompr Physiol20111152554023737184