Abstract
Background
COPD is a well-known risk factor for venous thromboembolism (VTE) development. However, recent data showed that it was not associated with VTE recurrence risk, which excluded cancer patients. This study investigated the association of airflow limitation and VTE recurrence in cancer patients with pulmonary embolism (PE).
Methods
This is a retrospective cohort study of cancer patients with newly diagnosed PE at a university hospital. PE was confirmed using contrast-enhanced computed tomography scan. Airflow limitation was defined as pre-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <0.7 within 2 years of PE diagnosis. VTE recurrence was defined as a composite of recurrence as PE or deep vein thrombosis or both.
Results
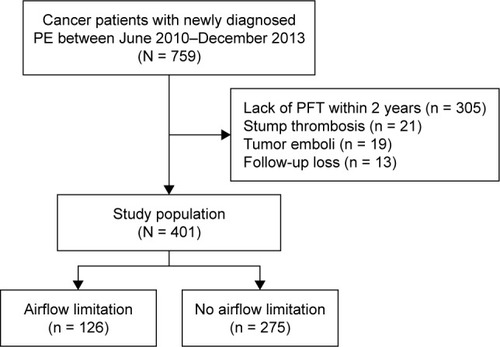
Among 401 cancer patients with newly diagnosed PE, spirometry-based airflow limitation was observed in 126 (31.4%) patients. Half of the patients had lung cancer, which was more common in the group with airflow limitation (65.1% vs 42.9%, p < 0.001). Symptomatic PE was present in less than half (45.4%) of the cases, and 62.6% of patients were treated for PE. During the median follow-up period of 9.7 months, VTE recurred in 49 (12.2%) patients. Compared with patients without airflow limitation, those with airflow limitation did not have an increased risk of VTE recurrence in univariate or multivariate analyses (adjusted hazard ratio, 1.29 [95% CI 0.68, 2.45]).
Conclusion
The presence of airflow limitation did not increase the risk of VTE recurrence in cancer patients with PE. Prospective studies are needed to validate this finding.
Introduction
VTE is a common cardiovascular disease with an annual incidence from 104 to 183 per 100,000 person-years.Citation1 It comprises DVT or PE or both. Acute PE is the most serious manifestation of VTE and results in high short-term mortality, chronic complications, and frequent recurrence.Citation2–Citation4 The cumulative incidence of VTE recurrence has been reported to reach 13% at 1 year, 23% at 5 years, and 30% at 10 years, and recurrent events can be fatal.Citation4,Citation5
COPD is a moderate risk factor for initial VTE and its prognosis.Citation6,Citation7 Several studies have shown that COPD patients have up to five times increased risk of PE or VTE compared with patients without COPD, and there is a significant association between VTE events and COPD severity.Citation8–Citation12 In addition, COPD patients with VTE have much higher mortality compared with COPD patients without VTE.Citation13 Likewise, several studies based on PE and VTE registries suggested that COPD is an unfavorable prognostic factor for bleeding and mortality.Citation2,Citation7,Citation14 However, VTE recurrence rates were similar between COPD patients and non-COPD patients during the 3-month follow-up period.Citation14 Recent data from a prospective cohort study indicated that COPD did not increase the long-term risk of recurrent VTE.Citation15 Although this study considered major provoking factors such as immobilization after surgery, use of estrogen-containing pills, or hormone replacement therapy, active cancer patients were not included.Citation15 Cancer is a strong provoking factor of VTE development and outcomes,Citation4,Citation16–Citation18 and the prevalence of cancer in COPD patients with PE was as high as 20% in a previous study.Citation14 We therefore investigated the association of spirometry-based airflow limitation with VTE recurrence among cancer patients with PE.
Methods
Study population
This is a retrospective cohort study conducted at Samsung Medical Center, Seoul, South Korea, between June 1, 2010, and December 31, 2013. Among patients who were diagnosed with PE during the study period, those who were undergoing treatment or follow-up for pathologically confirmed cancer were identified. Patients with stump thrombosis, tumor emboli, and a history of PE were excluded.
Measurements and data collection
PE diagnosis was confirmed by contrast-enhanced chest CT, and two authors (SHS and HC) reviewed the CT images for evaluation of initial diagnosis, burden, and recurrence. Data regarding basic demographics, cancer diagnosis and treatment, symptoms and signs of PE, treatment for PE, date of VTE recurrence, last follow-up, or death were collected from electronic medical records. Regarding airflow limitation, spirometry performed within 2 years of PE diagnosis was considered. Spirometry was performed as recommended by the American Thoracic Society/European Respiratory Society using Vmax 22 (SensorMedics, Yorba Linda, CA, USA).Citation19 Airflow limitation was defined as pre-bronchodilator FEV1/FVC < 70%.
The primary outcome was recurrence of VTE, defined as a composite of recurrence of PE or DVT or both. Diagnosis of recurrent PE was based on the CT scan, and the diagnosis of recurrent DVT was based on the duplex ultrasonography or CT venography. The institutional review board of Samsung Medical Center approved this study. Informed consent was waived due to the retrospective nature, and patient information was anonymized and de-identified prior to analysis.
Statistical analysis
Categorical variables were compared using the Chi-square test or Fisher’s exact test, and continuous variables were compared using Student’s t-test or Mann–Whitney U test. Cox proportional hazard models were used to evaluate the impact of airflow limitation on VTE recurrence. For multivariate analyses, we used three models. The first model was adjusted for age, sex, BMI, and smoking status. The second model was further adjusted for metastatic disease and recent surgery. The third model was further adjusted for PE treatment. All tests were two sided, and p-value <0.05 was considered statistically significant. All analyses were performed using Stata (version 13.0; Stata Corporation, College Station, TX, USA).
Results
Patient characteristics
Among cancer patients with newly diagnosed PE during the study period, we identified 401 patients who underwent spirometry within 2 years (); median time between spirometry and PE diagnosis was 92 days (IQR 47–231). Of the 401 patients, 126 (31.4%) had airflow limitation. Patients with airflow limitation were older (66.3 years vs 62.0 years, p < 0.001), more likely to be male (82.5% vs 52.4%, p < 0.001), and more likely to be a current smoker (66.7% vs 33.1%, p < 0.001) as compared with those without airflow limitation. History of DVT and comorbidities were not different between the groups. Among cancer patients, about half (49.9%) had lung cancer, which was significantly more common in patients with airflow limitation (65.1% vs 42.9%, p < 0.001). Although there were slightly more patients with metastatic disease in the group without airflow limitation, there were no differences between the two groups regarding recent surgery, ongoing chemotherapy, or radiotherapy ().
Table 1 Distribution of baseline characteristics in 401 cancer patients with PE according to airflow limitation
Clinical presentation, CT findings, and treatment of PE
At the time of PE diagnosis, only 45.4% (182/401) of patients were symptomatic. Patients with airflow limitation were more likely to report dyspnea (37.3% vs 26.6%, p = 0.029) as compared with those without airflow limitation. Signs at PE diagnosis, such as tachycardia, hypoxia, and shock, and the burden of PE on chest CT were not found to be significantly different according to airflow limitation (). Of the 251 (62.6%) patients who were treated for PE, only few were treated with embolectomy or thrombolysis along with anticoagulation treatment. Type and duration of anticoagulation did not differ significantly between the two groups ().
Table 2 Clinical presentation and CT findings of PE in 401 cancer patients according to airflow limitation
Table 3 Treatment for PE in 401 cancer patients according to airflow limitation
VTE recurrence according to airflow limitation
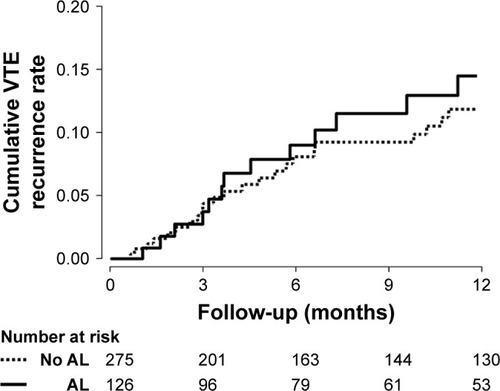
During the median follow-up period of 9.7 months (IQR 2.8–26.6), 49 (12.2%) patients experienced VTE recurrence (14% of patients with airflow limitation and 11% of those without airflow limitation). Among the patients with recurrent VTE, all (18/18) those with airflow limitation had PE (with or without DVT), whereas 19.4% (6/31) without airflow limitation had isolated DVT (p = 0.073).
In the univariate analysis, airflow limitation was not associated with the risk of VTE recurrence (HR 1.31 [95% CI 0.73, 2.34]) ( and ). The impact of airflow limitation on the risk of VTE recurrence remained insignificant after adjustment for patient-related characteristics (aHR 1.20 [95% CI 0.64, 2.26]), for cancer-related characteristics (aHR 1.26 [95% CI 0.66, 2.41]), and for PE treatment (aHR 1.29 [95% CI 0.68, 2.45]). The hazard remained unchanged in subgroup analysis with lung cancer patients (Table S1). Furthermore, recurrent VTE was not different according to the severity of airflow limitation (17.2%, 14.5%, and 7.1% in FEV1 ≥ 80% predicted, 50% ≤ FEV1 < 80% predicted, and FEV1 < 50% predicted, respectively) (p = 0.737).
Table 4 Impact of airflow limitation on VTE recurrence among PE patients with cancer
Discussion
In our study of 401 cancer patients with PE, about one-third of the patients had spirometry-based airflow limitation. During the median follow-up of 9.7 months, VTE recurred in 12.2% of patients. However, coexisting airflow limitation did not increase the risk of VTE recurrence in patients with cancer.
There have been few studies investigating VTE recurrence in COPD patients. One study was a post hoc analysis of a prospective management study in 673 hemodynamically stable PE patients. The study found that COPD was associated with increased bleeding and mortality, but not recurrent VTE over 3 months.Citation20 Another study was performed using the RIETE registry with 28,920 symptomatic VTE patients.Citation14 In that study, COPD patients experienced higher PE recurrence, minor bleeding, and higher rates of death by 3 months. However, recurrence of overall VTE was not different between patients with and without COPD. In both the studies, the results of the lung function tests were not available for all COPD patients, and patients could have been misclassified as COPD or non-COPD. A recent prospective cohort study of a large number of patients with long-term follow-up showed that patients with objectively measured COPD did not have an increased risk of VTE recurrence.Citation15 However, this study did not include active cancer patients, probably to minimize confounding factors.
Our study was restricted to cancer patients to explore the association between COPD and VTE recurrence, and we also confirmed that airflow limitation was not associated with the risk of VTE recurrence in cancer patients. Several factors should be considered in interpreting this finding. First, cancer patients undergo chest CT scan more frequently, which leads to more frequent findings of incidental PE.Citation21 Indeed, in our study, only 45.4% of cancer patients had symptomatic PE, indicating a high proportion of incidental PE among cancer patients. This might mask the effect of airflow limitation on VTE recurrence. Second, subtherapeutic anticoagulation treatment for PE is associated with the increment of VTE recurrence.Citation22 Approximately 38% of cancer patients did not receive anticoagulation in our study, which was at the discretion of the treating physicians. Among them, 85% had incidentally detected PE and 57% had PE in segmental or subsegmental pulmonary arteries. Although current guidelines recommend treating all cancer patients with PE,Citation23,Citation24 there is still debate over the use of anticoagulation in cancer patients with incidental PE.Citation25 In addition, treatment risk (ie, bleeding) over benefits could be considered, especially in patients with asymptomatic PE in segmental or subsegmental pulmonary arteries or those with short expected survival. Nevertheless, we performed subgroup analysis in patients with anticoagulation, which revealed the same result.
There are some limitations in our study. First, because this is a retrospective cohort study in a single referral hospital with a large volume of lung cancer patients, the result of our study may not be generalized to patients in other settings. Second, compared with a recent study of noncancer patients,Citation15 the follow-up duration was relatively short. This is largely attributable to high number of cancer-related deaths, which also contributed to the limited duration of anticoagulation in our study with a median duration of 4 months. However, analysis in which death was included as a competing risk of recurrence did not show a difference. Last, inclusion of patients with valid spirometry test results would have introduced selection bias. It is possible that PE patients in our study were more likely to have preexisting respiratory symptoms than PE patients who were not included in this study. Further prospective study with a larger and more heterogeneous cancer population is necessary to assess the generalizability of our results. Despite these limitations, our study used validated and standardized measurements to define airflow limitation and the diagnosis of PE and included cancer-related factors as well as treatment in the analyses.
Conclusion
Airflow limitation did not increase the risk of VTE recurrence in cancer patients with PE. Taken together with the result from patients without cancer,Citation15 there seems to be no evidence at present to support different management of PE in patients with COPD. Prospective studies are necessary to confirm whether the coexistence of airflow limitation deserves attention in the management of cancer patients with PE.
Abbreviations
| aHR | = | adjusted hazard ratio |
| CI | = | confidence interval |
| CT | = | computed tomography |
| DVT | = | deep vein thrombosis |
| FEV1 | = | forced expiratory volume in 1 second |
| FVC | = | forced vital capacity |
| HR | = | hazard ratio |
| IQR | = | interquartile range |
| PE | = | pulmonary embolism |
| RIETE | = | Registro Informatizado de la Enfermedad Thomboembolica venosa |
| VTE | = | venous thromboembolism |
Supplementary material
Table S1 Impact of airflow limitation on VTE recurrence among PE patients with lung cancer
Disclosure
The authors report no conflicts of interest in this work.
References
- HeitJAEpidemiology of venous thromboembolismNat Rev Cardiol201512846447426076949
- GoldhaberSZVisaniLDe RosaMAcute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER)Lancet199935391621386138910227218
- BecattiniCAgnelliGPesaventoRIncidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolismChest2006130117217516840398
- HeitJAMohrDNSilversteinMDPettersonTMO’FallonWMMeltonLJ3rdPredictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort studyArch Intern Med2000160676176810737275
- CarrierMLe GalGWellsPSRodgerMASystematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolismAnn Intern Med2010152957858920439576
- KonstantinidesSVTorbickiAAgnelliGTask Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)2014 ESC guidelines on the diagnosis and management of acute pulmonary embolismEur Heart J20143543303330693069a3069k25173341
- JiménezDAujeskyDMooresLRIETE InvestigatorsSimplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolismArch Intern Med2010170151383138920696966
- SidneySSorelMQuesenberryCPJrDeLuiseCLanesSEisnerMDCOPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care ProgramChest200512842068207516236856
- CurkendallSMDeLuiseCJonesJKCardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patientsAnn Epidemiol2006161637016039877
- SchneiderCBothnerUJickSSMeierCRChronic obstructive pulmonary disease and the risk of cardiovascular diseasesEur J Epidemiol201025425326020191376
- ChenWJLinCCLinCYPulmonary embolism in chronic obstructive pulmonary disease: a population-based cohort studyCOPD201411443844325010753
- MorganADHerrettEDe StavolaBLSmeethLQuintJKCOPD disease severity and the risk of venous thromboembolic events: a matched case-control studyInt J Chron Obstruct Pulmon Dis20161189990827175072
- BørvikTBrækkanSKEngaKCOPD and risk of venous thromboembolism and mortality in a general populationEur Respir J201647247348126585434
- BertolettiLQuenetSMismettiPRIETE InvestigatorsClinical presentation and outcome of venous thromboembolism in COPDEur Respir J201239486286821885395
- Le MaoRTromeurCBazireARisk of recurrent venous thromboembolism in COPD patients: results from a prospective cohort studyEur Respir J2017501
- HuttenBAPrinsMHGentMGinsbergJTijssenJGBüllerHRIncidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: a retrospective analysisJ Clin Oncol200018173078308310963635
- PrandoniPLensingAWPiccioliARecurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosisBlood2002100103484348812393647
- GussoniGFrassonSLa ReginaMDi MiccoPMonrealMRIETE InvestigatorsThree-month mortality rate and clinical predictors in patients with venous thromboembolism and cancer. Findings from the RIETE registryThromb Res20131311243023141849
- MillerMRHankinsonJBrusascoVATS/ERS Task ForceStandardisation of spirometryEur Respir J200526231933816055882
- NijkeuterMSöhneMTickLWChristopher Study InvestigatorsThe natural course of hemodynamically stable pulmonary embolism: clinical outcome and risk factors in a large prospective cohort studyChest2007131251752317296656
- DentaliFAgenoWBecattiniCPrevalence and clinical history of incidental, asymptomatic pulmonary embolism: a meta-analysisThromb Res2010125651852220451960
- HeitJALahrBDPettersonTMBaileyKRAshraniAAMeltonLJ3rdHeparin and warfarin anticoagulation intensity as predictors of recurrence after deep vein thrombosis or pulmonary embolism: a population-based cohort studyBlood2011118184992499921890644
- LymanGHKhoranaAAKudererNMAmerican Society of Clinical Oncology Clinical PracticeVenous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline updateJ Clin Oncol201331172189220423669224
- KearonCAklEAComerotaAJAntithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice GuidelinesChest20121412 Supple419Se496S22315268
- PerisMJiménezDMaestreARIETE InvestigatorsOutcome during and after anticoagulant therapy in cancer patients with incidentally found pulmonary embolismEur Respir J20164851360136827660517