Abstract
Background
The study was conducted to determine the impact of chronic obstructive pulmonary disease (COPD) in association with obstructive sleep apnea syndrome (OSAS) on cardiac autonomic control and functional capacity.
Subjects and methods
The study was a cross-sectional prospective controlled clinical study. Heart rate variability indices of 24 COPD (n = 12) and COPD+OSAS (n = 12) patients were evaluated and compared by electrocardiographic recordings acquired during rest, active postural maneuver (APM), respiratory sinus arrhythmia maneuver (RSA-m), and the 6-minute walk test (6MWT).
Results
The COPD group presented higher parasympathetic modulation during APM when compared to the COPD+OSAS group (P = 0.02). The COPD+OSAS group presented higher sympathetic modulation during RSA-m when compared to the COPD group (P = 0.00). The performance during 6MWT was similarly impaired in both groups, despite the greater severity of the COPD group.
Conclusion
Subjects with COPD+OSAS present marked sympathetic modulation, and the presence of OSAS in COPD subjects has a negative impact on functional capacity regardless of the severity of lung disease.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is a syndrome characterized by chronic airway obstruction, which is not completely reversible; it determines significant losses in pulmonary function, has systemic effects, and is associated with important comorbidities.Citation1 Among them, obstructive sleep apnea syndrome (OSAS), characterized by recurrent events of upper airway obstruction during sleep, affects most COPD patients.Citation2 COPD and OSAS are two of the most prevalent chronic respiratory diseases worldwide.Citation3,Citation4
According to Taranto-Montemurro et al,Citation5 the association of the two diseases is present in about 0.5%–1% of the general population; the prevalence of COPD in individuals with OSAS exceeds its prevalence in individuals who do not present with sleep disorders.Citation6 OSAS appears to be more commonly seen in subjects with COPD perhaps as a result of shared risk factors such as obesity, smoking, increased airway resistance, local and systemic inflammation, as well as anti-inflammatory therapy.Citation6
It is known that COPD+OSAS has a great impact on the systemic manifestations of affected patients, such as an increase in daytime sympathetic activity, with an increase in rest heart rate (HR) and a decrease in heart rate variability (HRV), which may lead to greater morbidity and mortality from other diseases.Citation5 On the other hand, other authors have demonstrated that COPD+OSAS determines marked parasympathetic hyperactivity in the airways and hypoxemia-related bronchoconstriction/vasoconstriction, among other neurohumoral effects.Citation6 These still contradictory autonomic imbalances could have a negative impact on static postural adjustments and responses to exercise. To our knowledge, however, no previous study has evaluated and compared such adjustments in these patients.
Thus, the main objective of this study was to determine the impact of the coexistence of OSAS and COPD on cardiac autonomic control in response to changes in breathing pattern, active postural maneuver (APM), and during submaximal exercise (6-minute walk test [6MWT]). We hypothesized that the coexistence of OSAS and COPD would be accompanied by greater cardiovascular, autonomic, and functional impairments than COPD alone.
Subjects and methods
Subjects
Twenty-four individuals of both sexes were screened at the Pulmonary Ambulatory of the School Health Unit (USE) of Federal University of São Carlos (UFSCar) and at the Municipal Health Specialty Center of São Carlos (CEME) with clinical diagnosis of COPD according to the criteria defined by the GOLD scientific committeeCitation7 with suspected diagnosis of OSAS.
Inclusion criteria
COPD group: clinical diagnosis of COPD according to the criteria defined by the GOLD scientific committee,Citation7 in regular treatment with optimized medication, current nonsmokers, people who were not in exacerbations period, and people whose after-home sleep examination did not confirm the suspicion of OSAS.
COPD+OSAS group: the same criteria for inclusion in COPD group were followed; however, the individuals presented a confirmed diagnosis of OSAS.
Subjects should not present clinical exacerbations or be on oral steroids for at least 3 months.
Exclusion criteria
Exclusion criteria included presence of diabetes mellitus, systemic arterial hypertension and uncontrolled pulmonary hypertension, use of medications capable of altering cardiac autonomic control, thyroid hormone alterations, use of home oxygen therapy or continuous positive airway pressure (CPAP) for the treatment of OSAS, pacemaker, cardiac arrhythmias interfering with >95% of heart beats, and neurological and orthopedic alterations that would prevent the performance of the proposed tests.
The study was approved by the Ethics and Research Committee of Federal University of São Carlos, São Paulo, Brazil (number 1.406.894/2016), and all the volunteers signed a written informed consent agreement in compliance with resolution number 466/2012 of the National Health Council.
Study protocol
All volunteers were subjected to two stages of evaluation: 1) investigation of the presence of OSAS in subjects with COPD and 2) evaluation of the autonomic function through HRV analysis and of the functional capacity through 6MWT application.
Measurements
In order to control the influence of circadian variations on the results, all tests were performed at the same time of the day in a laboratory with a mean temperature maintained between 20°C and 22°C and relative humidity in the range from 40% to 60%. All subjects were instructed to abstain from alcohol or stimulants on the day of the tests and not to practice physical or sports activities the day before and on the day of the tests.
Pulmonary function
Spirometric evaluation was performed with a CPFS/D® spirometer (Medgraphics, MGC Diagnostics Corporation, St Paul, MN, USA), according to the criteria of the Brazilian Society of Pulmonology and Tisiology,Citation8 and were staged according to the GOLD criteria.Citation7
Home sleep test
The apnea-hypopnea index (AHI) and the oxygen desaturation index (ODI) were obtained by an ApneaLink Plus™ equipment (ResMed Corporation, Poway, CA, USA), which is a portable sleep monitor validated to assess the presence of OSAS.Citation9 Subjects with AHI ≥15 were included in the COPD+OSAS group, while those with AHI <15 were included in the COPD group.
After group stratification, autonomic evaluation was initiated and included.
Recording of R-R intervals
The electrocardiographic signal was recorded through a BioAmp FE132 device (ADInstruments, Sydney, Australia), with electrodes placed in the MC5 lead configuration. Signals were recorded during the conditions shown in the following sections.
Active postural maneuver
The subjects remained in supine position for 10 minutes and were instructed not to talk, sleep, or move during this time. After this rest period, the subjects were instructed to position themselves in orthostatic position (10 minutes) and then in sitting position (10 minutes), following the instructions given for the rest period.
Respiratory sinus arrhythmia maneuver
After APM, the subjects, still seated, were subjected to respiratory sinus arrhythmia maneuver (RSA-m; controlled breathing with cycles of 10 seconds, divided into 5 seconds of inspiration and 5 seconds of expiration) during 4 minutes.
For HRV analysis during MPA and m-ASR, the R-R intervals (iRR) were collected at a rate of 500 samples/s. All signals were visually inspected to identify artifacts or noises where narrow peaks (<100 ms) were removed by linear interpolation. All signals were filtered with low pass filters with a cutoff frequency of 20 Hz.
Data were analyzed using Kubios HRV® software (Version 2.1, Matlab, Kuopio, Finland). The data collected during each MPA posture had a total period of iRR examined and, for analysis, the most stable and noise-free segment that contains 300 points was selected. In addition, the data collected during m-ASR had all the iRR contained within the 4 minutes of the maneuver used for the analysis.
Submaximal exercise using the 6-minute walk test
General test principles, criteria for discontinuation, as well as verbal stimuli were standardized based on the recommendations of the American Thoracic Society and the American College of Chest Physicians.Citation10 After a 4-minute rest period (2 minutes in sitting position and 2 minutes in orthostatic position), the volunteers were instructed to walk continuously, as far as possible, in a 30-meter walk for 6 minutes, and allowed to slow down and even interrupt the test if necessary. At the end, the distance traveled in meters as well as percentage in relation to the predicted was recorded.
HR was recorded by a Polar HR monitor (RX810, Kempele, Finland) during 4 minutes before the test (rest), throughout the test (exercise), and during 6 minutes after the test (recovery). For HRV analysis during the 6MWT, the data collected by the HR monitor were visually inspected to identify artifacts or noises where narrow peaks (<100 ms) were removed by linear interpolation. All signals were filtered with low pass filters with a cutoff frequency of 20 Hz.
Data were analyzed using Kubios HRV® software (Version 2.1, Matlab). The data were analyzed as follows:
Rest in the seated position: The 2 minutes of the seated rest period were analyzed.
Rest in the orthostatic position: iRR were analyzed from the 2 minutes of the standing rest period.
Walk: For analysis, we selected the most stable and noise-free segment that should have 3 minutes.
Recovery: The last 5 minutes of collected iRR were selected for analysis.
Selected sections were analyzed in the time and frequency domains and by means of non-linear analysis. In the time domain, the following indices were calculated: 1) SDNN: standard deviation of all iRR; 2) RMSSD: square root of the mean squared differences between adjacent iRR; 3) pnn50: percentage of adjacent iRR with duration difference greater than 50 ms; 4) TINN: iRR variability.Citation11 SDNN and TINN represent global autonomic activity.Citation11 RMSSD and pnn50 are representative of vagal modulation.Citation11 In the frequency domain, the following indices were calculated: 1) high frequency component (HF); frequency band between 0.15 Hz and 0.4 Hz; 2) low frequency component (LF); frequency band between 0.04 Hz and 0.15 Hz; and 3) LF/HF ratio.Citation11 The HF index represents cardiac parasympathetic modulation; the LF index represents the joint action of the sympathetic and parasympathetic components, with sympathetic predominance; the LF/HF ratio represents the sympathovagal balance.Citation11 In addition, the following non-linear indices were calculated: 1) SD1: standard deviation of instantaneous beat-to-beat variability; 2) SD2: long-term standard deviation of the continuous iRR.Citation11 SD1 index represents cardiac parasympathetic modulation and SD2 index represents global cardiac autonomic activity.Citation11
Statistical analysis
The results were presented as mean ± standard deviation, with a level of significance of 95%. Categorical variables were compared using the chi-square test. Two-way ANOVA (with Bonferroni post hoc test) was used for comparative analysis of the differences between the HRV indexes. Linear associations were evaluated with Pearson product-moment coefficients. Statistical analysis was performed using SPSS 19.0 (IBM, Armonk, NY, USA) and GraphPad Prism 6.0 (MacKiev Software, Boston, MA, USA).
Results
Population characteristics
One hundred and two subjects from the Pulmonary Ambulatory of the School Health Unit (USE) of Federal University of São Carlos (UFSCar) and from the Municipal Health Specialty Center of São Carlos (CEME) were screened. The groups were screened as described in .
Figure 1 Volunteer flowchart.
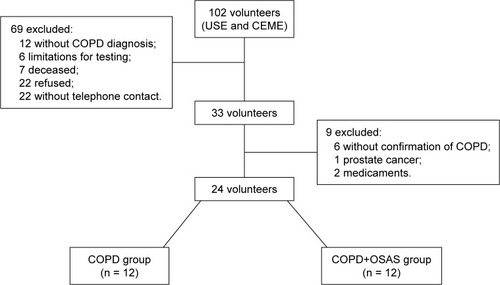
The baseline characteristics of the volunteers are shown in . The data contained in show that the lung function of the COPD group was worse when compared to the COPD+OSAS group, that is, the degree of respiratory commitment was higher for the COPD group.
Table 1 Volunteers’ baseline characteristics
Although the COPD+OSAS group presented higher levels of AHI and ODI, as expected due to the presence of OSAS, interestingly, the time spent with saturation below 90% (SAT < 90%) and below 80% (SAT < 80%) was much longer in the COPD group, confirming the great impact of the disease on nocturnal hypoxemia in these patients.
HRV indices at rest
The HRV indices during APM and RSA-m are represented in and , respectively. The COPD+OSAS group presented higher absolute and normalized LF values when compared to the COPD group (, P < 0.05). On the other hand, the COPD+OSAS group had lower normalized HF values when compared to the COPD group; taken together, data suggest greater sympathetic modulation in the COPD+OSAS group.
Table 2 HRV indices before and after active postural change from supine position to orthostatic position
Table 3 HRV indices in sitting position and during RSA-m
shows that both groups had higher SDNN and TINN values during RSA-m when compared to the sitting position, suggesting a greater global autonomic modulation for both groups during the maneuver (P < 0.05). However, there was no interaction between APM values for the two groups.
6MWT variables
The 6MWT parameters are shown in . shows that the COPD+OSAS group had higher SBP than the COPD group (P < 0.05). In addition, symptoms of dyspnea and lower limbs fatigue were worse in the COPD group than in the COPD+OSAS group (P < 0.05), even though we did not observe a worse performance in this group. Although there were no differences in SpO2 between groups during 6MWT (P = 0.21), the COPD group presented an average value below 90%, which indicates that desaturation was clinically more pronounced in this group.
Table 4 6MWT parameters
Strong correlations were observed between the HRV indices during 6MWT and nocturnal desaturation in these patients. We observed that the longer the time spent with saturation below 90%, the greater the parasympathetic modulation during walking, represented by the RMSSD and SD1 indices (R = 0.80, P < 0.05).
Discussion
To our knowledge, this is the first cross-sectional study comparing autonomic responses to APM, RSA-m, and walking, as well as submaximal exercise performance between subjects with COPD+OSAS versus subjects with COPD. The main findings of this study showed that individuals in the COPD+OSAS group presented less severe lung disease and shorter nocturnal hypoxemia time when compared to the COPD group.
In addition, the autonomic modulation during APM was impaired in both groups; however, the severity of COPD appears to lead to a greater parasympathetic modulation for the COPD group and the association of OSAS to a greater sympathetic activity for the COPD+OSAS group. During 6MWT, the groups presented similar performance impairments despite the greater severity of the COPD group; the time spent with saturation below 90% was associated with marked parasympathetic modulation during sub-maximal exercise.
Effects of autonomic tests in subjects with COPD and in the coexistence of COPD+OSAS
In our study, the COPD group presented higher parasympathetic modulation during APM when compared to the COPD+OSAS group. In contrast to findings from this study, some authors have reported reduced sympathetic and parasympathetic activity for individuals with COPD.Citation12,Citation13 By contrast, Volterrani et alCitation14 reported an increase in parasympathetic tone for this population, which is in accordance with the results obtained in this study, where higher normalized HF values were found during supine rest and after postural change to the orthostatic position. Stein et alCitation15 related the changes in the parasympathetic modulation of individuals with COPD to the severity of the disease, which also seems to happen in the present study, where autonomic alteration is present in the group with more severe pulmonary disease.
The COPD+OSAS subjects presented higher sympathetic modulation during APM when compared to the COPD group.Citation16 Taranto-Montemurro et alCitation5 observed increased sympathetic modulation in subjects with heart failure + OSAS during sleep. Our results are relevant, as we do not know any other study that has evaluated the coexistence of COPD+OSAS during postural change. Although marked sympathetic modulation was observed in both supine and orthostatic positions for the mixed group, none of the groups presented satisfactory postural adjustments (position effect: P > 0.05), demonstrating that postural adjustment is impaired in the presence of OSAS, even with COPD severity being lower for the COPD+OSAS group. Previous findings have shown that early neuropathy is common in both COPD and OSA subjects and is closely related to the disease severity.Citation17 Previous findings showed that hypoxemia contributes to autonomic impairment in COPD patients.Citation18 In our study, the COPD group presented longer desaturation time during sleep and this observation may help explain impaired postural adjustment in this group.
Effects of COPD and coexistence of COPD+OSAS during RSA-m and 6MWT
In the present study, only RSA-m and 6MWT produced autonomic responses in the subgroups, but without significant differences between the groups. We observed that in both groups RSA-m increased total HRV, as demonstrated by SDNN and TINN (P < 0.05). Previous findings from our group showed greater responses to the RSA-m in subjects with COPD and that altered responses were associated with greater COPD severity and poor diffusion capacity.Citation19 Similarly, in subjects with OSAS, one study showed that the component of respiratory sinus arrhythmia that is mediated by pulmonary vagal feedback remained intact, indicating that subjects presented a response to RSA-m.Citation20 Our findings showed that both the COPD group and the COPD+OSAS group presented an increase in total HRV; however, the indices representative of parasympathetic modulation did not change with the maneuver, suggesting possible mismatches of the coexistence of the diseases and the severity of COPD in front of maneuver.
When comparing the two groups, performance during 6MWT was similarly impaired, despite the greater COPD severity of the COPD group; this leads us to suggest that both the coexistence of OSAS and COPD and severe COPD can cause functional losses. In this context, it is known that the severity of COPD, as well as the severity of OSAS, is closely associated with impairments in functional capacity.Citation21 In the present study, only SDNN and SD2 (both representative of total HRV) were sensitive to HRV reduction during exercise. As for the other indexes, we did not observe any differences during walking. Exercise was shown to increase sympathetic modulation and reduce parasympathetic modulation and global HRV.Citation22 In subjects with COPD, physical training may improve exercise capacity, increase independence for activities of daily living, and improve cardiac autonomic control.Citation23 Results showed that in subjects with OSAS, sympathetic hyperactivity contributes to impairment in autonomic response during exercise.Citation24 However, we did not find any studies investigating the coexistence of COPD and OSAS. We believe, therefore, that both parasympathetic hyperactivity (present in COPD) and sympathetic hyperactivity (present in OSAS) contribute to impaired autonomic adjustment during exercise. These aspects are physiologically grounded because the integrity of the parasympathetic modulation is fundamental for fast HR adjustments (which occur in the first 30 seconds of exercise, as well as during the first minute of recovery) to allow for greater pulmonary blood flow at this early stage of exercise (cardiodynamic phase). At the same time, the increase of sympathetic modulation during exercise continuation is necessary to maintain cardiac work efficiency and meet exercise demands.
Therefore, the lack of responses during the autonomic tests applied in the present study may indicate great functional impairment. Both severity and coexistence of the diseases have a negative impact on cardiac autonomic control. These results are important, because they can lead to the development of important non-pharmacological rehabilitation strategies in these subjects with the objective to recover the integrity of the autonomic control or to minimize the negative impact of the diseases.Citation25–Citation27
Another interesting result of the present study was the strong correlation found between the time that subjects spent with saturation lower than 90% and the HRV indexes during the 6MWT (RMSSD and SD1, R = 0.80, P < 0.05), both representative of parasympathetic modulation. Our data reinforce that higher nocturnal hypoxemia is closely related to greater parasympathetic modulation during walking in both groups. During exercise, normal subjects are expected to present decreased parasympathetic modulation. It is known that cardiac sympathetic hyperactivity is related to airway vagal hyperactivity in subjects with COPD.Citation14 Therefore, we believe that the marked desaturation that occurred in COPD subjects in our study was related to greater parasympathetic modulation during exercise. These findings suggest that the use of nocturnal oxygen therapy may be appropriate to help minimize cardiac autonomic maladjustments in these patients.Citation28–Citation30
Study limitations
The present study has some limitations. In relation to the subjects involved in the study, it would be important to have groups with a greater number of individuals, which was not possible due to our rigid exclusion criteria and the difficulty in finding subjects with the coexistence of the diseases. Therefore, future clinical trials with a larger number of individuals are needed to confirm the findings of the present study.
Conclusion
In conclusion, the results of this study indicate that subjects with coexistence of COPD and OSAS have marked sympathetic hyperactivity. In addition, the results suggest that regardless of the degree of lung involvement caused by COPD, the presence of coexistence leads to a greater functional impairment than in the presence of pathologies alone. Therefore, it is suggested that COPD+OSA leads to greater cardiovascular, autonomic, and functional deficiencies.
Acknowledgments
Katiany Thays Lopes Zangrando was supported by a research grant from Capes (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasília, Brazil) and a research grant from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico – Brasil) (Process Number: 163789/2015-0), and a research grant from FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo, São Paulo, Brazil) (Process Number: 2015/26501-1). Luiz Carlos Soares de Carvalho Jr has a CNPq fellowship.
Disclosure
The authors report no conflicts of interest in this work.
References
- AndreasSAnkerSDScanlonPDSomersVKNeurohumoral activation as a link to systemic manifestations of chronic lung diseaseChest200512853618362416304321
- ParkJGRamarKOlsonEJUpdates on definition, consequences, and management of obstructive sleep apneaMayo Clin Proc2011866549554 quiz 554–55521628617
- McNicholasWTBonsigoreMRManagement Committee of EU COST ACTION B26Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research prioritiesEur Respir J200729115617817197482
- Sociedade Brasileira de Pneumologia e TisiologiaII Consenso Brasileiro sobre Doença Pulmonar Obstrutiva Crônica – DPOC [II Brazilian consensus on chronic obstructive pulmonary disease – COPD]J Bras Pneumol2004305152 Portuguese [with English abstract]
- Taranto-MontemurroLMessineoLPergerECardiac sympathetic hyperactivity in patients with chronic obstructive pulmonary disease and obstructive sleep apneaCOPD201613670671127383268
- IoachimescuOCTeodorescuMIntegrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndromeRespirology201318342143123368952
- Global Initiative for Chronic Obstructive Lung DiseaseGOLD 2017; Global Strategy for Diagnosis, Management, and Prevention of COPD Available from: htttp://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/Accessed April 11, 2018
- PereiraCATestes de função pulmonar [Pulmonary function tests]Proj Diretrizes Assoc Médica Bras e Cons Fed Med2001112 Portuguese [with English abstract]
- ErmanMKStewartDEinhornDGordonNCasalEValidation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording deviceJ Clin Sleep Med20073438739217694728
- CrapoROEnrightPLZeballosRJ(Writing Committee Members) ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk testAm J Respir Crit Care Med2002166111111712091180
- VanderleiLCMPastreCMHoshiRACarvalhoTDGodoyMFNoções básicas de variabilidade da frequência cardíaca e sua aplicabilidade clínica. [Basics of heart rate variability and its clinical applicability]Rev Bras Cir Cardiovasc2009242205217 Portuguese19768301
- BédardMEMarquisKPoirierPProvencherSReduced heart rate variability in patients with chronic obstructive pulmonary disease independent of anticholinergic or β-agonist medicationsCOPD20107639139721166626
- PantoniCBReisMSMartinsLECataiAMCostaDBorghi-SilvaAEstudo da modulação autonômica da freqüência cardíaca em repouso de pacientes idosos com doença pulmonar obstrutiva crônica [Study of the autonomic modulation of resting heart rate in elderly patients with chronic obstructive pulmonary disease]Rev Bras Fisioter20071113541 Portuguese
- VolterraniMScalviniSMazzueroGDecreased heart rate variability in patients with chronic obstructive pulmonary diseaseChest19941065143214377956396
- SteinPKNelsonPRottmanJNHeart rate variability reflects severity of COPD in PiZ alpha1-antitrypsin deficiencyChest199811323273339498947
- ChhabraSKDeSCardiovascular autonomic neuropathy in chronic obstructive pulmonary diseaseRespir Med200599112613315672861
- RestaORanaLProcacciVGuidoPPiccaVScarpelliFAutonomic dysfunction in normotensive awake subjects with obstructive sleep apnoea syndromeMonaldi Arch Chest Dis199853123299632903
- StewartAGWaterhouseJCHowardPCardiovascular autonomic nerve function in patients with hypoxaemic chronic obstructive pulmonary diseaseEur Respir J1991410120712141804668
- MazzucoAMedeirosWMSperlingMPRelationship between linear and nonlinear dynamics of heart rate and impairment of lung function in COPD patientsInt J Chron Obstruct Pulmon Dis2015101651166126316739
- JoJABlasiAValladaresEJuarezRBaydurAKhooMCDeterminants of heart rate variability in obstructive sleep apnea syndrome during wakefulness and sleepAm J Physiol Heart Circ Physiol20052883H1103H111215471971
- CholidouKGManaliEDKapsimalisFHeart rate recovery post 6-minute walking test in obstructive sleep apnea: cycle ergometry versus 6-minute walking test in OSA patientsClin Res Cardiol20141031080581524820928
- RoqueALValentiVEMassettiTChronic obstructive pulmonary disease and heart rate variability: a literature updateInt Arch Med201474325945125
- Borghi-SilvaAMendesRGTrimerRPotential effect of 6 versus 12-weeks of physical training on cardiac autonomic function and exercise capacity in chronic obstructive pulmonary diseaseEur J Phys Rehabil Med201551221122124594853
- CepedaFXToschi-DiasEMaki-NunesCObstructive sleep apnea impairs postexercise sympathovagal balance in patients with metabolic syndromeSleep20153871059106625669187
- CamilloCALaburu VdeMGonçalvesNSImprovement of heart rate variability after exercise training and its predictors in COPDRespir Med201110571054106221342757
- Borghi-SilvaAReisMSMendesRGNoninvasive ventilation acutely modifies heart rate variability in chronic obstructive pulmonary disease patientsRespir Med200810281117112318585024
- SinDDWongEMayersIEffects of nocturnal noninvasive mechanical ventilation on heart rate variability of patients with advanced COPDChest2007131115616317218570
- GongXHuangLLiuXCorrelation analysis between polysomnography diagnostic indices and heart rate variability parameters among patients with obstructive sleep apnea hypopnea syndromePLoS One2016116e015662827253187
- AeschbacherSBossardMSchoenTHeart rate variability and sleep-related breathing disorders in the general populationAm J Cardiol2016118691291727553103
- LewisMJAnnandaleJLewisKEInfluence of long-term oxygen therapy on heart rate and QT time-series in hypoxic patients with chronic obstructive pulmonary diseaseClin Physiol Funct Imaging200929643143919719731
