Abstract
Background
A prolonged QT interval is associated with increased risk of Torsade de Pointes and cardiovascular death. The prevalence and clinical relevance of QT prolongation in acute exacerbations of COPD (AECOPD), with high risk for cardiac morbidity and mortality, is currently unclear.
Methods
A dual cross-sectional study strategy was therefore designed. A retrospective study evaluated 140 patients with an AECOPD requiring hospitalization, half of which had prolonged QTc on the admission ECG. Univariate and multivariate analyses were conducted to determine associated factors; Kaplan–Meier and Cox regression analyses to assess prognostic significance. A prospective study evaluated 180 pulmonary patients with acute respiratory problems requiring hospitalization, to determine whether a prolonged QTc at admission represents an AECOPD-specific finding and to investigate the change in QTc-duration during hospitalization.
Results
Retrospectively, hypokalemia, cardiac troponin T and conductance abnormalities on ECG were significantly and independently associated with QTc prolongation. A prolonged QTc was associated with increased all-cause mortality (HR 2.698 (95% CI 1.032–7.055), p=0.043), however, this association was no longer significant when corrected for age, FEV1 and cardiac troponin T. Prospectively, QTc prolongation was observed in 1/3 of the patients diagnosed with either an AECOPD, lung cancer, pulmonary infection or miscellaneous acute pulmonary disease, and was not more prevalent in AECOPD. The QTc-duration decreased significantly during hospitalization in patients with and without COPD.
Conclusion
A prolonged QTc is a marker of underlying cardiovascular disease during an AECOPD. It is not COPD-specific, but a common finding during the acute phase of a pulmonary disease requiring urgent hospital admission.
Introduction
COPD is a major health concern worldwideCitation1 and is associated with significant morbidity and mortality.Citation2,Citation3 The disease is characterized by worsening airflow obstruction resulting in progressive breathlessness, and is complicated by the occurrence of acute exacerbations of COPD (AECOPD). These periods of increased respiratory symptoms beyond the normal day-to-day variations may result in hospitalization, respiratory failure and death.Citation4,Citation5 A recent study found a 4-year mortality rate of 45% after discharge and up to 10% in-hospital mortality in patients presenting with an AECOPD.Citation6–Citation8
As a complex systemic disease associated with multiple chronic conditions, many of these patients are more likely to die from comorbidity than from COPD itself.Citation9,Citation10 It is well established that patients with impaired pulmonary function due to COPD have an increased risk of cardiovascular disease,Citation11–Citation13 and there exists ample evidence of higher prevalence and incidence of hospitalization for major cardiovascular events.Citation14,Citation15 In case of an AECOPD, patients experience increased bronchoconstriction and mucus production, potentially resulting in hypoxemia and increased heart rate. These events correspond to periods of supplementary cardiovascular stress, potentially resulting in myocardial damage or even heart failure, reflected by the release of cardiac-specific troponin and natriuretic peptides, respectively. Numerous studies have investigated both biomarkers in stable COPD,Citation16 as well as AECOPD,Citation17–Citation19 resulting in a few systematic reviews and meta-analyses.Citation20,Citation21 Although results are not always comparable due to methodological heterogeneity, in general they indicate that elevation of one or both biomarkers is fairly common and corresponds to a strong and independent prognostic factor for mortality, in hospital and after discharge.
Alongside these biomarkers, electrocardiography serves as an important tool to investigate the cardiovascular effects of an AECOPD. It is a non-invasive and readily available procedure that carries important information concerning cardiac disease (eg, arrhythmias, ischemia, and conduction abnormalities) and its prognosis. The significance of a prolonged QT interval, a surrogate marker for the risk of malignant ventricular arrhythmias (eg, Torsade de Pointes) and sudden cardiac death, is under investigation in a number of cardiopulmonary diseases. For instance, it was recently shown to be an independent predictor of worse clinical outcomes in pulmonary hypertension.Citation22 Rigorous studies in patients with COPD, with coexisting cardiovascular risk factors and poly-pharmacologic treatment, are currently lacking. Findings are limited and conflicting,Citation23–Citation25 emphasizing the need for further investigation on the role of electrocardiogram (ECG) recording in the monitoring of patients with COPD.
The aim of this study was to provide insight into the clinical relevance of QT prolongation in a COPD subpopulation at high risk for cardiac morbidity and mortality. We therefore evaluated the patient characteristics and prognostic significance of a prolonged QT interval corrected according to Bazett’s formula (QTc) in patients hospitalized for an AECOPD in a retrospective patient sample. In a separate prospective cohort, we also investigated whether prolongation of the QT interval is specific for patients hospitalized for an AECOPD compared to admissions for other acute respiratory reasons that have less association with cardiovascular disease.
Methods
Study design and population
This study was conducted in accordance with the amended Declaration of Helsinki. The study protocol was approved by the local ethics committee (Commissie Medische Ethiek UZ-KU Leuven, s58766) for which obtaining informed consent was waived given the non-interventional, observational design of the protocol.
First, we conducted a retrospective analysis in a cohort of 140 patients with an AECOPD requiring hospitalization. The study population was derived from an existing database comprising patients with COPDCitation26 who were screened for eligibility in the Belgian trial with Azithromycin for acute COPD Exacerbations requiring hospitalization (BACE) (NCT2135354), a randomized placebo-controlled trial investigating long-term azithromycin for the prevention of AECOPD.Citation27 Our clinical cohort consisted of 70 patients who were considered not eligible based on a prolonged QTc on the admission ECG. They were selected as the first 70 in the same time window as the first 70 enrolled patients (who per protocol did not have QTc prolongation). All patients presented at the emergency department of the University Hospital Gasthuisberg (Leuven, Belgium) between August 2014 and September 2015.
Second, we outlined a prospective, non-interventional study. The clinical cohort consisted of 180 pulmonary patients who presented at the same emergency department with acute respiratory problems requiring hospitalization (168 unique patients) between December 2015 and February 2016. These patients were grouped based on the main admission diagnosis.
Clinical measurements
For both studies, positioned in clinical routine, available variables relating to the patient’s demographics, lung disease, comorbidity, concomitant respiratory, cardiovascular and QT-prolonging medication, and admission laboratory, ECG and chest X-ray (CXR) were collected. The presence of cardiovascular disease (yes/no), the CharlsonCitation28 and COPD index () were used to score comorbidity. The following was considered evidence for cardiovascular disease in patients aged ≥40 years: any 1 of coronary artery disease, peripheral vascular disease, stroke, myocardial infarction and diabetes with organ disease; in patients aged ≥60 years: any 2 of hypercholesterolemia, hypertension, diabetes and peripheral vascular disease. Laboratory variables at admission included hypokalemia (K+<3.0 mmol/L), hypomagnesemia (Mg2+<0.5 mmol/L), cardiac troponin T (cTnT, ≥0.014/0.014–0.052/≥0.052 ng/mL – Elecsys Troponin T hs assay, 13 ng/L 10% CV and 14 ng/L 99% URL; Roche Diagnostics, Mannheim, Germany), C-reactive protein and hypoxemia and respiratory acidosis determined through arterial blood gas analysis. Length of stay and total all-cause mortality were considered as outcome measures. Standard 12-lead resting ECGs (MAC-series 5000 and up, Marquette, GE Healthcare, Chicago, IL, USA – 25 mm/s paper speed, 10 mm/mV amplitude, 250 Hz sampling rate) were taken and inspected manually. Automated QTc values were originally calculated according to Bazett’s formula (correcting the QT interval to a value predicted at a heart rate of 60 bpm, QTc=QT/√RR [RR interval=60/heart rate]) representing the standard practice at our institution for the period in which patients were recruited. Where mentioned, the Fridericia formula was later applied to the collected data (QTcF=QT/Citation3√RR, QT interval corrected according to Fridericia’s formula). Predefined cutoff criteria, ie, >450 msec for male and >470 msec for femaleCitation29 were used to evaluate QTc prolongation.
Table 1 COPD comorbidity indexTable Footnote*
In the prospective study, we did not interfere with the course of clinical investigations during admission. We did, however, signal the presence of QTc prolongation to the treating physicians for them to decide freely whether a control ECG would be warranted.
Statistical analyses
Statistical analyses were performed using SPSS v20 (IBM Corporation, Armonk, NY, USA) and R v3.1.0 (R Core Team, Vienna, Austria). Two-sided p-values <0.05 were considered statistically significant.
Retrospective data
Continuous/ordinal data were compared using Mann–Whitney–Wilcoxon test, and categorical data were compared using Fisher’s exact test. For the multivariate logistic regression, potential meaningful variables were retained based on their significance in the univariate comparison. Age, sex and body mass index (BMI) were kept as covariates in all models. A stepwise approach was used starting from a full multivariate model from which variables were removed based on the Akaike information criterion. Using the mice package in R,Citation30 missing values were added for cTnT to allow for more robust model building. Kaplan–Meier and Cox regression analyses were conducted to assess survival. The latter was repeated with correction for age, forced expiratory volume in 1 second (FEV1) and cTnT. The study period was from hospital admission until death or December 31, 2015.
Prospective data
Continuous/ordinal data were compared using Kruskal–Wallis test, and categorical data were compared using Fisher’s exact test. p-values were estimated via Monte Carlo simulation with 5.000 replicates. McNemar and paired Mann–Whitney–Wilcoxon test assessed the change in QTc duration during hospitalization.
Results
Retrospective analysis – patient characteristics
The characteristics of the patients hospitalized for an AECOPD, according to whether they had prolonged QTc at admission, are summarized in .
Table 2 Characteristics of patients hospitalized for an AECOPD, according to prolonged QTc status on the admission ECGTable Footnote*
Patients with prolonged QTc were older, had a higher BMI and did not present with worse COPD. They had more severe (cardiovascular) comorbidity, as proven by the significantly higher scores for the Charlson and COPD comorbidity indices, and the presence of cardiovascular disease (). In particular, there was significantly more congestive heart failure (p=0.002), diabetes (p=0.006) and overall cardiovascular disease (p=0.010) (data not shown). There was no significant difference in the use of cardiovascular and QT-prolonging medication in general, neither when looking at the different subclasses separately (data not shown). The distribution of cTnT values was significantly different with higher values in patients with prolonged QTc (). The ECG-related variables showed that prolonged QTc was associated with increasing heart rate, and rhythm and conductance irregularities were significantly more prevalent. Extrasystoles (p=0.075), left (p=0.097) and right (p=0.021) bundle branch block were the most noteworthy (data not shown). Severely prolonged QTc (>500 msec) was documented in 17.1%. The CXR of patients with prolonged QTc showed significantly more often cardiomegaly and pleural fluid. There was no difference in length of stay, but there was a (non-significant) trend toward higher all-cause mortality in patients with prolonged QTc. Of all patients who died during follow-up, 16.6% had a prolonged QTc >500 msec.
Figure 1 Distribution of (cardiovascular) comorbidity according to prolonged QTc status in COPD patients hospitalized for an acute exacerbation, assessed by the (A) Charlson comorbidity index (p=0.034), (B) COPD comorbidity index (p=0.021) and (C) presence of cardiovascular disease (p=0.040).
Abbreviation: QTc, QT interval corrected according to Bazett’s formula.
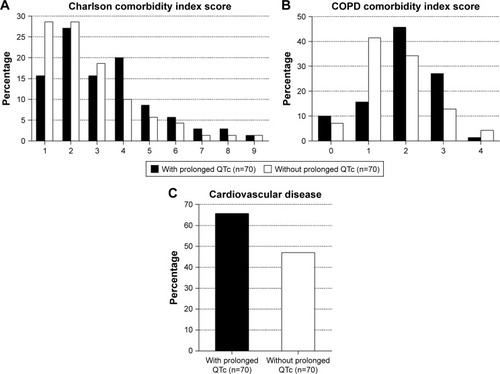
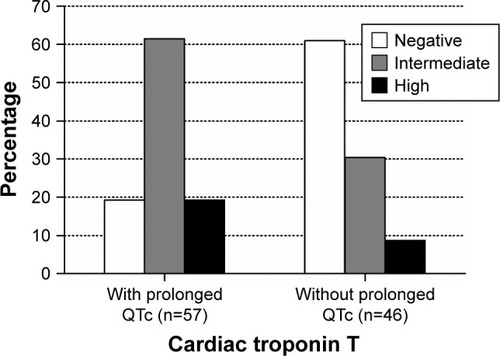
Figure 2 Distribution of cardiac troponin T value according to prolonged QTc status in COPD patients hospitalized for an acute exacerbation (p<0.001). Cutoff values: negative, ≤0.014 ng/mL; intermediate, 0.014–0.052 ng/mL; high, ≥0.052 ng/mL.
Determinants of prolonged QTc
A multivariate logistic regression model was fitted to gain insight into the determinants of prolonged QTc in patients hospitalized for an AECOPD (). There was no effect of the demographic variables. Notably, the model supported a strong association of hypokalemia, cTnT elevation and conductance abnormalities on ECG with prolonged QTc. The presence of pleural fluid on the CXR showed a borderline association with prolonged QTc, although not significant.
Table 3 Multivariate logistic regression model: determinants for prolonged QTc in patients hospitalized for an AECOPDTable Footnote*
Effect of prolonged QTc on survival
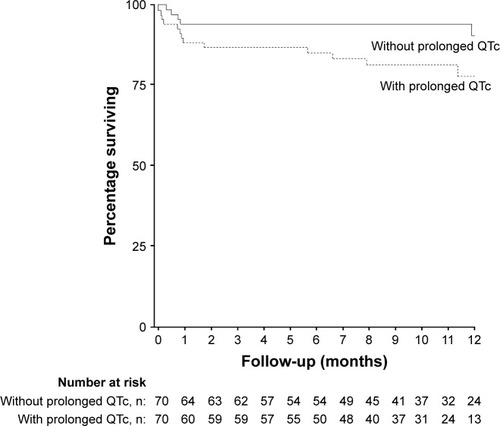
The Kaplan–Meier survival plot is shown in . Patients with prolonged QTc had a mean survival of 405.6 days (95% CI 362.8–448.4), which was significantly shorter compared to 471.9 days (95% CI 441.3–502.5) for patients without QTc prolongation (p=0.035). Prolonged QTc was associated with increased mortality risk (HR 2.698 [95% CI 1.032–7.055], p=0.043); however, it was no longer significant after correction for age, FEV1 and cTnT.
Prospective analysis – patient characteristics
The characteristics of the pulmonary patients hospitalized for acute respiratory problems, according to the main admission diagnosis, are summarized in . The cohort used for analysis consisted of 168 unique patients, 55 of which were admitted for an AECOPD, 34 for lung cancer and 32 for pulmonary infections. Due to low numbers, 7 admissions related to pulmonary hypertension, 12 to interstitial lung disease and 22 to lung transplantation were grouped under miscellaneous. Six patients were excluded as they could not be assigned to any of the groups.
Table 4 Characteristics of pulmonary patients hospitalized for acute respiratory problems, according to the main admission diagnosisTable Footnote*
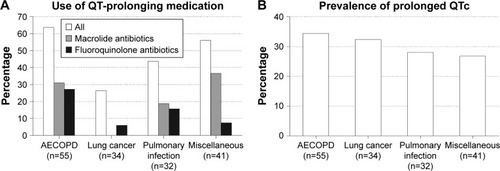
Lung disease and comorbidity variables revealed significant differences that were to be expected based on the inherent nature of the data. The use of QT-prolonging medication differed significantly, with higher use of macrolide and fluoroquinolone antibiotics in the AECOPD group and macrolide antibiotics in the miscellaneous group (). A prolonged QTc was equally prevalent in all groups, with ~30% of patients showing values above the sex-specific threshold (). Heart rate was significantly increased in the AECOPD group, while other ECG variables did not differ and the CXR generally showed less abnormalities. There was a (non-significant) trend toward higher all-cause mortality in the lung cancer and miscellaneous groups.
Figure 4 Distribution of the (A) concomitant use of QT-prolonging medication (p=0.004) and the (B) prevalence of prolonged QTc (p=0.858) among pulmonary patients hospitalized for acute respiratory problems according to the admission diagnosis.
QTc evolution
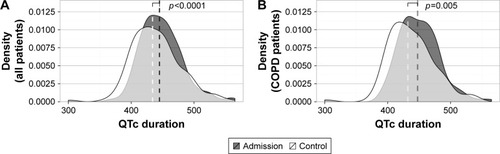
The ECGs taken before hospital discharge were examined to assess whether the QT interval remained prolonged during hospitalization. The patients for whom at least two ECGs were available were used for analysis. In the retrospective data (n=97), the proportion of patients with prolonged QTc remained stable: 33.0% at admission and 30.9% before discharge (p=0.80). In the prospective data (n=63), however, the proportion decreased significantly from 46.0% to 27.0% (p<0.001). There was no significant evolution of other ECG variables (data not shown) with the exception of the median heart rate, which decreased significantly from 88.0 (IQR 24.0) to 84.5 (IQR 22.0) bpm, p=0.004. When combining both data sets (n=160) and evaluating the QTc data in numeric (rather than categorical) form, the median QTc duration decreased significantly from 440.5 (IQR 43.3) to 434.5 (IQR 48.5) msec, p<0.0001. Similar results were found when limited to the established COPD patients (n=123) (p=0.005). The shift of the distribution toward lower QTc values is illustrated for both populations in . In the non-COPD patients, there was also a significant leftward shift (data not shown).
Figure 5 The median QTc duration, evaluated in (A) all pulmonary patients hospitalized for acute respiratory problems (n=160) and more specifically (B) COPD patients hospitalized for an acute exacerbation (n=123), decreases significantly during hospitalization.
Abbreviations: QTc, QT interval corrected according to Bazett’s formula; ECG, electrocardiogram.
Discussion
In this explorative study, we characterized patients hospitalized for an AECOPD with prolonged QTc as a distinct COPD subpopulation. They have significantly more comorbidity in general and more cardiovascular disease in particular, which reflects and highlights the intricate (and bidirectional) relationship between cardiovascular disease and AECOPD.Citation31
In a multivariate model, we identified hypokalemia, cTnT and conductance abnormalities on ECG to be strongly and independently associated with prolonged QTc. While hypokalemia is a recognized causal risk factor for QT prolongation,Citation32 the magnitude of risk associated with cTnT (a well-known prognostic factor associated with long-term and short-term mortality in AECOPD)Citation17–Citation19 may rather pinpoint to the presence of an unidentified underlying cardiovascular diseaseCitation33 or increased right ventricular strain during an AECOPD. Although borderline not significant, this is also reflected by the association with pleural effusion, which is most commonly caused by congestive heart failure, which in turn is a recognized risk factor for QT prolongation. Unfortunately, no echocardiogram was available that could have distinguished left from right heart failure. The association with conductance irregularities is likely explained by the lengthening of the QRS complex, which in itself results in a longer QT interval, for which Bazett’s formula does not correct.Citation34 The increased mortality risk associated with prolonged QTc further highlights that these patients represent a more fragile and comorbid subgroup. As its effect is not independent from other risk factors, prolonged QTc is to be considered as a sign of underlying cardiovascular (co) morbidity.
When comparing AECOPD to other acute respiratory problems, we found no significant differences in any of the ECG variables, specifically a prolonged QTc was not more prevalent in the AECOPD group. Earlier studies did reveal patients with COPD being more likely to have an abnormal ECG than the general population,Citation23,Citation25 but we found no earlier studies comparing COPD with other pulmonary diseases. In our population, 55.2% of patients with COPD had an abnormal ECG at admission, a number comparable to estimates in the literature.Citation31
A prolonged QTc was a rather common finding (~30%) that can partly be explained by the use of QTc values corrected according to Bazett’s formula, known to overcorrect at higher heart rates and not to correct for the QRS duration. The high prevalence leads us to question the predictive utility of current cutoff criteria and to support either the need for higher cutoff values than those cited in literature or to support the use of other correction methods.Citation34,Citation35 A scientific statement from the American Heart Association and the American College of Cardiology Foundation recommends that 470 msec for males and 480 msec for females should be considered abnormally prolonged.Citation36 While there is no consensus on the best correction method to be used in clinical practice, the Fridericia formula is currently considered to reflect a more accurate correction in patients with tachycardia.Citation34 Based on Bazett’s formula, we found QT prolongation to be more prevalent in COPD patients at hospital admission, with a significant decrease in the proportion of prolonged QTc on the ECG at discharge. Normalization of the QTc duration is likely related to the correction or stabilization of the acute respiratory event, which corresponds to periods of increased cardiovascular stress and tachycardia. Surprisingly, many patients were started on fluoroquinolone antibiotics (as an established treatment for AECOPD) regardless of their QT interval. This remarkable finding could indicate that QTc assessment is often overlooked or that the presence of prolonged QTc has little impact on the final decision to start with QT-prolonging medications.
The combined results of high prevalence and little clinical follow-up support the notion that the observed prevalence is subject to overestimation. Recalculating the QTc duration using the Fridericia formula in the prospective study resulted in a lower prevalence of only 11% for AECOPD, not significantly different when compared with other acute pulmonary diseases requiring hospitalization. Using QTcF in the retrospective sample resulted in 64% of the patients with “prolonged QTc” to be reclassified as “normal”. These reclassified patients are less likely at risk for malignant ventricular arrhythmias. Nevertheless, QT prolongation based on Bazett’s formula is still able to identify COPD patients with a distinct clinical profile characterized by significantly higher mortality and more underlying cardiovascular disease compared to similar COPD patients without QT prolongation.
Our study has several other limitations. Because we dealt with registry data, there were some missing observations, most notably cTnT. Moreover, it was considered as an ordinal variable (based on pertinent cutoff values), which impacts the results. The grouping of patients in the prospective data set was based on the admission diagnosis, using a non-systematic approach, oversimplifying the complex interplay of different pulmonary diseases in practice (eg, lung cancer or lung transplantation with underlying COPD). We used the automated QT value computed by the recording system and only used the Bazett’s formula for heart rate correction, with its inherent limitations.Citation34 In addition, the prospective protocol influenced the assessment of the control ECG and did not allow a systematic control of all ECGs before discharge, complicating straightforward interpretation and hypothesis testing concerning the evolution of QTc duration. Further research is necessary to systematically evaluate prolonged QTc in hospitalized patients with COPD and its potential consequences.
Conclusion
A prolonged QT interval corrected according to Bazett’s formula is a common finding in patients admitted with acute respiratory problems, non-specific for AECOPD. It is a sign of (cardiovascular) comorbidity in patients with COPD and is associated with increased mortality. Withholding QT-prolonging medication remains warranted in clinical practice, as the risk of developing ventricular arrhythmia is most pronounced in patients with preexisting cardiac disease on poly-pharmacologic treatment. It is valuable, however, to verify QT prolongation before discharge, either with another correction method or on another ECG, as many patients might have a normal QTc after correction or stabilization of the acute respiratory symptoms and could benefit from clinically indicated first-choice medication. The true validity of a prolonged QT interval in AECOPD, in terms of risk for ventricular arrhythmias and sudden cardiac death, requires further in-depth investigation.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by the Flemish Government Agency for Innovation by Science and Technology (IWT). This work was part of the Belgian trial with Azithromycin for acute COPD Exacerbations requiring hospitalization (BACE), which was funded by the Flemish Government Agency for Innovation by Science and Technology (grant number: IWT-TBM130233). The work was supported by the KU Leuven AstraZeneca Chair in Respiratory Pathophysiology. IWT nor AstraZeneca is involved in the study design; in the collection, analysis and interpretation of data; in the writing of the manuscript or in the decision to submit the manuscript for publication. KV and SE are supported as doctoral candidates by the IWT and the Fund for Scientific Research Flanders, respectively. RW and WJ are supported as postdoctoral clinical researchers by the Fund for Scientific Research Flanders. Data were used for a dissertation in the fulfillment of the degree of Master of Medicine. Data have been presented at the American Thoracic Society Conference (Washington, DC, 22 May 2017 – http://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2017.195.1_Meet-ingAbstracts.A3610).
Disclosure
RW has received research funding from Biotronik, Boston Scientific and Medtronic, and speakers and consultancy fees from Biotronik, Boston Scientific, Medtronic, St Jude Medical and Sorin. WJ has received research funding, speakers and consultancy fees from Boehringer Ingelheim, AstraZeneca, Novartis, Chiesi and GSK. The other authors report no conflicts of interest in this work.
References
- MathersCDLoncarDProjections of global mortality and burden of disease from 2002 to 2030PLoS Med2006311e44217132052
- HuiartLErnstPSuissaSCardiovascular morbidity and mortality in COPDChest200512842640264616236937
- RuttenFHMoonsKGCramerMJRecognising heart failure in elderly patients with stable chronic obstructive pulmonary disease in primary care: cross sectional diagnostic studyBMJ20053317529137916321994
- CelliBRMacNeeWAgustiAStandards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paperEur Respir J200423693294615219010
- DecramerMJanssensWMiravitllesMChronic obstructive pulmonary diseaseLancet201237998231341135122314182
- SuissaSDell’AnielloSErnstPLong-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortalityThorax2012671195796322684094
- PiquetJChavaillonJMDavidPMartinFBlanchonFRocheNFrench College of General Hospital Respiratory Physicians (CPHG)High-risk patients following hospitalisation for an acute exacerbation of COPDEur Respir J201342494695523349446
- AaronSDManagement and prevention of exacerbations of COPDBMJ2014349g523725245156
- SinDDAnthonisenNRSorianoJBAgustiAGMortality in COPD: role of comorbiditiesEur Respir J20062861245125717138679
- McGarveyLPJohnMAndersonJAZvarichMWiseRATORCH Clinical Endpoint CommitteeAscertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint CommitteeThorax200762541141517311843
- SinDDManSFWhy are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary diseaseCirculation2003107111514151912654609
- AgustíAGNogueraASauledaJSalaEPonsJBusquetsXSystemic effects of chronic obstructive pulmonary diseaseEur Respir J200321234736012608452
- FinkelsteinJChaEScharfSMChronic obstructive pulmonary disease as an independent risk factor for cardiovascular morbidityInt J Chron Obstruct Pulmon Dis2009433734919802349
- SinDDManSFChronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortalityProc Am Thorac Soc20052181116113462
- CurkendallSMDeLuiseCJonesJKCardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patientsAnn Epidemiol2006161637016039877
- NeukammAMHøisethADHagveTASøysethVOmlandTHigh-sensitivity cardiac troponin T levels are increased in stable COPDHeart201399638238723315609
- BrekkePHOmlandTHolmedalSHSmithPSøysethVTroponin T elevation and long-term mortality after chronic obstructive pulmonary disease exacerbationEur Respir J200831356357018032444
- HøisethADOmlandTHagveTABrekkePHSøysethVNT-proBNP independently predicts long term mortality after acute exacerbation of COPD – a prospective cohort studyRespir Res2012139723107284
- HøisethADNeukammAKarlssonBDOmlandTBrekkePHSøysethVElevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary diseaseThorax201166977578121653926
- ChangCLRobinsonSCMillsGDBiochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPDThorax201166976476821474497
- PavasiniRd’AscenzoFCampoGCardiac troponin elevation predicts all-cause mortality in patients with acute exacerbation of chronic obstructive pulmonary disease: systematic review and meta-analysisInt J Cardiol201519118719325965630
- RichJDThenappanTFreedBQTc prolongation is associated with impaired right ventricular function and predicts mortality in pulmonary hypertensionInt J Cardiol2013167366967622459397
- WarnierMJRuttenFHNumansMEElectrocardiographic characteristics of patients with chronic obstructive pulmonary diseaseCOPD2013101627123413894
- HoltzmanDAronowWSMellanaWMElectrocardiographic abnormalities in patients with severe versus mild or moderate chronic obstructive pulmonary disease followed in an academic outpatient pulmonary clinicAnn Noninvasive Electrocardiol2011161303221251131
- SieviNAClarenbachCFCamenGRossiVAvan GestelAJKohlerMHigh prevalence of altered cardiac repolarization in patients with COPDBMC Pulm Med20141415524690123
- VestboJHurdSSAgustíAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- VermeerschKGabrovskaMDeslypereGThe Belgian trial with azithromycin for acute COPD exacerbations requiring hospitalization: an investigator-initiated study protocol for a multicenter, randomized, double-blind, placebo-controlled trialInt J Chron Obstruct Pulmon Dis201611168769627099485
- CharlsonMEPompeiPAlesKLMacKenzieCRA new method of classifying prognostic comorbidity in longitudinal studies: development and validationJ Chronic Dis19874053733833558716
- GoldenbergIMossAJZarebaWQT interval: how to measure it and what is “normal.”J Cardiovasc Electrophysiol200617333333616643414
- van BuurenSGroothuis-OudshoornKMICE: multivariate imputation by chained equations in RJ Stat Softw2011453167
- LarattaCRvan EedenSAcute exacerbation of chronic obstructive pulmonary disease: cardiovascular linksBiomed Res Int2014201452878924724085
- Al-KhatibSMLaPointeNMKramerJMCaliffRMWhat clinicians should know about the QT IntervalJAMA2003289162120212812709470
- SøysethVBhatnagarRHolmedahlNHAcute exacerbation of COPD is associated with fourfold elevation of cardiac troponin THeart201399212212623024006
- VandenberkBVandaelERobynsTWhich QT correction formulae to use for QT monitoring?J Am Heart Assoc201656e00326427317349
- RautaharjuPMZhangZMPrineasRHeissGAssessment of prolonged QT and JT intervals in ventricular conduction defectsAm J Cardiol20049381017102115081446
- DrewBJAckermanMJFunkMAmerican Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, the Council on Cardiovascular Nursing, and the American College of Cardiology FoundationPrevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology FoundationCirculation201012181047106020142454