Abstract
Background
Health effects of electronic cigarette (EC) use in patients with chronic obstructive pulmonary disease (COPD) are largely unexplored.
Aim
We present findings from a long-term prospective assessment of respiratory parameters in a cohort of COPD patients who ceased or substantially reduced conventional cigarette use with ECs.
Methods
We prospectively re-evaluated COPD exacerbations, spirometric indices, subjective assessments (using the COPD Assessment Tool [CAT] scores), physical activity (measured by the 6-minute walk distance [6MWD]), and conventional cigarette use in EC users with COPD who were retrospectively assessed previously. Baseline measurements prior to switching to EC use were compared to follow-up visits at 12, 24, and 36 months. Age- and sex-matched regularly smoking COPD patients who were not using ECs were included as reference (control) group.
Results
Complete data were available from 44 patients. Compared to baseline in the EC-user group, there was a marked decline in the use of conventional cigarettes. Although there was no change in lung function, significant improvements in COPD exacerbation rates, CAT scores, and 6MWD were observed consistently in the EC user group over the 3-year period (p<0.01). Similar findings were noted in COPD EC users who also smoked conventional cigarettes (“dual users”).
Conclusion
The present study suggests that EC use may ameliorate objective and subjective COPD outcomes and that the benefits gained may persist long-term. EC use may reverse some of the harm resulting from tobacco smoking in COPD patients.
Introduction
Smoking is an important cause of avoidable premature mortality globally, mainly due to lung cancer, acute fatal complications of atherosclerotic cardiovascular disease, and chronic obstructive pulmonary disease (COPD).Citation1,Citation2 COPD is a progressive condition typified by ongoing airway inflammatory and remodeling responses resulting in respiratory symptoms, progressive lung function decline, respiratory failure, cor pulmonale, and death.Citation3–Citation5 The unique airway inflammatory response in COPD is largely assumed to be due to chronic exposure to a range of smoke toxicants.Citation6,Citation7
Stopping conventional tobacco use is the only evidence-based strategy that has been reported to enhance COPD prognosis.Citation8,Citation9 Prolonged abstinence from smoking attenuates the yearly lung function decline and respiratory symptoms and enhances health status.Citation10–Citation12 Moreover, smoking cessation decreases the chances of developing and consequently perishing from tobacco-related illnesses.Citation13
Although reducing the negative health burden of tobacco smoking is a clear priority for COPD patients who smoke, high failure rates are frequently reported in these patients.Citation14,Citation15 Moreover, approved smoking cessation therapies (ie, nicotine replacement therapy, bupropion, and varenicline) only seem to promote modest enduring cessation in smoking COPD patients.Citation16 This is because the subjects may find it challenging to completely stop using nicotine and/or require longer treatment regimen, support, or nicotine maintenance to possibly aid in attaining continued abstinence from smoking. For these individuals, tobacco harm reduction (THR), that is, the use of combustion-free nicotine delivery systems (ie, electronic cigarettes [ECs]) instead of cigarette smoking, could be a pragmatic compromise with the possibility of significant health gains. Although it is important to acknowledge that nicotine is a potent psycho-stimulant and young people should avoid its use, in conventional cigarettes, it is not nicotine but tobacco combustion chemicals that are the overwhelming cause of tobacco-related disease and death. As respiratory physicians, we should be more concerned of the damage associated with the harmful and potentially harmful constituents generated after combustion than nicotine consumption per se.
The EC has been proposed as a potential THR tool.Citation17 These products have been rapidly gaining ground over conventional cigarettes due to their efficiency in decreasing tobacco consumption, competitive price, the perception of being a much less harmful smoking alternative and also because they allow the smoker to maintain a “smoking experience without smoking.”Citation18–Citation20 ECs do not contain tobacco, create smoke, or rely on combustion to operate. They are not risk-free, but under normal conditions of use, the level of chemical constituents in their aerosol emissions is substantially lower compared to conventional cigarette smoke.Citation20–Citation22 Reducing conventional cigarette consumption by switching over to ECs is therefore expected to result in health benefits and may produce substantial health benefits. ECs by providing a much less harmful means to compete with (and even replace) combustible cigarettes may be saving more lives more rapidly than previously possible. Nonetheless, knowledge about the risk–benefit ratio of this strategy, including the use of ECs in smokers with COPD, is scarce.
According to the findings from the 2014 and 2015 National Health Interview Survey, EC use by COPD patients was significant with former smokers with COPD suggesting a reliance on ECs to prevent relapse to tobacco cigarettes.Citation23 Emerging evidence suggest that COPD smokers who quit or reduce tobacco consumption substantially by switching to EC use are likely to gain significant health benefits. Improvement in respiratory symptoms after switching was reported in 75.7% of 1,190 COPD EC users in a large cross-sectional survey, whereas worsening was reported in only 0.8%.Citation19
No negative impact in a retrospective study of COPD smokers who have been “vaping” (the acting of inhaling from ECs) regularly for at least 2 years.Citation24 Marked attenuation in annual COPD exacerbations and enhanced overall health status (assessed using the COPD Assessment Tool [CAT]) and physical activity (measured by the 6-minute walk distance [6MWD]) were also noted in the same study.Citation24 However, cross-sectional surveys and retrospective designs cannot establish health effects with certainty.
The aim of the present study was to verify these findings by reporting health outcomes of the third year follow-up in the same cohort of COPD patients who have continued to vape regularly for an additional year.
Methods
Patient population
All the patients in the index study were a cohort of COPD EC users; they were identified from medical records and regularly followed up for a period of 36 months. Another group of age-and sex-matched regularly smoking COPD patients (and not using ECs) was also selected over the same period as a reference (control) group. Details of these patients’ populations have been presented elsewhere.Citation24 COPD diagnosis was made according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria as per the prior published study.Citation24 In the current study, COPD patients from both the study groups (COPD EC users and COPD controls) were prospectively followed up for an additional 12 months, hence 36 months in total from baseline. The study was approved by the ethics review board of the coordinating center (Policlinico – Vittorio Emanuele Hospitals). We obtained written informed consent from each patient.
Study design and assessments
Details of the study design and assessments have been described previously.Citation24 Briefly, patients’ clinical notes were reviewed three times over 2 years: at baseline (when COPD patients in the EC group first reported EC use), at 12±1.5 months (follow-up visit 1; F/up1), and at 24±2.5 months (follow-up visit 2; F/up2) to acquire details about 1) their respiratory symptoms, 2) smoking status and conventional cigarette consumption per day (cig/day), 3) the number of severe COPD exacerbations in the prior 12 months, 4) post-bronchodilator lung function parameters (forced expiratory flow in 1 second [FEV1]; forced vital capacity [FVC]; expiratory ratio [FEV1/FVC]; as well as the annual rate of FEV1 decline), 5) CAT scores, and 6) 6MWD.
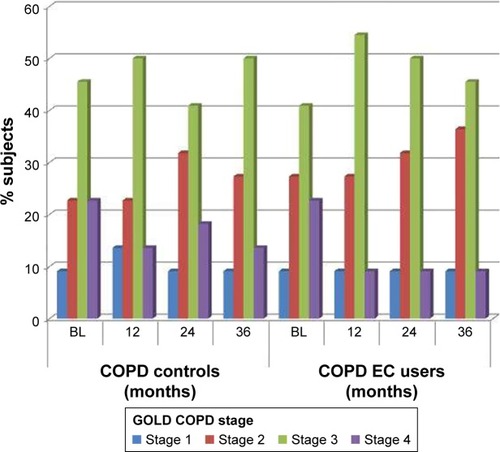
In the present study, COPD EC users and COPD controls were prospectively re-evaluated for changes in the same objective and subjective parameters at an additional follow-up at 36±3 months (follow-up visit 3; F/up3) compared to baseline. Changes in daily tobacco consumption were chemically confirmed using exhaled breath carbon monoxide (eCO), and EC use were also reviewed. Findings obtained at F/up3 were compared with those from baseline, F/up1 and F/up2. In addition, changes in the relative proportion of COPD GOLD stages over the study period were also evaluated.
Severe exacerbations were defined as respiratory symptoms that necessitated the use of antibiotics and/or oral corticosteroids through the primary care physician, emergency department attendance, and/or admission to hospital. For the latter two, nebulization may have also been administered to improve patient symptoms. CAT is a validated health status questionnaire for use in COPD patients with a 2 unit change considered to be of minimal clinical important difference.Citation25,Citation26 The 6MWD, which is a test conducted to assess patients’ overall ability to conduct daily activities, was only offered to patients who were amenable and physically able to do the test.Citation27
Smoking/vaping status
Smoking abstinence was defined as a complete self-reported cessation of tobacco smoking (not even a puff) since the previous study visit. This was also bio-chemically confirmed at F/up3 by eCO levels of ≤7 ppm. COPD EC users in this category are classified as quitters (single users). Patients who used both ECs and conventional cigarettes were classified as dual users.
Analyses
Means (± standard deviation [SD]) and medians (inter-quartile range [IQR]) were used to express parametric and non-parametric data, respectively. Data for single and dual users was also reviewed. Depending on whether the data were parametric or non-parametric, statistical analyses were conducted using student’s t-test and Wilcoxon-signed rank test, respectively. Similar statistical analyses were conducted on dual and single users within groups from baseline. Missing data were not considered in the analyses. With repeated parameter measurements over the study period, analysis of repeated measures with Bonferroni correction was conducted for between groups. A two-tailed p-value of <0.05 was considered to indicate statistical significance. All statistical evaluations were performed with the Statistical Package for Social Science (SPSS for windows version 18.0; SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Of the 48 COPD patients enrolled in the study at baseline, complete data sets at 36 months were obtained from 44 (37 male and 7 female) patients by the end of the study; data sets from two patients from the EC user group who relapsed to conventional cigarette smoking were not included, and updated clinical notes from two patients from the reference group were not available because one died and the other was lost to follow-up due to relocation. Patients’ demographics, objective and subjective parameters, as well as COPD GOLD staging at baseline are summarized in . No between-group differences were noted at baseline for all the parameters assessed. The patients enrolled had mild-to-severe COPD as per the GOLD guidelines and were managed accordingly.Citation24
Table 1 Baseline demographics of study participants (before switching to electronic cigarettes)
Smoking consumption and EC use
COPD EC users were characterized by a significant reduction in conventional cigarette use with a mean (±SD) cigarettes/day of 21.9 (±4.5) at baseline falling to 2 (±2.2) at F/up1, 1.6 (±2) at F/up2, and 1.5 (±2.4) at F/up3, respectively (p<0.001 for all three visits) (). No marked changes were observed among COPD controls. In the COPD EC user group, complete abstinence (quitters; exclusive EC users or single users) from daily conventional cigarette consumption was reported in 13/22 (59.1%) EC users at F/up3; tobacco smoking (dual users) in 9/22 (40.9%) (). A substantial decline in conventional cigarette use was also noted in dual users with the mean (±SD) cigarettes/day at baseline decreasing from 23.9 (±4.9) to 4 (±1.2) at F/up1 to 3.6 (±1.3) at F/up2 and to 3.8 (±1.1) at F/up3, respectively (p<0.001 for all three visits) (). Of note, all the dual users, at all three visits, managed to reduce their conventional cigarette use/day by ≥75% of their baseline consumption. Overall, a statistically significant decrease in conventional cigarettes smoked was consistently observed between the study groups over the 36-month observation period (p<0.001).
Table 2 Changes in study parameters from baseline at 12-, 24-, and 36-month follow-up visits in COPD controls and COPD EC users
Table 3 Changes in study parameters from baseline at 12-, 24-, and 36-month follow-up visits in single and dual users
COPD exacerbations
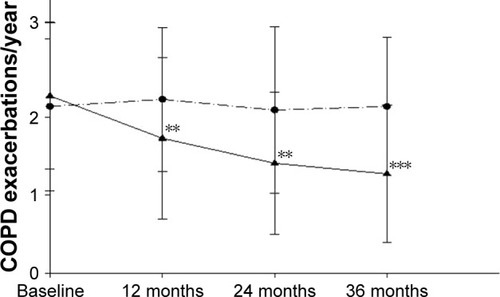
COPD EC users had a significant diminution in COPD exacerbations; with their mean (±SD) exacerbation rate falling from 2.3 (±0.9) at baseline to 1.7 (±1) at F/up1 (p=0.002), 1.4 (±0.9) at F/up2 (p=0.002), and 1.3 (±0.9) at F/up3 (p<0.001), respectively (). There were no significant changes in COPD exacerbation rates over the 3 years in the control group from baseline. A significant (p=0.004) between-group reduction in COPD exacerbations was seen over the 36-month period of the study (; ). Consistent reductions in COPD exacerbations were observed in the dual users as well, with their mean (±SD) exacerbation rate of 2.7 (±0.9) at baseline significantly falling to 1.5 (±0.9) at F/up2 (p=0.002) and 1.2 (±0.8) at F/up3 (p=0.001), respectively ().
Figure 1 Changes in the number of COPD exacerbations per year from baseline, at follow-up visit 1 (12±1.5 months), visit 2 (24±2.5 months), and visit 3 (36±3 months) separately for COPD EC users (closed triangles) and COPD controls (closed circles). All data are expressed as mean and error bars are standard deviation of the mean. The ** and *** indicate the within-group p-value of <0.01 and <0.001, respectively, compared to baseline.
Lung function assessments and COPD staging
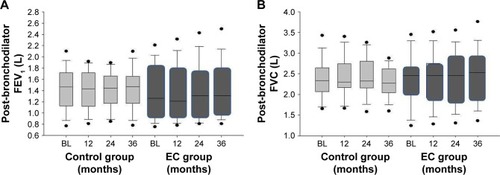
No significant changes in post-bronchodilator FEV1 and FVC from baseline were observed over the 36-month period in both the study groups (; ). In addition, no overall between study group differences in any spirometric assessments were observed. From baseline to F/up3, there was annual increase of 23.3 mL in FEV1 observed in the COPD EC user group compared to a decrease of 4.7 mL in the control group (p=0.139).
Figure 2 Changes in the FEV1 (A) and FVC (B) from baseline, at follow-up visit 1 (12±1.5 months), visit 2 (24±2.5 months), and visit 3 (36±3 months) separately for COPD EC users (dark gray boxes) and COPD controls (light gray boxes). The boxes represent the 25th to 75th percentiles; the line in the boxes indicates the median, and error bars are 5th and 95th percentiles.
Changes in GOLD COPD staging are depicted in . In the 3-year period, a number of COPD patients in the EC study group down-staged from GOLD COPD Stages 4 and 3 to Stages 3 and 2, respectively. In contrast, there was virtually a lack of change in the COPD GOLD stages in the control group over the observation period.
CAT scores and 6MWD
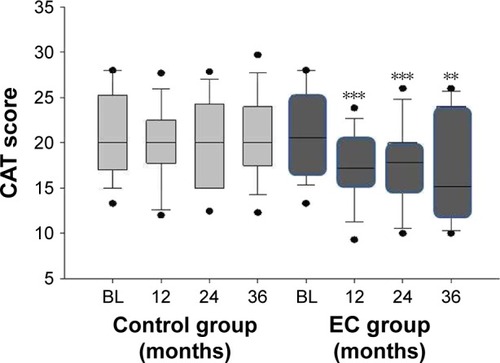
Subjective COPD assessment, evaluated using CAT scores, improved significantly in the COPD EC group throughout the study (p≤0.01 for all three visits). Improvements were of clinical relevance with a median CAT score reduction from baseline of 3.5, 3, and 5.5 units at F/up1, F/up2, and F/up3, respectively (). No significant changes in CAT scores were observed in the control group. Hence, significant (p=0.019) between-group reductions in CAT scores was seen over the 36-month period of the study (; ). Consistent and clinically relevant reductions in CAT scores were observed in the dual users as well ().
Figure 4 Changes in the CAT scores from baseline, at follow-up visit 1 (12±1.5 months), visit 2 (24±2.5 months), and visit 3 (36±3 months) separately for COPD EC users (dark gray boxes) and COPD controls (light gray boxes). The boxes represent the 25th to 75th percentiles; the lines in the boxes indicate the median, and error bars are 5th and 95th percentiles. The ** and *** indicate the within-group p-value of <0.01 and <0.001, respectively, compared to baseline.
Results of 6MWD were available for 13 subjects at F/up1 and F/up2 and for 11 subjects at F/up3 in the COPD EC group; while data from the COPD control group were available for 14 subjects at F/up1 and F/up2 and for 13 subjects at F/up3. Compared to baseline, at 36 months, the 6MWD improved by a median of 70 m (p=0.003) in the COPD EC user group whereas decreased by 7.5 m (p=0.087) in the COPD control group (). A significant (p=0.001) improvement in 6MWD was seen between study groups over the 36-month period of the study ().
Discussion
In a cohort of regular EC users with COPD, abstaining from smoking or substantially reducing cigarette consumption ameliorates quality of life as well as respiratory outcomes in COPD and that these positive effects persist long-term. This is in agreement with the notion that quitting smoking is a key strategy not only to prevent the onset of COPD but also to stop its progression into more severe disease stages.Citation8,Citation10–Citation13 These confirmatory findings are of thoughtful importance as many COPD patients continue their tobacco habit despite their symptoms and show little interest in relinquishing it;Citation15,Citation16,Citation28 a contradiction that may be justified by the highly addictive disposition of tobacco smoking and the fear of developing depressive symptoms.Citation28,Citation29
Over an observation period of ~3 years, only two (8.3%) patients from the COPD EC user group (both were dual users) relapsed to cigarette smoking. Relapse prevention may be another way by which ECs contribute to individual and public health. This is an important consideration, given that smokers with COPD are known to perform poorly in smoking cessation programs due to their high relapse rate.Citation16,Citation28,Citation29 Perhaps the fact that ECs reproduce the smoking experience and accompanying rituals with large compensatory effect at both physical and behavioral levels may explain the low relapse rates in this study of COPD smokers who switched to ECs. A similar mechanism might explain the low relapse rates observed among smokers not intending to quitCitation30,Citation31 as well as in smokers with schizophrenia, asthma, and high blood pressure after switching to EC use.Citation32–Citation34
This study corroborates previous observations of a lack of worsening in respiratory physiology (post-bronchodilator FEV1, FVC, and %FEV1/FVC) in patients with COPD who stopped or considerably reduced their conventional cigarette use by switching to EC use. The absence of marked changes in spirometric indices following smoking cessation is not unusual in COPD smokers and particularly in patients with advanced disease and irreversible airway obstructionCitation35,Citation36 as is the case in our study population.
The finding that COPD exacerbations were halved in patients who stopped or considerably reduced their smoking habit following switching to ECs was an important finding. This is in agreement with results from two large population studies: one reporting a 43% lower risk COPD-related hospitalizations in previous smokers compared with existing smokers;Citation37 and the other showing a 22% reduction in COPD exacerbation risk in ex-smokers compared with ongoing smokers when adjusted for comorbidity, COPD severity indices, and socioeconomic status.Citation38 In contrast, there have also been reports of a lack of any marked differences in hospital admissions between current smokers and ex-smokers with COPD.Citation39,Citation40 Importantly, these studies did not take into consideration important COPD exacerbation risk confounders such as smoking abstinence duration, severity of COPD, comorbidities, and age. These confounders were accounted for in the index study. Since chronic exposure to tobacco smoke is known to enhance susceptibility to airway infection,Citation41,Citation42 it is not surprising that abstention from cigarette smoking by swapping to ECs may result in marked attenuation of respiratory infections and COPD exacerbations.Citation43
Consistent improvements were observed in overall health status and physical activity in our EC-using COPD patient cohort who quit or reduced substantially their conventional cigarette consumption. These clinical changes in CAT and 6MWD confirm our previous observationsCitation24 and are compatible with those reported in undergoing intensive rehabilitation programs in COPD patients.Citation26,Citation44 The mechanism for these improved health outcomes may be associated with the substantial decline in CO exposure (as well as in carboxyhemoglobin levels) following smoking abstinenceCitation45 and to the linked time-dependent progression in exercise tolerance with abstaining from smoking.Citation46 Surprisingly, consistent improvements were also observed in dual users. This could be due to the fact that dual users in the index study significantly attenuated their daily smoking by at least 75% (ie, heavy reducers). Also a much larger proportion of less severe COPD GOLD stages patients were dual users, which may have favored the tendency toward harm reversibility.
There are limitations in our observations that need consideration. Our observations are in a small cohort of COPD patients, and hence the results need to be interpreted cautiously. Nonetheless, we observed consistent and clinically significant beneficial effects in several COPD health indicators. Also, there is the possibility that the patients in the index study may represent a self-selected sample, which may not be representative of all COPD smokers. Another shortcoming is that the 6MWD test was not performed in all study participants, as this was not the standard and some patients declined to do it.
The present study suggests that regular EC use ameliorates several health effect indicators in COPD and demonstrates that these beneficial effects may continue in the longer term. By markedly reducing the number of conventional cigarettes smoked per day and hence exposure to their numerous hazardous toxicants, EC use may not only enhance COPD outcomes, but may also bestow an overall health advantage.Citation47 Therefore, EC use may be exploited as a less harmful strategy to potentially halt or reverse COPD-related outcomes and, in general, to reduce the risk of smoking-related diseases or the harm from smoking-associated comorbidities. While the sample size in our study was relatively small, the results of this study may provide preliminary evidence that long-term use of ECs is unlikely to result in substantial health concerns in COPD patients. Additional studies in a larger and more diverse sample of COPD EC users are now needed to substantiate and elucidate the emerging role of the e-vapor category for smoking cessation and/or harm reversal in smoking COPD patients.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This research was supported by university grant number 21040100 of Ricerca Scientifica Finanziata dall’Ateneo di Catania [Scientific Research Funded by University of Catania].
Disclosure
In relation to RP’s work in the area of tobacco control and respiratory diseases, he has received lecture fees and research funding from Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, MSD, Boehringer Ingelheim, Novartis, Duska Therapeutics, and Forest Laboratories. He has also served as a consultant for Pfizer, Global Health Alliance for Treatment of Tobacco Dependence, CV Therapeutics, NeuroSearch A/S, Boehringer Ingelheim, Duska Therapeutics, Forest Laboratories, ECITA (Electronic Cigarette Industry Trade Association, in the UK), and Health Diplomat (consulting company that delivers solutions to global health problems with special emphasis on harm minimization). Lecture fees from a number of European EC industry and trade associations (including FIVAPE in France and FIESEL in Italy) were directly donated to vaper advocacy no-profit organizations on the behalf of RP. RP is also currently a scientific advisor for LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti-Smoking League) and Head of the European Technical Committee for Standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4). JBM has received honoraria for speaking and financial support to attend meetings/advisory boards from Wyeth, Chiesi, Pfizer, MSD, Boehringer Ingelheim, Teva, GSK/Allen & Hanburys, Napp, Almirall, AstraZeneca, Trudell and Novartis. The authors report no other conflicts of interest in this work.
References
- World Health OrganisationWHO report on the global tobacco epidemic: Warning about the dangers of tobacco Available from: http://www.who.int/tobacco/global_report/2011/en/Accessed July 18, 2018
- Office of the Surgeon General (US)Office on Smoking and Health (US)The Health Consequences of Smoking: A Report of the Surgeon GeneralAtlanta, GACenters for Disease Control and Prevention2004
- MacNeeWPathogenesis of chronic obstructive pulmonary diseaseProc Am Thorac Soc20052425826616267346
- MorjariaJBMalerbaMPolosaRBiologic and pharmacologic therapies in clinical development for the inflammatory response in COPDDrug Discov Today2010159–1039640520223295
- FalkJAKadievSCrinerGJScharfSMMinaiOADiazPCardiac disease in chronic obstructive pulmonary diseaseProc Am Thorac Soc20085454354818453369
- StrattonKShettyPWallaceRBondurantSClearing the smoke: the science base for tobacco harm reduction – executive summaryTob Control200110218919511387543
- Centers for Disease Control and Prevention (US)National Center for Chronic Disease Prevention and Health Promotion (US)Office on Smoking and Health (US)How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the surgeon generalAtlanta, GACenters for Disease Control and Prevention (US)2010
- The Health Benefits of Smoking CessationRockville, MarylandU.S. Department of Health and Human Services, Public Health Service. Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health1990
- HershCPDeMeoDLAl-AnsariEPredictors of survival in severe, early onset COPDChest200412651443145115539711
- AnthonisenNRConnettJEKileyJPEffects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health StudyJAMA199427219149715057966841
- BurchfielCMMarcusEBCurbJDEffects of smoking and smoking cessation on longitudinal decline in pulmonary functionAm J Respir Crit Care Med19951516177817857767520
- KannerREConnettJEWilliamsDEBuistASEffects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the Lung Health StudyAm J Med1999106441041610225243
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and HealthThe health consequences of smoking: 50 years of progress: a report of the sugeon generalAtlanta, GACentres for Disease Control and Prevention (US)2014
- van der MeerRMWagenaEJOsteloRWJacobsJEvan SchayckCPSmoking cessation for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20032CD002999
- Jimenez-RuizCAMasaFMiravitllesMSmoking characteristics: differences in attitudes and dependence between healthy smokers and smokers with COPDChest200111951365137011348940
- TashkinDPSmoking cessation in chronic obstructive pulmonary diseaseSemin Respir Crit Care Med201536449150726238637
- PolosaRRoduBCaponnettoPMagliaMRacitiCA fresh look at tobacco harm reduction: the case for the electronic cigaretteHarm Reduct J2013101924090432
- CaponnettoPRussoCBrunoCMAlamoAAmaradioMDPolosaRElectronic cigarette: a possible substitute for cigarette dependenceMonaldi Arch Chest Dis2013791121923741941
- FarsalinosKERomagnaGTsiaprasDKyrzopoulosSVoudrisVCharacteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumersInt J Environ Res Public Health20141144356437324758891
- FarsalinosKEPolosaRSafety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic reviewTher Adv Drug Saf201452678625083263
- GoniewiczMLKnysakJGawronMLevels of selected carcinogens and toxicants in vapour from electronic cigarettesTob Control201423213313923467656
- MarghamJMcAdamKForsterMChemical composition of aerosol from an e-cigarette: a quantitative comparison with cigarette smokeChem Res Toxicol201629101662167827641760
- KruseGRKalkhoranSRigottiNAUse of electronic cigarettes among U.S. adults with medical comorbiditiesAm J Prev Med201752679880428108191
- PolosaRMorjariaJBCaponnettoPEvidence for harm reduction in COPD smokers who switch to electronic cigarettesRespir Res201617116627986085
- JonesPWHardingGBerryPWiklundIChenWHKline LeidyNDevelopment and first validation of the COPD assessment testEur Respir J200934364865419720809
- KonSSCanavanJLJonesSEMinimum clinically important difference for the COPD assessment test: a prospective analysisLancet Respir Med20142319520324621681
- ATS statement: guidelines for the six-minute walk testAm J Respir Crit Care Med2002166111111712091180
- MorjariaJBMondatiEPolosaRE-cigarettes in patients with COPD: current perspectivesInt J Chron Obstruct Pulmon Dis2017123203321029138548
- ZhangMWHoRCCheungMWFuEMakAPrevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regressionGen Hosp Psychiatry201133321722321601717
- PolosaRMorjariaJBCaponnettoPEffectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational studyIntern Emerg Med20149553754623873169
- CaponnettoPCampagnaDCibellaFEffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design studyPLoS One201386e6631723826093
- CaponnettoPAuditoreRRussoCCappelloGCPolosaRImpact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot studyInt J Environ Res Public Health201310244646123358230
- PolosaRMorjariaJBCaponnettoPPersisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettesDiscov Med2016211149910827011045
- FarsalinosKCibellaFCaponnettoPEffect of continuous smoking reduction and abstinence on blood pressure and heart rate in smokers switching to electronic cigarettesIntern Emerg Med2016111859426749533
- ScanlonPDConnettJEWallerLASmoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health StudyAm J Respir Crit Care Med20001612 Pt 138139010673175
- TashkinDPRennardSTaylor HaysJLawrenceDMartonJPLeeTCLung function and respiratory symptoms in a 1-year randomized smoking cessation trial of varenicline in COPD patientsRespir Med2011105111682169021621992
- GodtfredsenNSVestboJOslerMPrescottERisk of hospital admission for COPD following smoking cessation and reduction: a Danish population studyThorax2002571196797212403880
- AuDHBrysonCLChienJWThe effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbationsJ Gen Intern Med200924445746319194768
- AnthonisenNRConnettJEEnrightPLManfredaJHospitalizations and mortality in the Lung Health StudyAm J Respir Crit Care Med2002166333333912153966
- KesslerRFallerMFourgautGMennecierBWeitzenblumEPredictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med199915911581649872834
- FeldmanCAndersonRCigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systemsJ Infect201367316918423707875
- SoporiMEffects of cigarette smoke on the immune systemNat Rev Immunol20022537237712033743
- CampagnaDAmaradioMDSandsMFPolosaRRespiratory infections and pneumonia: potential benefits of switching from smoking to vapingPneumonia (Nathan)201684 eCollection 2016
- GreulichTKoczullaARNellCEffect of a three-week inpatient rehabilitation program on 544 consecutive patients with very severe COPD: a retrospective analysisRespiration201590428729226340440
- CampagnaDCibellaFCaponnettoPChanges in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettesEur J Clin Invest201646869870627322745
- BerkovitchAKivitySKlempfnerRTime-dependent relation between smoking cessation and improved exercise tolerance in apparently healthy middle-age men and womenEur J Prev Cardiol201522680781424817697
- MorjariaJBPolosaRThe holistic perspective of chronic obstructive pulmonary disease: doubt some moreTher Adv Chronic Dis201012374123251727