Abstract
Background
COPD has been identified as an etiology or related disease of bronchiectasis, and bronchiectasis has been classified as a comorbidity of COPD. In this study, we investigated the prevalence of bronchiectasis in different phenotypes of COPD subjects and the correlation between bronchiectasis and different phenotypes, especially emphysema.
Methods
COPD patients were recruited from April 2012 to December 2015. The presence of bronchiectasis and related information were statistically analyzed. COPD subjects were separated into subgroups in two ways: COPD with and without bronchiectasis groups and emphysema-predominant (emphysema index, EI≥9.9%) and non-emphysema-predominant (EI<9.9%) groups.
Results
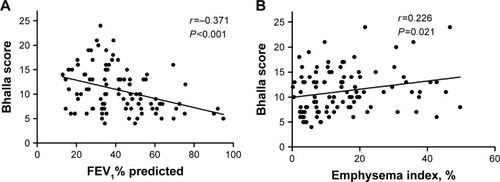
In total, 1,739 COPD patients were incorporated into the study, among which 140 cases (8.1%) were accompanied with radiological bronchiectasis. COPD patients with concomitant bronchiectasis presented worse pulmonary function (FEV1% predicted, P<0.001), higher EI (15.0% vs 13.4%, P<0.001), and higher proportion of pulmonary hypertension and cor pulmonale (6.4% vs 2.4%, P=0.005 and 23.6% vs 16.1%, P=0.022) than patients without bronchiectasis. Of all the COPD patients, 787 with EI data were divided into emphysema-predominant (n=369) and non-emphysema-predominant groups (n=418). The proportion of bronchiectasis was 16.5% and 10.3% (P=0.01), respectively. Severity of bronchiectasis increased as the degree of airflow limitation (r=−0.371, P<0.001) and emphysema increased (r=0.226, P=0.021). After adjusting confounding factors, FEV1% predicted (OR, 1.636; 95% CI, 1.219–2.197; P=0.001) and EI (OR, 1.993; 95% CI, 1.199–3.313; P=0.008) were significantly related with the presence of bronchiectasis in COPD patients.
Conclusion
The proportion of bronchiectasis is higher in emphysema-predominant COPD subjects. Emphysema measured by EI and FEV1% predicted are independent predictors for bronchiectasis in COPD subjects, while the underlying mechanism deserves further investigation.
Introduction
Bronchiectasis is defined as an irreversible and progressive dilation of the airways due to chronic airway injury.Citation1 It is not an independent disease, and many other diseases that affect the defense function of airway could induce bronchiectasis. With the extensive use of high-resolution computed tomography (HRCT), COPD has been considered as one of the etiologies or related diseases of bronchiectasis.Citation1 Bronchiectasis has been classified as the comorbidity of COPD in Global Initiative for Chronic Obstructive Lung Disease (GOLD) since 2014.Citation2 Thereafter, the updated version of GOLD emphasizes the impact that bronchiectasis has on the natural course and prognosis of COPD.Citation3,Citation4 In recent years, studies about coexisting COPD and bronchiectasis have caught increasing attention. The content mainly involved the influence of coexisting diseases on acute exacerbation frequency, severity of airflow limitation, prognosis, and characteristics of pathogenic microorganism.Citation5–Citation8 Owing to the heterogeneity of both diseases, limited information is known about the relationship between bronchiectasis and different phenotypes of COPD subjects.
Emphysema and chronic bronchitis are two classical phenotypes of COPD, which show different characteristics of symptom, sign, and prognosis.Citation9 Previous studies focusing on the phenotype of COPD often excluded bronchiectasis as a confounding factor. Nowadays, coexisting COPD and bronchiectasis has been put forward as not only a comorbidity but also an underlying phenotype.Citation10 Just as in COPD, key inflammatory cytokines and proteases, such as neutrophil elastases, were found at elevated levels in bronchiectasis.Citation11 This reminds us there may be a common pathway for the two diseases, especially bronchiectasis and emphysema. Emphysema index (EI) measured by computer has been considered as a convenient way to differentiate COPD subjects into emphysema-predominant and non-emphysema-predominant groups.Citation12,Citation13 In the present study, we explored the relationship between bronchiectasis and emphysema, which was quantified by EI.
Methods
Study population
In this retrospective study, COPD patients were consecutively retrieved from April 2012 to December 2015 in Qilu Hospital, Shan Dong University. On the basis of GOLD guidelines,Citation14 COPD was diagnosed with persistent respiratory symptoms and airflow limitation (post-BD FEV1/FVC <0.70). Patients with chest computed tomography (CT) and pulmonary function test (PFT) at stable status within 12 months were included. An experienced respiratory physician and a respiratory radiologist confirmed the diagnosis of bronchiectasis, according to 1) lack of tapering of bronchi, 2) dilation of bronchi if the internal diameter was larger than that of the adjacent pulmonary artery, or 3) visualization of the peripheral bronchi within 1 cm of the pleural surface.Citation7,Citation8,Citation15 Bronchiectasis in a single pulmonary segment was not included, and lingula was considered as an independent lobe. The Bhalla score was used to quantify the severity of bronchiectasis.Citation16 Exclusion criteria include asthma; interstitial lung disease; pneumoconiosis; pneumonia or pulmonary masses >3 cm on chest CT; bronchiectasis occurring before the age of 40 years, with a history of measles and pertussis, or with a definite history of pulmonary tuberculosis and original site of tuberculosis overlapping with bronchiectasis from CT scans.
Demographic and clinical characteristics were collected, including age, gender, smoking history, history of illness, comorbidities, peripheral blood tests (blood routine, albumin [ALB], erythrocyte sedimentation rate, and fibrinogen), HRCT scans, and PFT data.
The study was approved by the Ethics Committee of Qilu Hospital of Shandong University (No 2015091). All the data and analysis were performed anonymously. The Ethics Committee of our hospital agreed that for retrospective studies written informed consent from participants was not required.
PFT
PFTs were performed by experienced technicians according to the American Thoracic Society and European Respiratory Society (ATS/ERS) recommendations.Citation17 Computerized spirometer (MasterScreen, Jaeger, Hoechberg, Germany) was used. Parameters including basic information (age, height, weight, and body mass index) and spirometry data (FEV1, FVC, FEV1% predicted, and FEV1/FVC) were collected. Post-BD FEV1/FVC <0.70 was considered as airflow limitation.
HRCT
A 64-slice spiral CT scanner (SOMATOM Definition AS; Siemens Healthcare, Erlangen, Germany) was used for chest HRCT. Subjects who followed the procedure of chest CT performed at full inspiration. Tube voltage was 120 kV and tube current varied between 20 and 500 mA by automatic regulation. Exposure time was 0.5 second and matrix size was 512×512 pixels. Consecutive images were reconstructed with 1 mm slice thickness.
Quantitative measurement of emphysema
Emphysema was defined by the percentage of voxels below −950 HU (%LAA-950) at inspiration as assessed using Airway Inspector software (Surgical Planning Laboratory at Brigham and Women’s Hospital, Boston, MA, USA).Citation13 As reported in previous work,Citation12,Citation13 %LAA-950 exceeding 9.9% was responsible for high EI. Based on the EI value, subjects were divided into two groups: emphysema-predominant group (%LAA-950≥9.9%) and non-emphysema-predominant group (%LAA-950<9.9%).
Statistical analyses
Statistical analyses were performed using SPSS version 19.0 (IBM Corporation, Armonk, NY, USA). Data are presented as mean ± SD for continuous variables and percentage for categorical variables. The Student’s t-test or Mann–Whitney U-test was used for normally distributed values or non- normally distributed values. Considering categorical variables, chi-square test was performed to determine the statistical differences between proportions. The Pearson’s r coefficient was used to investigate the correlation between the severity of bronchiectasis and airflow limitation or EI. Multivariate logistic regression analysis was performed to identify predictive factors for bronchiectasis in COPD patients. P<0.05 was considered statistically significant.
Results
Participants’ characteristics
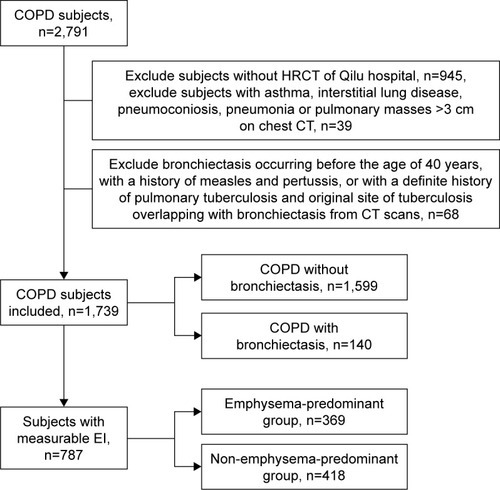
As shown in , a total of 2,791 COPD patients were analyzed retrospectively, 984 patients of whom meet the exclusion criteria or did not have HRCT scans. Another 68 patients meet the exclusion criteria of bronchiectasis. In the end, 1,739 COPD patients were included in the present study. The mean age was 68.57±9.76 years, and 79.8% were male. There were 74.1% current or former smokers. Mean value of FVC, FEV1.0, and FEV1.0/FVC was 2.63 L, 1.49 L, and 54.94%, respectively. Considering the EI, mean %LAA-950 was 13.58%. Of all the subjects, 8.1% were diagnosed with radiological bronchiectasis. Other corresponding comorbidities are shown in .
Table 1 Characteristics of COPD patients
Characteristics of COPD patients with or without bronchiectasis
Patients were divided into two groups: COPD with (n=140) and without (n=1,599) bronchiectasis groups. As shown in , there were less male in the group of subjects with bronchiectasis (72.9% vs 80.4%, P=0.034). Patients with both COPD and bronchiectasis had worse status of nutrition (ALB, P=0.046), more severe airflow obstruction (FEV1.0% predicted, P<0.001), and more extensive emphysema (%LAA-950, P=0.011) than patients without bronchiectasis. Regarding comorbidities, more patients had pulmonary hypertension (6.4% vs 2.4%, P=0.005) and cor pulmonale (23.6% vs 16.1%, P=0.022) in COPD and bronchiectasis coexistence group.
Table 2 Comparison between subjects with and without bronchiectasis
Comparison of characteristics between emphysema-predominant group and non-emphysema-predominant group
There were 787 subjects with measurable EI in the present study. Based on the %LAA-950 value, patients were divided into emphysema-predominant group (%LAA-950≥9.9%, n=369) and non-emphysema-predominant group (%LAA-950,9.9%, n=418). Subjects in emphysema-predominant group had higher proportion of bronchiectasis (16.5% vs 10.3%, P=0.01), higher EI (24.5% vs 4.0%, P<0.001), and more severe airflow limitation (FEV1% predicted, 38.0% vs 56.7%, P<0.001). Other clinical characteristics of the two groups are shown in .
Table 3 Comparison of characteristics between emphysema-predominant group and non-emphysema-predominant group
As shown in , correlations between the severity of bronchiectasis and airflow limitation or emphysema were also performed. The Bhalla score increased as the severity of airflow limitation (r=−0.371, P<0.001) and emphysema increased (r=0.226, P=0.021).
Predictors of bronchiectasis in COPD subjects
We performed multivariate logistic regression analysis that included sex, age, smokers, ALB, and FEV1% predicted in model 1 and sex, age, smokers, ALB, and EI in model 2 to identify factors associated with bronchiectasis among COPD subjects (). In the two models, FEV1% predicted (OR, 1.636; 95% CI, 1.219–2.197; P=0.001) and EI (OR, 1.993; 95% CI, 1.199–3.313; P=0.008) were significantly related with the presence of bronchiectasis in COPD subjects.
Table 4 Logistic regression analysis for predictors of bronchiectasis among COPD subjects
Discussion
As the importance of chest CT in the assessment of COPD becomes more and more prominent, bronchiectasis is found in 4%–57% of COPD patients.Citation8,Citation18–Citation20 However, the linkage mechanism underlying two diseases remains unclear. As previously reported by Blasi et al,Citation10 three hypothetical conditions might be considered: 1) COPD leads to the development of bronchiectasis on account of airway inflammation or infection; 2) bronchiectasis leads to fixed airflow limitation, which meets the diagnostic criteria of COPD; and 3) bronchiectasis and COPD are independent diseases coexisting in one subject. Identification of the three mentioned conditions is of great significance, which might guide the etiology and therapy of diseases. In the present study, we focused on the first condition. As a result, primary bronchiectasis occurring before the age of 40 years, with a history of measles and pertussis or traction bronchiectasis induced by tuberculosis, was all excluded.
Conflicting results were showed in several studies that the proportion of bronchiectasis in patients with COPD ranged from 4% to 57%.Citation6,Citation8,Citation20 The prevalence of bronchiectasis among COPD subjects was 8.1% in the present research. The main reason for different prevalence might lie in the different inclusion and exclusion criteria of COPD subjects and different sample composition. As shown in previous reports,Citation5 proportion of bronchiectasis increases with the severity of GOLD stages. Martinez-Garcia et al reported that 57.2% COPD patients presented with bronchiectasis, while the total number of COPD patients in their study was only 201.Citation7 In our research, 1,739 COPD patients were included and there were 49.1% with GOLD stages III and IV. What’s more, the conditions of bronchiectasis that are included in research are considerably different. Several studies did not exclude the second condition mentioned earlier.Citation6,Citation8 Other studies included bronchiectasis caused by tuberculosis traction,Citation18 which might occur in subjects without COPD. These reasons may partly explain the disparities in prevalence.
High prevalence of bronchiectasis in COPD patients might correlate with the mechanism of COPD. The development of COPD involves small airway, alveoli, and also pulmonary vessels,Citation3,Citation9 while the impairment of small airway occurs early.Citation9,Citation21 Recently, a newly accepted hypothesis was proposed: airway basal cell-specific dysfunction of regeneration and remodeling in susceptible subjects might be the driver of COPD development.Citation22,Citation23 COPD airway epithelial cells regenerate and differentiate poorly, reducing the function of host defense and airway barrier, which makes it susceptible to infections and results in persistent inflammation and remodeling. The same pathogenesis may also induce the development of bronchiectasis. In this sense, it seems that bronchiectasis can be considered as a part of pathological changes of the airway in COPD subjects.
Comparison between patients with and without bronchiectasis shows that the degree of airflow limitation and frequency of comorbidities were higher, and the extent of emphysema was larger in coexisting patients. This implies that exploring the mechanism of coexisting COPD and bronchiectasis may help clinicians to understand the pathology and pathophysiology of COPD. Given sufficient time course, dysfunction of regeneration in pulmonary parenchyma and airway due to the imbalance of protease/anti-protease may aggravate impairment of lung function.Citation24 Importantly, clinicians should pay attention to the clarification and therapy of coexisting COPD and bronchiectasis, especially the treatment for improving lung function and delaying complications.
In the present study, it is the first time that EI is used to investigate the correlation between bronchiectasis and different phenotypes in COPD patients. Subjects are divided into two groups: emphysema-predominant and non-emphysema-predominant groups. It turns out that the proportion of bronchiectasis in emphysema-predominant group is higher than in non-emphysema-predominant one (16.5% vs 10.3%, P=0.010). Traditionally, chronic bronchitis, due to the airway mucus hypersecretion, poor drainage, and repeated infections, is more easy to complicate with bronchiectasis. However, data in the present research remind us that correlation between bronchiectasis and emphysema is more close, which is different from previous work.Citation25 Explanation of the phenomenon may involve two aspects. On one hand, the degree of airflow limitation is more severe in emphysema-predominant group. Patients with higher GOLD stages have poorer drainage and are more susceptible to infections, which makes them more likely to develop bronchiectasis.Citation19 On the other hand, one may consider that the imbalance of protease/anti-protease of emphysema influences the regeneration and remodeling of airway wall.Citation26–Citation28 The higher proportion of smokers and larger amount of smoking pack-years in emphysema-predominant group of the present study make this explanation more possible, while the verification of this hypothesis and the investigation of mechanism deserve more attention.
Limitations
The present research has some limitations. First, as a retrospective study, part of the clinical data is incomplete and information bias is unavoidable. Patients took CT examinations under several conditions: examination before operations, voluntary examination, and COPD assessment performed by clinicians. The single-center study also influenced the efficacy of analysis results. However, the number of samples is relatively large, and nonsmokers are also included in our study. Just as described in GOLD 2018,Citation4 particulate matter also correlates with the development of COPD. Thus, the source of our sample is representative for the general population. To elucidate the conclusions presented in this article, future prospective study involving multicenter data is needed. Second, accurate etiology examinations of bronchiectasis were not performed. The underlying etiologies of bronchiectasis are complicated, including congenital factors and acquired factors such as tuberculosis. The patients with bronchiectasis as such might develop airflow limitation, which could confuse the diagnosis of COPD. The total study population included in this study fulfilled the GOLD criteria of COPD. Thus, bronchiectasis occurring before the age of 40 years or overlapping with original site of tuberculosis from CT scans was excluded in the present study. Future studies involving different etiologies of bronchiectasis are necessary. Other limitation lies in the fact that it is hard to investigate in depth the linkage mechanism of COPD and bronchiectasis based on available data. Considering the inextricable correlation between emphysema and bronchiectasis in COPD subjects, further studies on molecular mechanisms are closely needed.
Conclusion
COPD patients with concomitant bronchiectasis present poorer lung function and higher risk of complication, which should be considered a phenotype of COPD. The occurrence of bronchiectasis in COPD patients correlates with not only a higher degree of GOLD stage but also a higher EI from CT scans. The underlying mechanism deserves further investigation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81370148).
Disclosure
The authors report no conflicts of interest in this work.
References
- PasteurMCBiltonDHillATBritish thoracic society guideline for non-CF bronchiectasisThorax201065757720627912
- Global Initiative for Chronic Obstructive Lung Disease2014Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease Available from: http://wwwgoldcopdorg/Accessed February 1, 2018
- Global Initiative for Chronic Obstructive Lung Disease2017Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease Available from: http://goldcopdorgAccessed December 5, 2017
- Global Initiative for Chronic Obstructive Lung Disease2018Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease Available from: http://wwwgoldcopdorg/Accessed November 15, 2017
- AgustiACalverleyPMCelliBCharacterisation of COPD heterogeneity in the ECLIPSE cohortRespir Res20101112220831787
- Martinez-GarciaMASoler-CatalunaJJDonat SanzYFactors associated with bronchiectasis in patients with COPDChest201114051130113721546440
- Martinez-GarciaMAde la Rosa CarrilloDSoler-CatalunaJJPrognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187882383123392438
- MaoBLuHWLiMHThe existence of bronchiectasis predicts worse prognosis in patients with COPDSci Rep201551096126077673
- HoggJCPathophysiology of airflow limitation in chronic obstructive pulmonary diseaseLancet2004364943570972115325838
- BlasiFChalmersJDAlibertiSCOPD and bronchiectasis: phenotype, endotype or co-morbidity?COPD201411660360425384083
- RussellDWGaggarASolomonGMNeutrophil fates in bronchiectasis and alpha-1 antitrypsin deficiencyAnn Am Thorac Soc201613Suppl 2S123S12927115946
- CuiLJiXXieMDouSWangWXiaoWRole of inspiratory capacity on dyspnea evaluation in COPD with or without emphysematous lesions: a pilot studyInt J Chron Obstruct Pulmon Dis2017122823283029033563
- XieMWangWDouSCuiLXiaoWQuantitative computed tomography measurements of emphysema for diagnosing asthma-chronic obstructive pulmonary disease overlap syndromeInt J Chron Obstruct Pulmon Dis20161195396127226711
- Global Initiative for Chronic Obstructive Lung Disease2012Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease Available from: http://wwwgoldcopdorg/Accessed January 1, 2018
- NaidichDPMcCauleyDIKhouriNFStitikFPSiegelmanSSComputed tomography of bronchiectasisJ Comput Assist Tomogr1982634374447096687
- BhallaMTurciosNAponteVCystic fibrosis: scoring system with thin-section CTRadiology199117937837882027992
- LaszloGStandardisation of lung function testing:helpful guidance from the ATS/ERS task forceThorax200661974474616936234
- JinJYuWLiSLuLLiuXSunYFactors associated with bronchiectasis in patients with moderate-severe chronic obstructive pulmonary diseaseMedicine (Baltimore)20169529e421927442646
- DuQJinJLiuXSunYBronchiectasis as a comorbidity of chronic obstructive pulmonary disease: a systematic review and meta-analysisPLoS One2016113e015053226978269
- NiYShiGYuYHaoJChenTSongHClinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysisInt J Chron Obstruct Pulmon Dis2015101465147526251586
- McDonoughJEYuanRSuzukiMSmall-airway obstruction and emphysema in chronic obstructive pulmonary diseaseN Engl J Med2011365171567157522029978
- CrystalRGAirway basal cells. The “smoking gun” of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2014190121355136225354273
- ShaykhievRCrystalRGEarly events in the pathogenesis of chronic obstructive pulmonary disease. Smoking-induced reprogramming of airway epithelial basal progenitor cellsAnn Am Thorac Soc201411Suppl 5S252S25825525728
- StollerJKAboussouanLSA review of alpha1-antitrypsin deficiencyAm J Respir Crit Care Med2012185324625921960536
- GatheralTKumarNSansomBCOPD-related bronchiectasis; independent impact on disease course and outcomesCOPD201411660561424983298
- ParrDGGuestPGReynoldsJHDowsonLJStockleyRAPrevalence and impact of bronchiectasis in alpha1-antitrypsin deficiencyAm J Respir Crit Care Med2007176121215122117872489
- GrieseMScheuchGDelivery of alpha-1 antitrypsin to airwaysAnn Am Thorac Soc201613Suppl 4S346S35127564672
- ChanEDIsemanMDSignificance of bronchiectasis in patients with alpha1-antitrypsin deficiencyAm J Respir Crit Care Med2008178220818594121