Abstract
Introduction
Acute exacerbation of COPD (AECOPD) and left heart failure (LHF) commonly exist together in clinical practice. However, the identification of AECOPD concurrent with LHF is currently challenging. Our study aimed to investigate the role of plasma N-terminal brain natriuretic pro-peptide (NT-proBNP) in diagnosing elderly patients with AECOPD associated with LHF.
Methods and results
LHF was diagnosed in patients with AECOPD according to echocardiographic criteria, and the levels of NT-proBNP in plasma were measured by quantitative electrochemiluminescence assay. Among the 655 patients with AECOPD, 158 (24.1%) had comorbid LHF, whether systolic (n=108, 68.4%) or diastolic (n=50, 31.6%). The plasma concentrations of NT-proBNP in elderly patients with AECOPD associated with LHF were markedly elevated, compared with those with only AECOPD (4,542.5 and 763.0 ng/L, respectively, P<0.01). The receiver operating characteristic curve indicated a diagnostic cutoff value of 1,677.5 ng/L of NT-proBNP in plasma for ascertaining the presence of LHF in AECOPD, with a sensitivity of 87.9%, a specificity of 88.5%, and an accuracy of 88.4%.
Conclusion
The plasma level of NT-proBNP may be a useful indicator in diagnosing AECOPD associated with LHF.
Introduction
COPD is a common, heterogeneous disorder of the lungs characterized by increasing airflow limitation that is irreversible, and is always progressive.Citation1 This debilitating disorder remains a major contributor to the disease burden worldwide, with acute exacerbations of COPD (AECOPD) being a formidable driver of mortality and morbidity.Citation2 AECOPD is considered an acute event, which disrupts the natural course of the disease, characterized by the worsening of respiratory symptoms, deterioration of dyspnea, cough, and an alteration in sputum purulence and abundance.Citation2 Although dominated by viral or bacterial infection of the tracheobronchial tree, the causes of AECOPD are not identified in nearly one-third of these patients.Citation3,Citation4
Furthermore, AECOPD and left heart failure (LHF) may commonly exist together in clinical settings.Citation5,Citation6 This may be partly owing to the usual association of two disorders sharing the same risk factors, including male predominance in an aging population and cigarette smoking,Citation6,Citation7 and the complex interplay between chronic low-level systemic inflammation and oxidative stress may also attribute to the development of both progressive COPD and LHF.Citation6–Citation9 Consequently, it was hypothesized that the incidence of LHF in elderly patients with AECOPD is probably frequent. However, a diagnosis of AECOPD with comorbid LHF is extremely difficult, as manifestations may be very similar.Citation5,Citation6
N-terminal brain natriuretic pro-peptide (NT-proBNP) is a 76-amino acid N-terminal fragment, derived from the pro-BNP prohormone, and this biologically inactive fragment has the advantage of being easily and affordably assayed.Citation10 Plasma NT-proBNP is widely used clinically for diagnosis, risk stratification, and management in heart failure.Citation11,Citation12 Additionally, elevated plasma levels of NT-proBNP are present in various conditions, including chronic pulmonary diseases, infectious diseases and sepsis, critical illness and shock, liver cirrhosis, renal failure, hyperthyroidism, and intracranial pathologies.Citation10 However, the clinical performance of plasma NT-proBNP for the determination of coexisting LHF in the specific context of AECOPD is less well known. The previous reports showed that the plasma concentrations of NT-proBNP can be increased in COPD, especially during acute exacerbations, although not as high as in manifest heart failure.Citation13,Citation14 Therefore, we hypothesized that evaluating plasma levels of NT-proBNP may be useful in diagnosing elderly AECOPD patients suspected of possessing comorbid LHF.
The goal of this cross-sectional observational study was to evaluate the diagnostic utility of plasma NT-proBNP for identifying AECOPD concurrent with LHF.
Patients and methods
This study was performed between November 2013 and May 2017 in the Department of Respiratory Medicine of Renmin Hospital of Wuhan University (Wuhan, China). The investigation accorded with the principles listed in the Declaration of Helsinki, and the study design was approved by the Ethics Committee of Renmin Hospital of Wuhan University. Written informed consent was provided by each participant in accordance with the Ethics Board of this hospital.
Inclusion and exclusion criteria
Patients aged 65–88 years who previously had a diagnosis of COPD and were in an acute exacerbation of the disorder were included. The diagnosis of COPD was previously confirmed by available spirometric tests, with FEV1/FVC <70% after inhalation of bronchodilators, according to the guidelines established in the Global Initiative for Chronic Obstructive Lung Disease (GOLD).Citation2 AECOPD is defined as an event in the disorder course, characterized by an alteration in the patient’s baseline cough, dyspnea, and/or sputum that is beyond normal day-to-day changes, is acute in onset, and may warrant an alteration in routine medication in any patient with underlying COPD, according to the GOLD guidelines.Citation2 Patients with AECOPD who had available data of plasma NT-proBNP and echocardiography were included.
Patients with any of the characteristics below were not included: renal dysfunction (serum creatinine>2.8 mg/dL), acute myocardial infarction, cardiogenic shock, valvular heart disease, pyemia, and severe endocrine or hepatic dysfunction. Patients with an obvious etiology of exacerbations (pneumothorax or pneumonia on the basis of chest X-ray, and pulmonary embolism diagnosed through computerized tomography scan) or recent treatment with diuretics were excluded in this study. Patients with a known history of LHF were ineligible. Subjects who were nonechogenic based on echocardiographic assessment or who did not undergo pulmonary function tests (PFTs) were also excluded from our analysis.
In addition to the selected AECOPD cohort, 40 healthy elderly individuals were recruited from the check-up department and served as normal control group. Those age-matched healthy participants had no obvious respiratory or cardiac symptoms or disorders. Moreover, none of them met any of the above exclusion criteria.
Study protocol
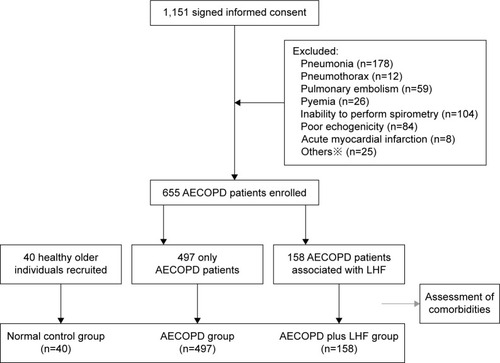
This was a prospective cross-sectional observational study, and the study flow-chart is outlined in . LHF was diagnosed in patients with AECOPD according to echocardiographic criteria, and the levels of NT-proBNP in plasma were measured by quantitative electrochemiluminescence assay.
Diagnosis of AECOPD and routine examination
At screening, enrolled patients underwent total history taking, thorough physical examination, emphasizing the signs of COPD exacerbations, LHF, and cor pulmonale. Auscultation abnormalities were coarse crackles (rhonchi), fine crackles (crepitations), and wheezing. PFTs were performed according to the standard operating procedureCitation15 and measured using a Vmax229 Pulmonary Function Instrument (SensorMedics, Yorba Linda, CA, USA). FEV1 and FVC were measured using the Vmax229 (SensorMedics). Total lung capacity (TLC) was determined using a body plethysmograph (6200 Autobox; SensorMedics). Diffusion capacity for carbon monoxide (DLCO) was determined by the single breath methodCitation15 using an infrared analyzer (Vmax229; SensorMedics). PFT results were expressed as percentages of predicted normal values. Other findings, including arterial blood gas analysis, hematology, biochemistry, electrocardiogram (ECG), chest radiography, and computerized tomography scan data, were also collected. ECG abnormalities concerning left heart dysfunction mainly include: T-wave inversion, pathological Q-wave, or loss of R, left ventricular hypertrophy, complete or incomplete left bundle branch block, QRS axis deviation, and ST depression or elevation. All clinical data were recorded on the first day of enrollment.
AECOPD was diagnosed based on all relevant clinical data, according to the diagnostic criteria of the GOLD guidelines, by two pulmonologists. Cor pulmonale was established in patients who had clinical and instrumental evidence of right ventricle (RV) abnormalities secondary to COPD, using standard criteria.Citation16,Citation17
Assessment of LHF and comorbidities
Although invasive cardiac catheterization is considered the gold standard for showing impaired filling and relaxation, the balance of pros and cons as well as financial burden argues against its routine use in clinical practice.Citation18 Doppler echocardiography has assumed the principal role in noninvasive evaluation of heart failure.Citation17 On the first day of enrollment, all patients with AECOPD were subjected to transthoracic echocardiographic examination, which was accomplished by two experienced cardiac sonographers blinded to the plasma NT-proBNP values, using color Doppler, and M-mode, two-dimensional imaging with commercially available instruments, working at 2.0–3.5 MHz (Siemens Medical Solutions, Erlangen, Germany). An expert panel (involving one pulmonologist and two cardiologists) analyzed all relevant clinical data, except each patient’s plasma NT-proBNP levels. The final identification of LHF was established according to consensus among all three independent physicians, according to the American Society of Echocardiography Guidelines.Citation17 Furthermore, the clinically important comorbidities in all subjects with AECOPD were analyzed in conjunction with a thorough workup, referring to corresponding clinical guidelines.Citation19–Citation21
Detection of NT-proBNP in plasma
Blood samples from all patients who were diagnosed as having AECOPD and those who served as normal controls were obtained to determine baseline NT-proBNP concentrations within 6 hours on the first day of enrollment. The levels of NT-proBNP in plasma were measured using quantitative electrochemiluminescence assay (Elecsys NT-proBNP; Roche Diagnostics, Basel, Switzerland) on an Elecsys 2010 analyzer (Roche Diagnostics) following established methods. The investigator responsible for the measurements was not aware of the subjects’ clinical parameters.
Statistical analysis
All continuous variables are expressed as mean±SD or median (interquartile range), and are compared using independent sample t-tests and Wilcoxon–Mann–Whitney tests, respectively. Categorical variables are expressed as absolute numbers and proportions. Receiver operating characteristic (ROC) curves and areas under the curves were obtained, with the values giving the best combination of specificity and sensitivity (maximum sum of both) being considered indications of the diagnostic accuracy of plasma NT-proBNP in the patient groups. P<0.05 is considered statistically difference. All statistical analyses were performed in SPSS software version 20.0 (IBM Corporation, Armonk, NY, USA).
Results
Baseline characteristics of patients with AECOPD
A total of 1,151 patients with AECOPD were enrolled. Four hundred and ninety-six patients were excluded for these reasons: AECOPD owing to infectious pneumonia (n=178), pulmonary embolism (n=59), or pneumothorax (n=12); acute myocardial infarction (n=8); pyemia (n=26); inability to undergo echocardiography (n=84) or PFTs (n=104); other reasons (n=25). Six hundred and fifty-five patients met our inclusion criteria and completed this study according to the protocol. The patients with only AECOPD were defined as the AECOPD group, and patients with AECOPD associated with LHF were defined as the AECOPD plus LHF group.
The baseline characteristics of all subjects are shown in . Age, gender ratio, smoking history (pack-years), body mass index, or SBP were similar in the normal control group, AECOPD group, and AECOPD plus LHF group (all P>0.05). The patients in the AECOPD plus LHF group had significantly increased concentrations of high-sensitivity C-reactive protein (hs-CRP), compared with those in the AECOPD group (40.5±17.1 and 32.8±15.3 mg/L, respectively; P<0.01), but the serum levels of hemoglobin or albumin were not statistically different between the two groups (all P>0.05). The levels of hydrogen ion concentration (pH), arterial oxygen saturation (O2Sat), bicarbonate, and partial pressure of oxygen in arterial blood (PaO2) in the AECOPD group were comparable to those in the AECOPD plus LHF group (all P>0.05). However, the values of partial pressure of carbon dioxide in arterial blood (PaCO2) differed significantly between the two groups (49.1±20.2 and 55.6±20.7 mmHg, respectively; P<0.01). In the AECOPD plus LHF group, 39.9% of patients suffered from corpulmonale, as compared to 32.8% of patients in the AECOPD group. ECG abnormalities, mainly ST depression or elevation (12.1%), complete/incomplete left bundle branch block (9.6%), and T-wave inversion (6.6%), occurred in 34.1% of the subjects: three (7.5%) patients in the normal control group, 138 (27.8%) in the AECOPD group, and 96 (60.8%) in the AECOPD plus LHF group. About 10.9% of the subjects had more than one abnormal result. Auscultation abnormalities were common in the AECOPD patients: 31.4% in the AECOPD group and 42.4% in the AECOPD plus LHF group.
Table 1 Patient baseline characteristics for the individual groups
Pulmonary function tests
The results of the PFTs are shown in . The mean values of the FEV1 predicted, FEV1/FVC (%), and DLCO predicted in the 655 patients with AECOPD were significantly lower, compared with those in healthy controls (all P<0.01). However, patients with AECOPD exhibited markedly higher percentage of TLC predicted than the healthy individuals (P<0.05). The mean values of the FEV1 predicted, FEV1/FVC (%), or TLC predicted were similar in the AECOPD group and the AECOPD plus LHF group (all P>0.05). Noticeably, patients in the AECOPD plus LHF group suffered poorer diffusion capacity: the DLCO predicted was 52.5%±20.8% in the AECOPD group and was 47.1%±24.5% in the AECOPD plus LHF group (P<0.01).
Table 2 Pulmonary function tests and echocardiographic parameters in 655 patients with acute exacerbation of COPD
Diagnosis of LHF in patients with AECOPD and comorbidities
The echocardiographic findings are shown in . In our cohort, a total of 655 subjects were included, of whom 158 (24.1%) had concurrent echocardiographic heart failure, whether systolic (n=108, 68.4%) or diastolic (n=50, 31.6%).
The patients with AECOPD associated with systolic heart failure had markedly decreased levels of left ventricular ejection fraction (LVEF), as compared with those associated with diastolic heart failure or with only AECOPD (P<0.01). Furthermore, the ratio of peak velocities of blood flow between early diastolic filling and atrial contraction filling (E/A ratio) and left ventricular isovolumetric relaxation time (IVRT) were significantly different among the three groups (all P<0.01). However, the E wave deceleration time (EDT) had similar performance among the above groups (P>0.05). Pulmonary artery pressure was markedly different among the three groups: the level of pulmonary artery systolic pressure (PASP) was 39.5±9.2 mmHg in patients with only AECOPD, 51.9±20.1 mmHg in those associated with systolic heart failure, and 57.1±17.3 mmHg in those associated with diastolic heart failure (P<0.01). In addition, the values of LVEF, E/A ratio, IVRT, and EDT in the AECOPD group were similar to those in the normal control group. However, the level of PASP was markedly higher in patients with AECOPD only (39.5±9.2 mmHg) compared with the healthy controls (25.3±6.1 mmHg) (P<0.01).
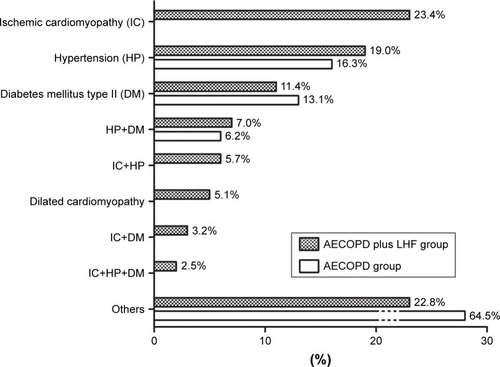
After carefully analyzing the comorbidities in all the subjects with AECOPD in conjunction with detailed clinical data, we demonstrated that patients in the AECOPD plus LHF group suffered ischemic cardiomyopathy, hypertension, diabetes mellitus type II, or dilated cardiomyopathy, with an involvement of 23.4%, 19.0%, 11.4%, and 5.1%, respectively. Thirty-six (22.8%) patients in the AECOPD plus LHF group may have other clinically unimportant comorbidities or have no comorbidities, as shown in . Moreover, our findings showed that 29 (18.4%) patients in the AECOPD plus LHF group had two or three comorbidities, and 31 (6.2%) patients in the AECOPD group simultaneously suffered hypertension and diabetes mellitus type II.
Plasma concentrations of NT-proBNP
The plasma concentrations of NT-proBNP are shown in . The plasma concentrations of NT-proBNP in elderly patients with AECOPD associated with LHF were markedly elevated, as compared with those with AECOPD only (4,542.5 and 763.0 ng/L, respectively, P<0.01). In the AECOPD plus LHF group, patients with systolic heart failure had significantly higher levels of NT-proBNP than patients with diastolic heart failure (4,903.7 and 3,762.3 ng/L, respectively, P<0.05). The 655 aged patients with AECOPD exhibited markedly enhanced levels of NT-proBNP in plasma, as compared to the healthy controls (P<0.01).
Figure 3 NT-proBNP level in different groups and its accuracy in identifying patients with AECOPD co-occurring with LHF.
Abbreviations: AECOPD, acute exacerbation of COPD; LHF, left heart failure; NT-proBNP, N-terminal brain natriuretic pro-peptide; AUC, area under the curve.
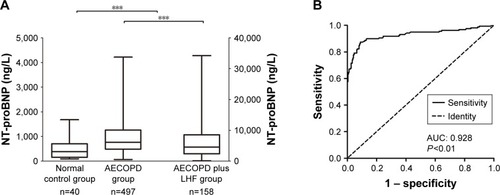
ROC curve was obtained using plasma levels of NT-proBNP as a diagnostic test for AECOPD associated with LHF, as shown in . The ROC curve demonstrated an area of 0.928 (95% CI 0.898–0.958, P<0.01). The optimal threshold point for plasma NT-proBNP levels was 1,677.5 ng/L, which yielded the maximum sum of both sensitivity and specificity. At this cutoff, the sensitivity for diagnosing AECOPD plus LHF was 87.9%, specificity was 88.5%, positive predictive value was 70.9%, negative predictive value was 95.9%, and accuracy was 88.4%, as outlined in .
Table 3 Sensitivity, specificity, positive and negative predictive values, and accuracy of plasma NT-proBNP in identifying left heart failure at different cutoff points
Discussion
In the current study, 158 (24.1%) of the 655 AECOPD patients had concurrent LHF, of which 108 (68.4%) had systolic heart failure, and 50 (31.6%) had diastolic heart failure. The plasma concentrations of NT-proBNP in elderly patients with AECOPD The plasma concentrations of NT-proBNP in elderly patients with AECOPD concurrent with LHF were markedly elevated, as compared with those with AECOPD only. For the diagnosis of AECOPD associated with LHF, a plasma level of NT-proBNP of 1,677.5 ng/L, established by ROC analysis, yielded the optimal combination of sensitivity and specificity (87.9% and 88.5%, respectively) with an area under the ROC curve of 0.928. Therefore, our data demonstrated that the plasma level of NT-proBNP may be a useful indicator in diagnosing AECOPD associated with LHF.
Heart failure is frequently associated with COPD, affecting up to one-third of COPD patients.Citation5,Citation6,Citation22 In the present study, 24.1% of the elderly AECOPD patients had comorbid LHF, suggesting an increased frequency of cardiac dysfunction in this senior population. Nevertheless, the pathogenesis underlying the frequent coexistence of AECOPD and LHF is multifaceted. Common risk factors, including older age, cigarette smoking, and the male predominance, do not fully explain the high incidence of the two diseases.Citation6–Citation9 It is believed that chronic low-grade inflammation associated with COPD can be regarded as a systemic disorder, which is involved in the formation of atherosclerosis, manifested by high concentrations of circulating inflammatory biomarkers, like acute phase protein, in patients.Citation6–Citation8 Consequently, it is inferred that the systemic inflammatory state related to COPD exacerbations may impair the endothelial function and accelerate the atherosclerosis progression, resulting in increased incidence of ischemic heart disease.Citation9,Citation23 Our data showed that patients with AECOPD concurrent with LHF exhibited significantly enhanced levels of hs-CRP, as compared with those with AECOPD only. Furthermore, our findings demonstrated that 37 (23.4%) subjects in the AECOPD plus LHF group simultaneously suffered ischemic heart disease. Therefore, it is possible that the interrelationship between COPD exacerbations and LHF may be related to low-grade systemic inflammation, but future studies are required to clarify this.
Unfortunately, due to an overlap in clinical presentation between AECOPD and LHF, including exertional dyspnea, easy fatigability, nocturnal cough, wheezing, etc., the timely identification of AECOPD associated with LHF is currently challenging and needs a high degree of clinical suspicion.Citation5–Citation7 In the current study, the majority of the enrolled patients experienced dyspnea or decreased exercise tolerance, and abnormal pulmonary sounds were common. However, clinical examinations with sufficient accuracy to diagnose AECOPD coexisting with LHF are not always available in routine practice. Our data showed that about 21.0% of the unqualified patients failed to perform PFTs. Interestingly, apart from the diffusion capacity, such as the DLCO predicted, variables of FEV1/FVC, FEV1 predicted, or TLC predicted were similar in patients with AECOPD only and those with AECOPD associated with LHF, suggesting that PFTs cannot reliably facilitate a differential diagnosis between geriatric patients with AECOPD only and those with concurrent LHF. Transthoracic echocardiography is a noninvasive and nonradioactive method for accurately assessing heart failure in the clinical practice.Citation17 However, it is not effective in patients with AECOPD, since hyperinflation of the chest will alter sound-wave transmission through the chest, resulting in an impaired acoustic window.Citation17,Citation24 Our data revealed that diagnostic echocardiographic results could not be obtained in 16.9% of the excluded subjects. As such, a reliable but simple method to objectify these events is required.
Plasma NT-proBNP is derived from a 134-amino acid precursor pre-pro-BNP, which is relatively stable in vitro, and has the advantages of sensitive detection, and less individual difference.Citation10 The main stimulus responsible for increasing NT-proBNP production is myocardial wall stress.Citation10 Plasma NT-proBNP is currently widely used in the biochemical detection of heart failure.Citation11,Citation12 Additionally, it has prognostic value and serves as a guide for titrating treatments in this setting.Citation11,Citation12 A threshold value of plasma NT-proBNP of 900 pg/mL is accurate for making a diagnosis of heart failure in individuals between 50 and 75 years old.Citation22 However, the specific setting of AECOPD is linked to a lower rise in plasma levels of NT-proBNP.Citation13,Citation14 It has been reported that severe hypoxemia induced by bronchoconstriction, mucus hyper-secretion, and alveolar hypoxia in AECOPD could lead to pulmonary vasoconstriction, resulting in increased pulmonary arterial systolic pressure, greater RV afterload, and elevated plasma concentrations of NT-proBNP.Citation25,Citation26 Furthermore, the low oxygen tension may cause an increase in the synthesis of plasma NT-proBNP.Citation26 Our results have shown that elderly patients with AECOPD exhibit markedly enhanced plasma concentrations of NT-proBNP, and experience hypoxemia and elevated PASP, as compared to healthy controls. Therefore, the enhanced plasma concentrations of NT-proBNP in geriatric patients with AECOPD may be associated with the existence of hypoxemia and PASP in those patients. It has been reported that AECOPD could in itself cause sufficient strain on the heart to induce necrosis of myocardial cells.Citation6 Besides the prolonged hypoxemia, secondary pulmonary hypertension, and subsequent RV stress, lung hyperinflation due to ventilation impairment may, to some extent, lead to elevated levels of NT-proBNP in plasma.Citation25–Citation28 The hyperinflation mechanics during exacerbations might reduce ventricular filling and impede venous return, leading to a lower stroke volume and cardiac dysfunction.Citation28 Moreover, the potential cardiopulmonary interactions may also contribute to the result.Citation7 In the current study, the plasma concentrations of NT-proBNP in the elderly patients with AECOPD associated with LHF were markedly elevated, as compared with those with AECOPD only. ROC curves for the plasma concentrations of NT-proBNP were obtained as a diagnostic test for LHF in the elderly patients with AECOPD. The optimal cutoff point for plasma NT-proBNP was 1,677.5 ng/L, at which the sum of specificity and sensitivity values was the maximum, as shown in . For this value, the area under the ROC curve was 0.928. Therefore, the measurement of the plasma levels of NT-proBNP may provide an important method for the detection of AECOPD co-occurring with LHF.
Limitations
Our study has some limitations. First, we did not obtain data regarding GOLD staging during the acute exacerbations of COPD, since multiple factors, such as bronchoconstriction, respiratory muscle fatigue, and mucus hypersecretion and obstruction would affect the final results of the PFTs. The GOLD grading standard of airflow obstruction may be even more unreliable when AECOPD patients have concurrent LHF. Second, we did not assess the correlation between the AECOPD and respiratory infections. According to several epidemiological studies, respiratory tract infections, either viral or bacterial, are major triggering factors in AECOPD, and airway inflammation is significantly associated with an elevated plasma level of NT-proBNP.Citation4,Citation10 Thus, we excluded those patients with infectious pneumonia or pyemia. Third, potential biomarkers, such as cardiac troponin,Citation29 galectin-3,Citation30 soluble suppression of tumorigenicity-2 (sST2),Citation31 and soluble AXL (sAxl),Citation32 that may lead to faster diagnosis of heart failure were not characterized in our study. Although these emerging biomarkers may have a conspicuous role in cardiovascular physiology,Citation29–Citation31 and may even be associated with the progression and severity of heart failure,Citation33,Citation34 their use in heart failure is not yet established. Finally, the enrollment of COPD patients based on spirometric-test-confirmed criteria may have missed some eligible subjects. However, our data were not short of statistical power to detect an association between plasma concentrations of NT-proBNP and LHF in the elderly patients with AECOPD.
Conclusion
In summary, the present study has indicated that a relatively large proportion of elderly AECOPD patients have concurrent LHF. The plasma concentrations of NT-proBNP in older patients with AECOPD associated with LHF are markedly elevated, as compared with those with AECOPD only. Therefore, we have concluded that plasma NT-proBNP, which can be readily assessed in most medical settings, may be a useful indicator in identifying patients with AECOPD concurring with LHF.
Author contributions
AW designed the research; XG, HN, QC, ND, SC, RL, XD, SH, and AW performed the research and analyzed the data; XG, HN, and QC prepared the tables and figures; XG, HN, QC, and AW wrote and revised the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no relationships that could be construed as a conflict of interest.
References
- RussellRNorcliffeJBafadhelMChronic obstructive pulmonary disease: management of chronic diseaseMedicine2016445310313
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) [webpage on the Internet]The Global Strategy for the Diagnosis, Management and Prevention of COPD, updated 2013 Available from: http://www.goldcopd.org/Accessed December 20, 2013
- SapeyEStockleyRACOPD exacerbations. 2: aetiologyThorax200661325025816517585
- KoFWChanKPHuiDSAcute exacerbation of COPDRespirology20162171152116527028990
- ZengQJiangSUpdate in diagnosis and therapy of coexistent chronic obstructive pulmonary disease and chronic heart failureJ Thorac Dis20124331031522754671
- BhattSPDransfieldMTChronic obstructive pulmonary disease and cardiovascular diseaseTransl Res2013162423725123727296
- BafadhelMRussellREAre COPD and cardiovascular disease fundamentally intertwined?Eur Respir J20164751307130927132259
- UkenaCMahfoudFKindermannMThe cardiopulmonary continuum systemic inflammation as ‘common soil’ of heart and lung diseaseInt J Cardiol2010145217217620570377
- de Miguel DíezJChancafe MorganJJiménez GarcíaRde MigueMJChancafeMJJiménezGRThe association between COPD and heart failure risk: a reviewInt J Chron Obstruct Pulmon Dis2013830531223847414
- TsaiSHLinYYChuSJHsuCWChengSMInterpretation and use of natriuretic peptides in non-congestive heart failure settingsYonsei Med J201051215116320191004
- don-WauchopeACMckelvieRSEvidence based application of BNP/NT-proBNP testing in heart failureClin Biochem2015484–523624625448029
- OremusMMckelvieRdon-WauchopeAA systematic review of BNP and NT-proBNP in the management of heart failure: overview and methodsHeart Fail Rev201419441341924953975
- PavasiniRTavazziGBiscagliaSAmino terminal pro brain natriuretic peptide predicts all-cause mortality in patients with chronic obstructive pulmonary disease: Systematic review and meta-analysisChron Respir Dis201714211712627956645
- RalucaHMariaAMagdaBNatriuretic peptides in heart failure coexisting with chronic obstructive pulmonary diseaseExp Clin Cardiol20142016061614
- MillerMRHankinsonJBrusascoVStandardisation of spirometryEur Respir J200526231933816055882
- KilcoyneMMDavisALFerrerMIA dynamic electrocardiographic concept useful in the diagnosis of corpulmonale. Result of a survey of 200 patients with chronic obstructive pulmonary diseaseCirculation19704259039244249377
- LangRMBadanoLPMor-AviVRecommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular ImagingEur Heart J Cardiovasc Imaging201516323327125712077
- de Oliveira CastroYTBRolimILTPSilvaACOSilvaLDCKnowledge and meaning of cardiac catheterization from the perspective of cardiac patientsRev Rene20161712935
- FihnSDBlankenshipJCAlexanderKPACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic SurgeonsCirculation2014130191749176725070666
- JamesPAOparilSCarterBLEvidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8)JAMA2014311550752024352797
- GarberAJAbrahamsonMJBarzilayJIAACE/ACE comprehensive diabetes management algorithm 2015Endocr Pract201521443844725877012
- MacdonaldMIShafuddinEKingPTChangCLBardinPGHancoxRJCardiac dysfunction during exacerbations of chronic obstructive pulmonary diseaseLancet Respir Med20164213814826781000
- AndersonWJLipworthBJRekhrajSStruthersADGeorgeJLeft ventricular hypertrophy in COPD without hypoxemia: the elephant in the room?Chest20131431919722797769
- ReichekNCorpulmonaleparvus: patting the elephantJ Am Coll Cardiol201464192010201225440096
- KawutSMPoorHDParikhMACor Pulmonale Parvus in Chronic Obstructive Pulmonary Disease and EmphysemaJ Am Coll Cardiol201464192000200925440095
- AbrougFOuanes-BesbesLDetection of acute heart failure in chronic obstructive pulmonary disease patients: role of B-type natriuretic peptideCurr Opin Crit Care200814334034718467897
- ArjamaaONikinmaaMNatriuretic peptides in hormonal regulation of hypoxia responsesAm J Physiol Regul Integr Comp Physiol20092962R257R26419005014
- JörgensenKMüllerMFNelJUptonRNHoultzERickstenSEReduced intrathoracic blood volume and left and right ventricular dimensions in patients with severe emphysema: an MRI studyChest200713141050105717426209
- ShahKSMaiselASFonarowGCTroponin in Heart FailureHeart Fail Clin2018141576429153201
- LalaRIPuschitaMDarabantiuDPilatLGalectin-3 in heart failure pathology – “another brick in the wall”?Acta Cardiol201570332333126226706
- MccarthyCPJanuzziJLSoluble ST2 in Heart FailureHeart Fail Clin2018141414829153199
- BatlleMRecarte-PelzPRoigEAXL receptor tyrosine kinase is increased in patients with heart failureInt J Cardiol2014173340240924681018
- SrivatsanVGeorgeMShanmugamEUtility of galectin-3 as a prognostic biomarker in heart failure: where do we stand?Eur J Prev Cardiol20152291096111025268020
- Bayes-GenisAde AntonioMVilaJHead-to-head comparison of 2 myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 versus galectin-3J Am Coll Cardiol201463215816624076531