Introduction
It has been increasingly recognized that the numbers of blood eosinophils (eos) might be an important biomarker in patients with COPD to identify patients at risk for exacerbations and for treatment to inhaled corticosteroid (ICS) treatment or anti-interleukin-5 therapy.Citation1–Citation3 However, data about the stability of blood eos counts over time are rare. We used data from the multicenter COSYCONET study to analyze the variability of eos by strata over a period of 18 months.Citation4
Methods
The German COPD and Systemic Consequences-Comorbidities Network (COSYCO-NET) cohort study is a multicenter, longitudinal, prospective, observational study, into which 2,741 patients with the diagnosis of COPD were recruited between 2010 and 2013 in 31 study centers throughout Germany.Citation4 Eos were collected in a number of centers as part of routine clinical assessments. To determine the longitudinal stability of their counts, we included all patients in whom a differential blood cell count was available at the study visits V1–V3 (baseline, 6 months, 18 months). These 334 patients were more prone to exacerbations and were more likely to have an ICS-containing treatment regimen than the remaining part of the COSYCONET population ().
Table 1 Baseline data from 334 patients in whom a differential blood cell blood count was available at visits V1–V3 as compared to 2,407 patients without differential blood cell count available at all time points V1–V3
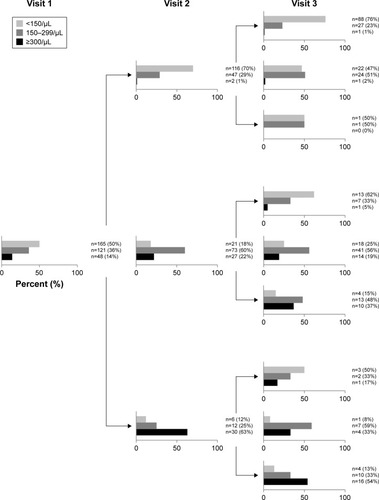
First, we determined the absolute number and proportion of patients, who exhibited eos of <150 cells/µL (rated as non-eosinophilic), 150–299 cells/µL (intermediate), and ≥300 cells/µL (eosinophilic) at visit 1. Next, we analyzed the longitudinal stability of the three strata from visit 1 to visit 2, and from visit 2 to visit 3. Finally, we displayed the results in analogy to the stability analysis performed by Hurst for the susceptibility to exacerbations.Citation5
Results
At visit 1, 165 patients (50%) were non-eosinophilic, 121 (36%) intermediate, and 48 (14%) eosinophilic. The overall distribution remained fairly stable over time (visit 2: 43%, 40%, 18%, respectively; visit 3: 46%, 40%, 14%, respectively). The changes between strata over consecutive visits and the resulting distributions are shown in . Putting the data from the three visits together, 26% of patients were persistently non-eosinophilic (<150 eos/µL), which implies that 74% exhibited ≥150 eos/µL at least at one occasion. Conversely, 28% exhibited ≥300 eos/µL at least once within 18 months, but only 5% of patients were persistently eosinophilic (≥300 eos/µL) at all three study visits. Excluding patients whose status regarding corticosteroid treatment (on/off) changed from one visit to another (n=63) did not change the results significantly (data not shown).
Figure 1 Absolute number and proportion of patients according to blood eos count at visits 1, 2, and 3.
Abbreviation: eos, eosinophil.
Discussion
The main findings of this analysis are (1) that 26% of COPD patients in the study cohort were persistently non- eosinophilic, (2) 5% were persistently eosinophilic, and (3) 28% exhibited ≥300 eos/µL at least once in three observations over a period of 18 months.
Few longitudinal studies evaluated the robustness of eos strata in COPD before.Citation6,Citation7 Oshagbemi et al found the stability of counts higher in patients showing <340 eos/ µL compared to patients showing ≥340 eos/µL.Citation6 Using a lower cut-off level of 2%, Singh et al observed that the majority of patients showed variations around a value of 2%.Citation7 When defining three different strata in absolute eos numbers, we observed that among those that were robust over time the non-eosinophilic stratum (<150 eos/ µL) was the most frequent one. Still it comprised only about one quarter of the population, while the majority of patients were in the intermediate or high eosinophilic group at least at one occasion. More than one quarter of the population exhibited ≥300 eos/µL at least once in three visits.
Even though our data may not be fully representative for the entire COSYCONET cohort (eg, higher exacerbation rate, higher percentage of patients on any ICS-containing regimen) and some groups at visit 3 included a small number of patients (making the results somewhat preliminary), they help to assess the stability of the eos signal, which will potentially be used in the future to come to treatment decisions.
Conclusion
Our analysis demonstrates that in COPD non-eosinophilia in blood is more robust over time than eosinophilia defined as count ≥300 eos/µL. These observations might be helpful for the design of studies that address the question, whether rational and effective treatment decisions should better refer to persistent or to occasional eosinophilia.
Disclosure
Dr T Greulich reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, CSL-Behring, GlaxoSmithKline, Grifols, MedUpdate, Mundipharma, and Novartis. S Mager and Dr T Lucke report no conflicts of interest. Professor Dr AR Koczulla reports grants from Boehringer-Ingelheim, Astra Zeneca, Novartis, Grifols, CSCSL, Roche, Teva, Berlin-Chemie, Chiesi und Novotec, outside the submitted work. Professor Dr R Bals reports grants from BMBF, AstraZeneca GmbH, Bayer Schering Pharma AG, Boehringer Ingelheim Pharma GmbH & Co. KG, Chiesi GmbH, GlaxoSmithKline, Grifols Deutschland GmbH, MSD Sharp & Dohme GmbH, Mundipharma GmbH, Novartis Deutschland GmbH, Pfizer Pharma GmbH, Takeda Pharma Vertrieb GmbH & Co. KG., during the conduct of the study; as well as grants from Wilhelm-Sander-Stiftung, grants from Deutsche Krebshilfe and grants from Schwiete-Stiftungoutside the submitted work. Dr S Fähndrich reports grants from Grifols, CSL Behring, and AstraZeneca, outside the submitted work. Dr RA Jörres reports grants from Federal Ministry of Education and Research (grant number 01GI0881, 01GI0882), during the conduct of the study. Professor Dr P Alter reports grants from German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET), grants from AstraZeneca GmbH, grants and non-financial support from Bayer Schering Pharma AG, grants, personal fees and non-financial support from Boehringer Ingelheim Pharma GmbH & Co. KG, grants and non-financial support from Chiesi GmbH, grants from GlaxoSmithKline, grants from Grifols Deutschland GmbH, grants from MSD Sharp & Dohme GmbH, grants and personal fees from Mundipharma GmbH, grants, personal fees and non-financial support from Novartis Deutschland GmbH, grants from Pfizer Pharma GmbH, grants from Takeda Pharma Vertrieb GmbH & Co. KG, outside the submitted work. Dr AM Kirsten reports personal fees from Astra Zeneca and Boehringer Ingelheim, outside the submitted work. Professor Dr CF Vogelmeier reports grants from AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Grifols, Menarini, Mundipharma, Novartis, Teva, Cipla, Omniamed, and MedUpdate. Dr H Watz reports personal fees from AstraZeneca, personal fees from Takeda, personal fees from BerlinChemie, personal fees from BoehringerIngelheim, personal fees from Chiesi, personal fees from Novartis, personal fees from GlaxoSmithKline, personal fees from Roche, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- BafadhelMPavordIDRussellREKEosinophils in COPD: just another biomarker?Lancet Respir Med20175974775928601554
- WatzHTetzlaffKWoutersEFBlood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trialLancet Respir Med20164539039827066739
- PavordIDChanezPCrinerGJMepolizumab for eosinophilic chronic obstructive pulmonary diseaseN Engl J Med2017377171613162928893134
- KarchAVogelmeierCWelteTThe German COPD cohort COSYCONET: aims, methods and descriptive analysis of the study population at baselineRespir Med2016114273727109808
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- OshagbemiOABurdenAMBraekenDCWStability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline countsAm J Respir Crit Care Med2017195101402140428165763
- SinghDKolsumUBrightlingCEEosinophilic inflammation in COPD: prevalence and clinical characteristicsEur Respir J20144461697170025323230
