Abstract
Purpose
This study evaluated the efficacy of tiotropium/olodaterol vs tiotropium on lung function, exercise capacity, and physical activity in patients with COPD.
Patients and methods
A total of 184 patients aged ≥40 years with COPD (Global Initiative for Chronic Obstructive Lung Disease stage II–IV) received tiotropium/olodaterol for 6 weeks, then tiotropium for 6 weeks, or vice versa. The primary endpoint was inspiratory capacity (IC) at peak post-dose.
Results
Adjusted mean IC after 6-week treatment was 1.990 L with tiotropium/olodaterol vs 1.875 L with tiotropium (difference: 115 mL; 95% CI: 77, 153; p<0.0001). Forced expiratory volume in 1 s (difference: 105 mL; 95% CI: 88, 123), forced vital capacity (difference: 163 mL; 95% CI: 130, 197), and slow vital capacity (difference: 134 mL; 95% CI: 91, 176) improved with tiotropium/olodaterol (all p<0.0001). Adjusted mean 6-min walk distance was similar between treatments in the overall population but was significantly increased with tiotropium/olodaterol in the subgroup with Global Initiative for Chronic Obstructive Lung Disease stage III/IV at baseline (difference: 18.1 m; 95% CI: 2.3, 33.9; p=0.0254). In a post hoc analysis, tiotropium/olodaterol improved the values for ≥2.0 metabolic equivalents (difference: 5.0 min; 95% CI: 0.4, 9.7; p=0.0337).
Conclusion
Tiotropium/olodaterol significantly improved IC compared with tiotropium and potentially enhanced the exercise capacity in COPD patients. A slight improvement in physical activity of relatively more than moderate intensity was also seen with tiotropium/olodaterol.
Keywords:
Introduction
The prevalence of COPD is increasing worldwide, with an estimated 210 million cases in 2007.Citation1 A sharp rise in the prevalence of COPD has been reported in developing countries, largely attributed to a combination of risk factors, particularly tobacco use.Citation2 The World Health Organization reported that ~3 million deaths secondary to COPD occurred in 2015, and that the disease affects men and women almost equally.Citation3
Bronchodilators are the first-line treatment for COPD, and inhaled long-acting bronchodilator use in newly diagnosed patients is associated with fewer hospital admissions and lower medical costs.Citation4
Previous studies have shown that combination therapy of tiotropium, a long-acting muscarinic antagonist (LAMA), and olodaterol, a long-acting beta agonist (LABA), provides significantly improved lung function and quality of life, compared with either therapy used individually.Citation5–Citation7
Other Phase III studies in Western countries showed significant improvements in lung hyperinflation with an increase in inspiratory capacity (IC) after 6 or 12 weeks of treatment compared with monotherapy or placebo.Citation8,Citation9
However, there is limited evidence of significant improvements in exercise capacity with LAMA/LABA combination therapy, and few studies have compared the effects of the combination therapy with the respective monotherapy on physical activity;Citation10–Citation13 thus, a need remains for therapeutics that improve exercise capacity in patients with COPD.
This study investigated the efficacy of tiotropium/olodaterol in terms of lung volume, exercise tolerability, and physical activity compared with that of tiotropium in COPD patients.
Patients and methods
Patients
Inclusion criteria were as follows: male and female Japanese patients aged ≥40 years with COPD and stable airway obstruction with post-bronchodilator forced expiratory volume in 1 s (FEV1) <80% of predicted normal; Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade II–IV, and post-bronchodilator FEV1/forced vital capacity (FVC) <70% at Visit 1; current or ex-smokers with a smoking history of >10 pack years; modified Medical Research Council ≥1; 6-min walk distance (6MWD) test <400 m; and a score ≥4 on the modified Borg scale of breath discomfort at the end of the 6MWD test at Visit 2.
The main exclusion criteria were the presence of a significant disease other than COPD; a clinically significant abnormality in hematology, blood chemistry, or urinalysis; and concurrent bronchial asthma. Patients who used daytime oxygen therapy for >1 hour per day were also excluded. Further details have been described elsewhere.Citation14 Written informed consent was obtained from all patients prior to the study, which was approved by the institutional review board at each participating center (details of all institutional review boards are provided in Table S1).
Study design
This was a multicenter, randomized, double-blind, active-controlled, two-way crossover trial (ClinicalTrials.gov Identifier NCT2629965). Details of the study design have been published previously.Citation14
Patients were randomized in a 1:1 ratio via interactive response technology to receive tiotropium for 6 weeks followed by tiotropium/olodaterol for 6 weeks, or vice versa. Randomization was not stratified. The study sponsor (Boehringer Ingelheim) generated and stored the randomization schedule and prepared and coded the medication in a blinded fashion. Investigators and all individuals involved in trial conduct or analysis remained blinded to the randomized treatment until after data lock.
Treatment
Oral doses of tiotropium/olodaterol 5/5 μg inhalation solution (2.5/2.5 μg per actuation) and tiotropium 5 μg inhalation solution (2.5 μg per actuation) were administered by RESPIMAT inhaler based on the marketed dose in Japan. Visit 1 consisted of a screening process carried out over a period of 2 weeks. At Visit 2, patients were randomly allocated to each group. The intervention group received once-daily tiotropium/olodaterol 5/5 μg inhalation solution for 6 weeks, and patients in the second arm of the study received tiotropium 5 μg inhalation solution over the same period. There was no placebo comparator in this trial.
Patients were instructed to inhale two puffs from the RESPIMAT inhaler, once a day, in the morning. All instances of trial medication taken were recorded using patient diaries indicating the number of puffs of salbutamol metered dose inhaler used. At Visit 3, patients received the crossover treatment without a washout period. Restricted treatments prior to the start of the study included any oral and patch β-adrenergic therapies, and oral corticosteroid medication at unstable doses (ie, <6 weeks on a stable dose) with doses in excess of the equivalent of 10 mg prednisone per day or 20 mg every other day.
Study assessments
Pulmonary function testing (Flowscreen; eResearch Technology GmbH, Estenfeld, Germany), 6MWD, and physical activity assessment occurred in visits 1–4/end of treatment (EOT); visits 1 and 4/EOT also included a physical examination of patients, including smoking status and, for female participants, a pregnancy test.
Lung capacity was assessed using IC at rest, measured at 60 min post-dose after 6 weeks of treatment. Exercise capacity and physical activity were measured using the 6MWD test and a 3-axis accelerometer (Active style PRO HJA-750C, HJA-750C; OMRON, Kyoto, Japan), respectively. The patients were required to wear the accelerometer on the waist.
At the start of screening (Visit 1), Visit 2, Visit 3, and Visit 4/EOT, a complete physical examination was performed by the investigator. Follow-up examinations were scheduled for Visit 5, if there were any clinically significant findings at Visit 4/EOT.
Standard 12-lead electrocardiogram (ECG) and vital sign monitoring at rest were performed on all patients at visits 1–4/EOT.
Efficacy outcomes
Lung function
The primary endpoint was IC at rest, measured at 60 min post-dose after 6 weeks of treatment.
The secondary endpoints were lung function (FEV1, 30 min post-dose; FVC, 30 min post-dose; slow vital capacity [SVC], 60 min post-dose).
The 6-min walk test
The 6-min walk test (6MWT) was used to measure exercise capacity associated with dynamic hyperinflation. The analysis of the 6MWD was also conducted in several subgroups. The 6MWT was terminated when percutaneous oxygen saturation by pulse oximetry (SpO2) decreased to <83% at any time after the patient began walking. 6MWD was also analyzed only in patients who completed the 6MWT.
Physical activity
Physical activity was measured with a 3-axis accelerometer using average number of steps/day, average daily duration (min) of ≥4, ≥3, and ≥2 metabolic equivalents (METs), and average daily active strength (METs min) of ≥3 METs during the last 2 weeks of 6 weeks of treatment. In this study, 24 hours of measurement were performed each day, except during bathing or water sport activities. Because it has been reported that exclusion of non-wearing time may increase the accuracy of physical activity measurement, we conducted a post hoc analysis based on previously reported methodologies; in this analysis, data for patients with <8 hours wearing time and less than two valid days were excluded.Citation15,Citation16
Safety
Safety endpoints were all adverse events (AEs) (including physical examination, vital sign monitoring, 12-lead ECG, and laboratory tests) until the end of the study, in addition to heart rate and SpO2 in conjunction with the 6MWT.
Statistical methods
Details of the study populations and sample size calculations (Supplementary materials) are given in the published study design paper.Citation14
Primary endpoint analysis (IC at rest) was conducted using a mixed-effects model repeated-measures (SAS procedure MIXED) approach, with treatment and period as categorical fixed effects, study baseline (Visit 2) as a covariate, and patient as a random effect.
Adjusted mean values and treatment contrasts are presented with 95% CIs.
Secondary and further endpoints were analyzed using a similar model with descriptive statistics provided for both treatments.
A two-sided significance level of 5% was used to test the primary endpoint. The analysis of secondary and further endpoints was not adjusted for multiplicity, and the corresponding p-values for treatment comparisons are descriptive (nominal p-values). All statistical analyses were performed using validated SAS (SAS Institute Inc., Cary, NC, USA) macros, customized at Boehringer Ingelheim (Ingelheim am Rhein, Germany).
Ethics approval and informed consent
Written informed consent was obtained from all patients prior to the study, which was approved by the institutional review board at each participating center. The study was conducted according to the principles of the International Conference on Harmonisation and Good Clinical Practice, Declaration of Helsinki, Japanese Good Clinical Practice, and all relevant local regulatory, legal, and ethical requirements.
Results
Patients
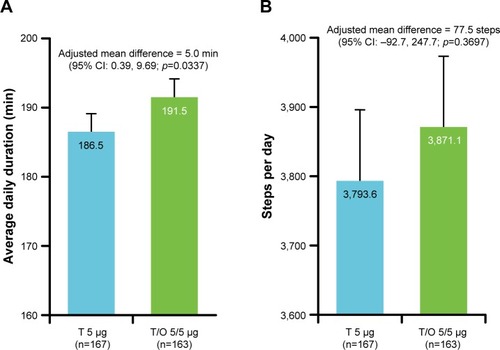
In total, 334 patients from 44 medical institutions provided written informed consent. Of these, 184 patients were entered into the investigational treatment period and 150 patients were excluded at screening. Reasons for the exclusions were a 6MWD of >400 m, a modified Borg scale score of <4, achieving ≥80% FEV1 in pulmonary testing, experiencing an AE, and withdrawal of consent. After randomization, seven patients discontinued treatment, four because of AEs, two withdrew consent, and one for other reasons ().
The majority of the study participants were male (89.7%). Mean (SD) age was 72.8 (7.1) years, mean (SD) body mass index was 22.2 (4.0) kg/m2 (), and mean (SD) duration of COPD was 5.49 (4.28) years.
Table 1 Patient characteristics and demographics
Study medication compliance for tiotropium/olodaterol was 98.9%, with 97.8% recorded for tiotropium.
Primary endpoint
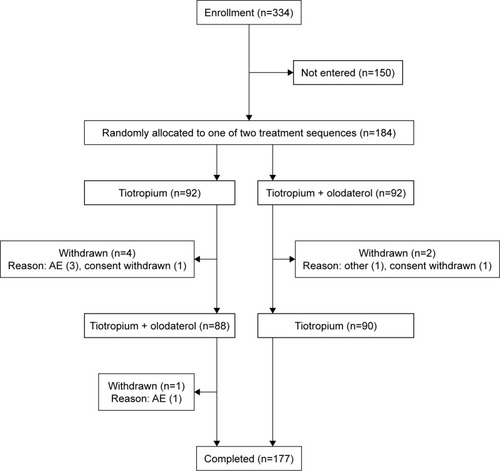
Significant improvements were achieved for tiotropium/olodaterol vs tiotropium: the adjusted mean IC after 6 weeks of treatment was 1.990 vs 1.875 L, respectively, corresponding to an increase of 115 mL (95% CI: 77 mL, 153 mL) in IC with tiotropium/olodaterol (p<0.0001; ).
Figure 2 IC and lung function parameters after 6 weeks of treatment with T or T/O.
Abbreviations: FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IC, inspiratory capacity; SVC, slow vital capacity; T, tiotropium; T/O, tiotropium/olodaterol.
Secondary endpoints
Significant improvements were seen for tiotropium/olodaterol vs tiotropium in FVC (difference: 163 mL; 95% CI: 130 mL, 197 mL; ), FEV1 (difference: 105 mL; 95% CI: 88 mL, 123 mL; ), and SVC (difference: 134 mL; 95% CI: 91 mL, 176 mL; ), respectively (all p<0.0001).
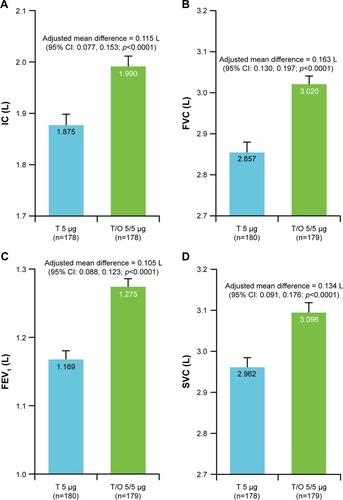
The adjusted mean 6MWD after 6 weeks of treatment was similar between tiotropium/olodaterol and tiotropium in the overall population; the adjusted mean difference was 4.2 m (95% CI: −6.2 m, 14.5 m; p=0.4291). Subgroup analyses according to GOLD stage were pre-specified, and the differences in adjusted mean 6MWD between tiotropium/olodaterol and tiotropium treatments, by GOLD stage subgroup, are shown in Table S2.
Because only 16 patients (8.7% of total) were categorized as GOLD IV, the combined GOLD III and IV subgroups were analyzed post hoc. An increase in 6MWD with tiotropium/olodaterol vs tiotropium was observed in the GOLD stage III/IV subgroup (adjusted mean difference=18.1 m [95% CI: 2.3 m, 33.9 m; p=0.0254; ]). Assessment of the 6MWD was terminated if the patient’s SpO2 fell below 83%, in order to assure patient safety. In total, 57 patients (32.8%) could not complete the 6MWD test during the treatment period. In a post hoc analysis, patients who completed the 6MWT during the treatment period without desaturation achieved 357.3 m (tiotropium/olodaterol) vs 346.6 m (tiotropium), as shown in ; between treatment difference=10.7 m (95% CI: 4.2 m, 17.3 m; p=0.00014).
Figure 3 6MWD after 6 weeks of treatment with T or T/O.
Abbreviations: 6MWD, 6-min walk distance; GOLD, Global Initiative for Chronic Obstructive Lung Disease; T, tiotropium; T/O, tiotropium/olodaterol.
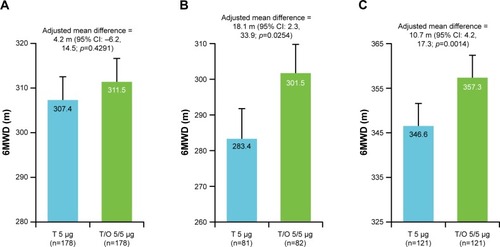
No significant differences were found for physical activity between the two treatments in terms of mean number of steps per day or daily duration of activity. The results are presented in the Supplementary materials. However, in a post hoc analysis, excluding the non-wearing time (mean wearing time of accelerometer was 14.3 hours in tiotropium/olodaterol and 14.2 hours in tiotropium; tiotropium/olodaterol: n=163, tiotropium: n=167), tiotropium/olodaterol improved the values for 2.0 METs (186.5 vs 191.5 min, difference: 5.0 min; 95% CI: 0.4 min, 9.7 min; p=0.0337), but no difference was seen for steps (3,871 vs 3,794 steps, difference:78 steps; 95% CI: −93 steps, 248 steps) compared with tiotropium ().
Figure 4 Physical activity after 6 weeks of treatment with T or T/O, excluding individuals with <8 hours wearing time or less than 2 valid days.
Abbreviations: METs, metabolic equivalents; T, tiotropium; T/O, tiotropium/olodaterol.
AEs
A total of four patients discontinued the study owing to AEs.
The incidences of AEs, serious AEs, drug-related AEs, and AEs leading to discontinuation were similar between tiotropium/olodaterol and tiotropium treatments. The most frequent AEs were viral upper respiratory tract infection (tiotropium/olodaterol: 10.0%, tiotropium: 6.0%), followed by worsening COPD (tiotropium/olodaterol: 5.0%, tiotropium: 4.9%). Individual events with an incidence ≥2% are shown in .
Table 2 Adverse events
There were no differences in pulse rate or SpO2 in conjunction with the 6MWT. Mean (SD) pulse rate for the treated population was 75.4 (11.9), 97.2 (14.7), and 77.1 (13.0) beats per minute before, after, and 5 min after the end of the 6MWT, respectively.
SpO2 was 94.0% for tiotropium/olodaterol and 94.0% for tiotropium just before the test, 88.4% for tiotropium/olodaterol and 88.5% for tiotropium just after the test, and 94.8% for tiotropium/olodaterol and 94.8% for tiotropium 5 min after the test.
There were no clinically relevant changes in vital signs, laboratory parameters, or 12-lead ECG parameters.
Discussion
In the VESUTO® study, Japanese patients with COPD were treated with tiotropium/olodaterol to evaluate the effects on lung hyperinflation, exercise capacity, and physical activity levels.
Results for the primary outcome of IC (60 min post-dose) after 6 weeks of treatment with tiotropium/olodaterol showed a significant increase of 115 mL compared with tiotropium (p<0.0001); thus, tiotropium/olodaterol therapy reduced lung hyperinflation in Japanese patients with COPD.
This result is comparable with that achieved in the MORACTO studies for 5/5 μg tiotropium/olodaterol vs tiotropium: study 1, IC (120 min post-dose) adjusted mean (standard error)=114 mL (0.027); study 2, IC (120 min post-dose) adjusted mean (standard error)=88 mL (0.025).Citation9
Improvements were also realized for tiotropium/olodaterol vs tiotropium in lung function for FEV1, SVC, and FVC. Although certain baseline characteristics of Japanese patients with COPD differ from those of Western patients, this study shows that the effects of tiotropium/olodaterol on lung function are consistent across Japanese and Western patients.
The VESUTO study is the first head-to-head comparison trial between tiotropium/olodaterol and tiotropium, and provided the first evidence for the evaluation of IC in Japanese patients with COPD.
The 6MWD assessment is a common tool to evaluate restricted exercise capacity caused by lung hyperinflation and an important IC correlation measurement and predictor of increased mortality in COPD.Citation17–Citation19 To our knowledge, no other randomized controlled trial has compared combined LAMA/LABA therapy to a monotherapy on the 6MWD.Citation20
It has been demonstrated that IC improved more in patients with severe GOLD stages,Citation8,Citation21 which suggests that the 6MWD could also be improved more in such patients; therefore, analyses on 6MWD in subgroups of GOLD stages were considered to be a clinically meaningful evaluation of 6MWD. Subgroup analyses in VESUTO provide evidence that 6MWD was improved more in the GOLD III/IV patients receiving tiotropium/olodaterol than in those receiving tiotropium (by 18 m; 95% CI: 2.3 m, 33.9 m; p=0.0254), although the adjusted mean of 6MWD after 6 weeks of treatment was similar between tiotropium/olodaterol and tiotropium in the overall population.
The improvement seen in exercise capacity did not reach the minimum clinically important difference for 6MWD in COPD (25–33 m),Citation22 indicating that bronchodilator treatment alone may not be sufficient to provide clinical benefit in exercise capacity.Citation19 However, bronchodilator treatment taken over longer periods in combination with pulmonary rehabilitation may further improve exercise capacity.Citation23
In this study, desaturation prevented 57 patients (32.8%) from completing the 6MWT during the treatment periods. SpO2 levels were carefully monitored throughout the 6MWT, and the 6MWT was immediately terminated if SpO2 levels fell below 83%, although the patients could continue the 6MWT. Careful monitoring of SpO2 is required during the 6MWT due to increased mortality levels in patients who experience desaturation, although the underlying mechanisms are unclear.Citation24
Physical activity decreases in patients with COPD with increasing age, and this may be the greatest risk factor for death. Without intervention, patients experience a worsening in quality of life and prognosis.Citation25,Citation26
GOLD recommends maintenance of exercise capacity,Citation27 and the COPD guidelines of the Japanese Respiratory Society also recommend improvement of exercise capacity and physical activity as a treatment goal.Citation28
Previous studies on the effects of several different bronchodilators on physical activity showed inconsistent findings.Citation29 In particular, two studies on LAMA/LABA combination therapy were placebo controlled.Citation12,Citation13
The VESUTO study is the first to compare the effects of LAMA/LABA combination therapy to LAMA or LABA monotherapy on physical activity without any behavioral intervention.
In our study, no significant differences were noted between tiotropium/olodaterol and tiotropium treatments in the mean number of steps/day, or the mean daily duration of activity ≥2, 3, and 4 METs during the last 2 weeks of the 6-week treatment in the overall population. One reason for this might be that patients had low baseline activity and were unlikely to achieve improvement in activity with pharmacologic intervention alone (baseline activity: 3,723 steps; 2 METs, 182.5 min; 3 METs, 47.9 min, 10.8 min; 4 METs).
We conducted a post hoc analysis based on previously reported methodologies, excluding data for patients with <8 hours wearing timeCitation15 and less than 2 valid days.Citation16 The analysis showed greater separation between tiotropium/olodaterol and tiotropium treatments in 2 METs. Although a clinically meaningful difference is unclear, these factors need to be considered in order to assess physical activity in further studies.
Evidence from previous studies indicates that improvements in physical activity levels of patients with COPD can be achieved through patient education, rehabilitation, and motivation.Citation30,Citation31 The outcomes of the VESUTO study indicate that a comprehensive approach including pharmacologic treatment may strengthen these results.
Limitations
The study population was limited to Japanese patients – mostly males – with COPD with a modified Medical Research Council ≥1, 6MWD <400 m, and modified Borg scale ≥4, and was relatively short, meaning long-term investigation is needed to confirm the results. These characteristics limit generalizability to other populations and females. All tests were performed at post-dose levels, and future studies are needed to investigate the 24-hour trough efficacy for exercise capacity. However, our results support and expand on current knowledge.
Conclusion
Tiotropium/olodaterol therapy demonstrated a significant improvement in hyperinflation compared with tiotropium and showed a potential enhancement of exercise capacity in COPD patients. A slight improvement in physical activity of relatively more than moderate intensity was also seen in tiotropium/olodaterol; however, additional studies may be needed to further explore physical activity. There were no safety concerns or serious adverse effects reported.
Author contributions
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript. MI, YM, TM, JU, TS, TA, and KH contributed to the study design, YG contributed to the data collection, TA analyzed data, and all authors contributed to the interpretation of the data. MI led the drafting of the manuscript. All authors contributed toward data analysis, drafting and revising the paper, and agree to be accountable for all aspects of the work.
Acknowledgments
We thank all the patients involved in this study. We also thank the following investigators for their assistance in conducting this study: Osamu Hataji, Matsusaka City Hospital, Matsusaka, Mie, Japan; Yuji Higashimoto, Kindai University Hospital, Osakasayama, Osaka, Japan; Yasuhiro Kondoh, Tosei General Hospital, Seto, Aichi, Japan; Masaru Suzuki, Hokkaido University Hospital, Sapporo, Hokkaido, Japan; Hiroyuki Ohbayashi, Tohno Chuo Clinic, Mizunami, Gifu, Japan; Takefumi Saito, NHO Ibarakihigashi National Hospital, Naka-gun, Ibaraki, Japan; Kazuhisa Asai, Osaka City University Hospital, Osaka, Japan; Motohiko Miura, Japan Organization of Occupational Health and Safety Tohoku Rosai Hospital, Sendai, Miyagi, Japan; Naoki Miyao, Medical Corporation Kokankai Kokan Clinic, Kawasaki, Kanagawa, Japan; Hirotaka Nagashima, Shinjuku Research Park Clinic, Shinjuku-ku, Tokyo, Japan; Hisatoshi Sugiura, Tohoku University Hospital, Sendai, Miyagi, Japan; Toshiyuki Harada, Japan Community Health Care Organization Hokkaido Hospital, Sapporo, Hokkaido, Japan; Tetsuo Hiramatsu, Hiramatsu Internal and Respiratory Medicine Clinic, Komaki, Aichi, Japan; Michiko Tsuchiya, Rakuwakai Otowa Hospital, Kyoto, Japan; Takashi Kinoshita, Kurume University Hospital, Kurume, Fukuoka, Japan; Tohru Tsuda, Kirigaoka Tsuda Hospital, Kitakyushu, Fukuoka, Japan; Shigeo Muro, Kyoto University Hospital, Kyoto, Japan; Yu Utsumi, Iwate Medical University Hospital, Morioka, Iwate, Japan; Toshio Ichiwata, Tokyo Medical University Hachioji Medical Center, Hachioji, Tokyo, Japan; Masahiro Kaneko, Kobe City Hospital Organization Kobe City Medical Center West Hospital, Kobe, Hyogo, Japan; Takuya Samukawa, Kagoshima University Hospital and Dental Hospital, Kagoshima, Japan; Takashi Motegi, Respiratory Care Clinic, Nippon Medical School, Chiyoda-ku, Tokyo, Japan; Yoshiaki Minakata, NHO Wakayama Hospital, Hidaka-gun, Wakayama, Japan; Satoshi Fuke, KKR Sapporo Medical Center, Sapporo, Hokkaido, Japan; Motokazu Kato, Kishiwada City Hospital, Kishiwada, Osaka, Japan; Yasuhiro Gon, Nihon University Itabashi Hospital, Itabashi-ku, Tokyo, Japan; Atsushi Nagai, Shinyurigaoka General Hospital, Kawasaki, Kanagawa, Japan; Hiromasa Harada, Yao Tokushukai General Hospital, Yao, Osaka, Japan; Keisuke Tomii, Kobe City Medical Center General Hospital, Kobe, Hyogo, Japan; Yasuko Harada, Nishi Fukuoka Hospital, Fukuoka, Japan; Masahiko Saito, Uji Tokushukai Medical Center, Uji, Kyoto, Japan; Tadashi Sato, Juntendo University Hospital, Bunkyo-ku, Tokyo, Japan; Hironori Sagara, Showa University Hospital, Shinagawa-ku, Tokyo, Japan; Hiroyuki Nakamura, Sakaide City Hospital, Sakaide, Kagawa, Japan; Yusuke Shikama, Showa University Fujigaoka Hospital, Yokohama, Kanagawa, Japan; Shinichi Osaki, Osaki Internal and Respiratory Clinic, Kitakyushu, Fukuoka, Japan; Koichi Nishimura, National Center for Geriatrics and Gerontology, Obu, Aichi, Japan; Hiroyuki Koba, Teine Keijinkai Clinic, Sapporo, Hokkaido, Japan; Keisuke Miki, NHO Toneyama National Hospital, Toyonaka, Osaka, Japan; Yuriko Mizobe, Nihonbashi Sakura Clinic, Chuo-ku, Tokyo, Japan; Yojiro Onari, Mazda Hospital, Aki-gun, Hiroshima, Japan; Hiroshi Hayakawa, NHO Tenryu Hospital, Hamamatsu, Shizuoka, Japan; Tetsuji Kawamura, NHO Himeji Medical Center, Himeji, Hyogo, Japan; Takeshi Isobe, Shimane University Hospital, Izumo, Shimane, Japan. Medical writing assistance was provided by Allison Kirsop, PhD, and Marion Barnett of Edanz Medical Writing, with publication management and editorial support by Daisuke Kuroki of Nippon Boehringer Ingelheim. This study was funded by Nippon Boehringer Ingelheim Co., Ltd. Nippon Boehringer Ingelheim contributed to the design of the study, data collection, analysis, and interpretation of the study results. Nippon Boehringer Ingelheim also funded the medical writing support, publication charges, and open access fee for publication of this manuscript.
Supplementary materials
Analysis groups
The primary analysis was performed in the full analysis set, comprising all patients who had signed informed consent, were randomized, and had taken any dose of study medication, and had non-missing baseline data at Visit 2 and non-missing post-dose baseline measurements of inspiratory capacity (IC) at rest for the primary endpoint.
The safety analysis was conducted in the analysis set that consisted of all treated patients.
Determination of sample size
For the primary endpoint of IC at rest after Week 6, results from the recent two Boehringer Ingelheim studies (data on file) suggested that the SD was ~0.4 L. However, a conservative 0.41 L was deemed more appropriate due to uncertain prediction of drop-outs during tests for physical activities. In addition, the IC at rest after Week 6 was estimated at 0.1 L for the difference between tiotropium/olodaterol and tiotropium. To detect a difference of 0.1 L with the SD of 0.41 L in the IC at rest between the two treatments with 90% power at the two-sided alpha of 0.05, 180 patients were required. Nquery Advisor nTerim ver.2.0 (MOT0-1, one-sample t-test) was used for the sample size calculation.
Statistical analysis
The primary analysis was conducted using mixed-effects model repeated measures including treatment and period as categorical fixed effects, study baseline as a covariate, and patient as a random effect. Compound symmetry was used as a covariance structure for within-patient variation. The SAS procedure MIXED was used, involving the restricted maximum likelihood estimation and the Kenward–Roger approximation for denominator degrees of freedom. Adjusted mean values as well as treatment contrasts were presented together with the 95% CIs and p-values.
Concomitant diagnoses
Of the total number of patients in the study, 174/184 (94.6%) had concomitant diagnoses and >50% had vascular disorders. Metabolic and nutritional disorders (46.2%), gastrointestinal disorders (31.0%), and cardiac disorders (17.4%) were also commonly reported.
The 6-min walk distance (6MWD)
The 6-min walk test was performed according to the methodology described by American Thoracic Society guidelines.
The criterion of the 6MWD <400 m is referred to in studies that evaluated the combination therapy of tiotropium and formoterol compared to tiotropium and the ECLIPSE study which considered the relation of 6MWD with survival rate.
Physical activity
As a secondary endpoint, physical activity was compared between treatments. The adjusted mean values of average steps per day, average daily activity durations of ≥4, ≥3, and ≥2 metabolic equivalents (METs), and average daily active strength in the 2 weeks prior to Week 6 were similar between the treatments. The treatment difference was 9.5 (95% CI: −155.7 steps, 174.7 steps; p=0.9098) steps/day for the average number of steps; −0.3 min (95% CI: −1.2 min, 0.6 min; p=0.5338), 0.9 min (95% CI: −1.0 min, 2.9 min; p=0.3524) and 2.3 min (95% CI: −3.0 min, 7.5 min; p=0.3949); 2.4 min (95% CI: −4.6 min, 9.4 min) for average daily duration ≥4 METs, ≥3 METs, ≥2 METs; and average daily active strength (METs min) of ≥3 METs, respectively.
Table S1 Investigator, site, and IRB list
Table S2 Treatment difference between tiotropium/olodaterol and tiotropium by GOLD stage subgroup
Disclosure
TS and SN are employees of Nippon Boehringer Ingelheim. MI has received honoraria from AstraZeneca, Nippon Boehringer Ingelheim, and Novartis Pharma. YG has received honoraria from Nippon Boehringer Ingelheim, AstraZeneca, Novartis Pharma and KYORIN Pharmaceutical Co., Ltd. TM reports honoraria from Nippon Boehringer Ingelheim and Fukuda Life Tech. JU has received honoraria from Nippon Boehringer Ingelheim, Hoshi Iryo-Sanki, and Teijin Pharma. YM and KH have received honoraria from Nippon Boehringer Ingelheim. TA received compensation from Nippon Boehringer Ingelheim for statistical analysis service. The authors report no other conflicts of interest in this work.
References
- BousquetJKhaltaevNGlobal Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive ApproachGenevaWorld Health Organisation2007
- BarnesPJChronic obstructive pulmonary diseasePreface Clin Chest Med201435113
- WHO [webpage on the Internet]COPD factsheet Published November 2016. Available from: http://www.who.int/mediacentre/factsheets/fs315/en/Accessed October 8, 2017
- KimJKimKKimYThe association between inhaled long-acting bronchodilators and less in-hospital care in newly-diagnosed COPD patientsRespir Med2014108115316123993445
- BuhlRMaltaisFAbrahamsRTiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4)Eur Respir J20154596997925573406
- BeehKMWestermanJKirstenAMThe 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary diseasePulm Pharmacol Ther201532535925956072
- SinghDFergusonGTBolitschekJTiotropium + olodaterol shows clinically meaningful improvements in quality of lifeRespir Med2015109101312131926320402
- MaltaisFKirstenAMHamiltonADe SousaDVoßFDecramerMEvaluation of the effects of olodaterol on exercise endurance in patients with chronic obstructive pulmonary disease: results from two 6-week crossover studiesRespir Res20161717727383762
- O’DonnellDECasaburiRFrithPEffects of combined tiotropium/olodaterol on inspiratory capacity and exercise endurance in COPDEur Respir J2017494 pii: 1601348
- BeehKMKornSBeierJEffect of QVA149 on lung volumes and exercise tolerance in COPD patients: the BRIGHT studyRespir Med2014108458459224534204
- MaltaisFSinghSDonaldACEffects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomized, double-blind clinical trialsTher Adv Respir Dis20148616918125452426
- WatzHTroostersTBeehKMACTIVATE: the effect of aclidinium/formoterol on hyperinflation, exercise capacity, and physical activity in patients with COPDInt J Chron Obstruct Pulm Dis20171225452558
- WatzHMailänderCBaierMKirstenAEffects of indacaterol/glycopyrronium (QVA149) on lung hyperinflation and physical activity in patients with moderate to severe COPD: a randomised, placebo-controlled, crossover study (The MOVE Study)BMC Pulm Med20161619527301417
- IchinoseMMinakataYMotegiTStudy design of VESUTO®: efficacy of tiotropium/olodaterol on lung hyperinflation, exercise capacity, and physical activity in Japanese patients with chronic obstructive pulmonary diseaseAdv Ther20173471622163528537001
- ByromBRoweDAMeasuring free-living physical activity in COPD patients: deriving methodology standards for clinical trials through a review of research studiesContemp Clin Trials20164717218426806669
- WatzHPittaFRochesterCLAn official European Respiratory Society statement on physical activity in COPDEur Respir J20144461521153725359358
- CamilloCALangerDOsadnikCRSurvival after pulmonary rehabilitation in patients with COPD: impact of functional exercise capacity and its changesInt J Chron Obstruct Pulmon Dis2016112671267927822029
- AndrianopoulosVWoutersEFPinto-PlataVMPrognostic value of variables derived from the six-minute walk test in patients with COPD: results from the ECLIPSE studyRespir Med201510991138114626143282
- CelliBTetzlaffKCrinerGCOPD Biomarker Qualification ConsortiumThe 6-minute-walk distance test as a chronic obstructive pulmonary disease stratification tool. Insights from the COPD biomarker qualification consortiumAm J Respir Crit Care Med2016194121483149327332504
- CalzettaLOraJCavalliFRoglianiPO’DonnellDECazzolaMImpact of LABA/LAMA combination on exercise endurance and lung hyperinflation in COPD: a pair-wise and network meta-analysisRespir Med201712918919828732830
- CasaburiRMaltaisFPorszaszJEffects of tiotropium on hyperinflation and treadmill exercise tolerance in mild to moderate chronic obstructive pulmonary diseaseAnn Am Thorac Soc20141191351136125289942
- Puente-MaestuLPalangePCasaburiRUse of exercise testing in the evaluation of interventional efficacy: an official ERS statementEur Respir J201647242946026797036
- CasaburiRKukafkaDCooperCBWitekTJJrKestenSImprovement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPDChest2005127380981715764761
- WaatevikMJohannessenAGomez RealFOxygen desaturation in 6-min walk test is a risk factor for adverse outcomes in COPDEur Respir J2016481829127076586
- VaesAWGarcia-AymerichJMarottJLChanges in physical activity and all-cause mortality in COPDEur Respir J20144451199120925063247
- WaschkiBKirstenAHolzOPhysical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort studyChest2011140233134221273294
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD)2017 [homepage on the Internet]. Available from: http://goldcopd.orgAccessed October 30, 2017
- Committee for the 4th edition of the COPD guidelines of the Japanese Respiratory SocietyGuidelines for the diagnosis and treatment of COPD (chronic obstructive pulmonary disease)4th editionTokyo, JapanThe Japanese Respiratory Society2013 Japanese
- MantoaniLCRubioNMcKinstryBMacNeeWRabinovichRAInterventions to modify physical activity in patients with COPD: a systematic reviewEur Respir J2016481698127103381
- MendozaLHortaPEspinozaJPedometers to enhance physical activity in COPD: a randomised controlled trialEur Respir J201545234735425261324
- PleguezuelosEPérezMEGuiraoLImproving physical activity in patients with COPD with urban walking circuitsRespir Med2013107121948195623890958