Abstract
Introduction
Long-term oxygen therapy (LTOT) improves survival and may reduce hospital admissions in patients with chronic obstructive pulmonary disease (COPD) and severe hypoxemia, but the impact of daily duration of LTOT on hospitalization rate is unknown. We aimed to estimate the association between the daily duration of LTOT (24 vs 15 h/d) and hospital admissions in patients with LTOT due to COPD.
Materials and methods
A population-based, cohort study included patients who started LTOT due to COPD between October 1, 2005 and June 30, 2009 in the Swedish national register for respiratory failure (Swedevox). Time to first hospitalization from all causes and from respiratory or nonrespiratory disease, using the National Patient Registry, was analyzed using Fine–Gray regression, adjusting for potential confounders.
Results
A total of 2,249 patients with COPD (59% women) were included. LTOT 24 h/d was prescribed to 539 (24%) and LTOT 15–16 h/d to 1,231 (55%) patients. During a median follow-up of 1.1 years (interquartile range, 0.6–2.1 years), 1,702 (76%) patients were hospitalized. No patient was lost to follow-up. The adjusted rate of all-cause hospitalization was similar between LTOT 24 and 15–16 h/d (subdistribution hazard ratio [SHR] 0.96; [95% CI] 0.84–1.08), as was cause-specific hospitalizations analyzed for respiratory disease (SHR: 1.00; 95% CI: 0.86–1.17) and nonrespiratory disease (SHR: 0.92; 95% CI: 0.75–1.14).
Conclusion
LTOT prescribed for 24 h/d was not associated with decreased hospitalization rates compared with LTOT for 15–16 h/d in patients with oxygen-dependent COPD. The results should be validated in a randomized controlled trial.
Introduction
Chronic obstructive pulmonary disease (COPD) is the most common cause of hypoxic respiratory failure. Long-term oxygen therapy (LTOT) improves survival in patients with chronic severe hypoxemia due to COPD.Citation1,Citation2 The current recommendation to use LTOT at least 15 h/d but preferably 24 h/d is based on the Medical Research Council (MRC) and the Nocturnal Oxygen Therapy Trial (NOTT) studies performed in the 1970s.Citation1,Citation2 In the MRC trial, LTOT 15 h/d was compared with no oxygen, and in the NOTT, LTOT 24 h/d was compared with nocturnal LTOT 12 h/d.Citation1,Citation2 In an observational comparison of the treatment arms of these 2 trials, the crude mortality rate was lower for the 18 h/d group in NOTT than for the 15 h/d group in the MRC trial.Citation1,Citation2,Citation3 The comparison was unadjusted and involved only 290 patients.Citation1,Citation2,Citation3 A recent observational study of 2,249 patients with oxygen-dependent COPD found no survival benefit from LTOT 24 vs 15 h/d.Citation4
Patients with oxygen-dependent COPD form a vulnerable group with impaired exercise tolerance and health-related quality of life,Citation5 high risk of anxiety and depression, and high risk of hospitalizations associated with suffering and high health care costs.Citation6 Thus, it is critical to explore factors influencing these measurements. LTOT has been reported to be associated with reduced hospital admissions in patients with COPD and hypoxic respiratory failure.Citation7 However, LTOT in moderate hypoxemia,Citation8 intermittent oxygen use at home,Citation9 or oxygen treatment at exerciseCitation10 do not prevent hospital admissions. The impact of LTOT duration on hospital admissions is unknown. This issue is of clinical importance as higher duration or continuous LTOT may pose an unnecessary burden and limitation for many patients. Dependence on LTOT has been shown to be associated with increased feelings of anxiety, and shame, potentially leading to social isolation and restrictions in activities in daily life.Citation11,Citation12
The aim of the present study was to estimate the association between LTOT daily duration and hospital admissions in patients with COPD and hypoxic respiratory failure. We hypothesized that hospitalizations would not differ in people prescribed LTOT 24 h/d compared with LTOT 15 h/d.
Materials and methods
Study design and data collection
This was a population-based, retrospective cohort study of prospectively collected data from the Swedish national register for respiratory failure (Swedevox). The structure, validity, and coverage of Swedevox have been described elsewhere.Citation13 All 48 Swedish centers prescribing LTOT follow the national guidelines that LTOT should be prescribed for 15 h/d or more, and that LTOT is indicated when arterial blood gas tension of oxygen (PaO2) breathing ambient air is <7.4 kPa; or PaO2 7.4–8.0 kPa together with signs of right-sided heart failure/pulmonary hypertension and/or secondary polycythemia erythrocyte volume fraction >0.54.Citation13
The present study included all registered patients who started LTOT due to COPD and hypoxic respiratory failure between October 1, 2005 and June 30, 2009. Patients with COPD and a comorbid diagnosis of lung cancer before starting LTOT were excluded. For patients who started LTOT more than once during the period, only the most recent treatment was included in the analysis.
Patient characteristics and measures
Variables were registered prospectively in Swedevox at the start of LTOT including on resting arterial PaO2 and blood gas tension of carbon dioxide (PaCO2) breathing air and oxygen, forced expiratory volume in 1 second (FEV1), body mass index (BMI), World Health Organization (WHO) performance status, prescribed oxygen dose (L/min), and prescribed LTOT duration (h/d). Data on comorbid conditions and number of hospitalizations during the 4-year period before baseline were obtained from the Swedish National Inpatient Register, which covers more than 99% of all hospital admissions and about 80% of all hospital-based outpatient care during period.Citation14 Comorbid conditions were defined as doctor’s diagnoses coded according to the tenth revision of the International Classification of Disease. Diagnoses of interest were anxiety, renal failure, and cardiovascular disease. The latter was expressed as number of cardiovascular diseases (cerebrovascular disease, heart failure, hypertension, ischemic heart disease, peripheral artery disease, pulmonary embolism, or other circulatory disease). Data on all dispensed drug prescriptions in outpatient care after July 1, 2005 were obtained from the Swedish Prescribed Drug Register.Citation15 Vital status and cause of death were obtained from the National Board of Health and Welfare’s Cause of Death Register.Citation16
Ethical considerations
All patients participating in the study were informed according to directives from the authorities. Participants provided their verbal consent when registering in Swedevox, and the consent procedure and the study was approved by the Regional Ethical Review Board of Lund (DNr 157/2007 and 350/2008), the Swedish National Board of Health and Welfare, and the Swedish Data Inspection Board.
Statistical analyses
Statistical analyses were conducted using the software packages Stata, version 13 (StataCorp LP; College Station, TX, USA), and SAS, version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Continuous baseline variables were presented as means with standard deviations (SD) or medians with range or interquartile range (IQR) for normal and skewed distribution, respectively. Categorical variables were expressed as frequencies and percentages.
The follow-up period was from start of LTOT until first hospital admission, with censoring due to death, LTOT withdrawal, or study end at the 30th of June, 2009. The primary endpoint was time to first hospitalization, and secondary endpoints were time to first hospitalization due to respiratory disease (tenth revision of the International Classification of Disease, J00–J99) or nonrespiratory disease including cardiovascular diseases and other diseases. Analyses were conducted using Fine–Gray regression, accounting for the competing risk of death. Prescribed LTOT daily duration was analyzed both by comparing 24 h/d with 15 h/d (n=1,770 patients) and as a continuous variable (n=2,249), with adjustment for potential confounders. The confounders were chosen a priori using subject matter knowledge and prior mortality analysesCitation17–Citation19 and included baseline age, sex, prescribed oxygen dose (L/min), PaO2 (on air), PaCO2 (on air), FEV1, WHO performance status (in 5 categories), BMI (in 4 categories), maintenance treatment with oral gluco-corticoids, anxiety, renal failure, number of cardiovascular diagnoses (3 categories), and number of hospitalizations in 4 years before baseline (continuous variable). Missing data were imputed for PaO2 (air), PaCO2 (air), FEV1, BMI, and WHO performance status.Citation17 The model estimates were robust to the imputations. Statistical significance was defined as 2-sided P-value <0.05.
Results
Patient characteristics
During October 1, 2005–December 31, 2009; a total of 2,249 patients with COPD (59% women) started LTOT and were included in the analysis. Due to the comprehensive coverage of the Swedish National Inpatient Register, follow-up was complete. LTOT 24 h/d was prescribed to 539 (24%) patients, LTOT 15–16 h/d was prescribed to 1,231 (55%) patients, and other daily durations were prescribed to 470 patients (21%). Patients receiving LTOT 24 h/d had significantly lower PaO2 on air and worse WHO performance status at the start of LTOT, as compared with patients with oxygen 15 h/d (). The aimed PaO2 at above 8 kPa on oxygen was achieved in the majority of patients, with similar rates for LTOT 24 h/d (77%) and LTOT 15–16 h/d (80%).
Table 1 Baseline characteristics
Hospital admissions
Median follow-up time was 1.1 years (IQR, 0.6–2.1 years), and 1,702 (76%) patients were hospitalized at least 1 time during the follow-up period. Of these, 8 patients were hospitalized during the whole study period and excluded from regression analysis. The remaining patients were censored due to death before any hospital admission (n=190; 8%) or due to withdrawal from LTOT (n=357; 16%). In the majority of the patients, their first hospitalization was due to worsening of respiratory disease (n=1,105). In 16 of the included hospitalization events, a main diagnosis code was missing, and these patients were excluded from cause-specific regression analysis.
Hospital admissions in LTOT 24 vs 15–16 h/d
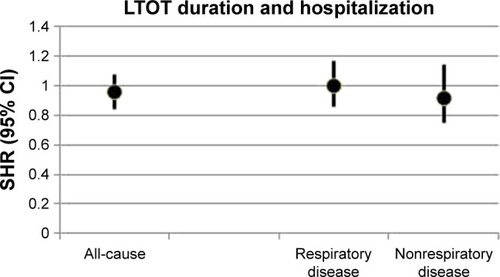
In the crude analysis, comparing LTOT 24 h/d and 15–16 h/d (n=1,763), there was no difference in all-cause hospitalizations (subdistribution hazard ratio [SHR]: 0.96; 95% CI: 0.86–1.08). In the multivariate model with adjustment for potential confounders, the result remained almost exactly the same (SHR: 0.96; 95% CI: 0.84–1.08) (). The adjusted cumulative function plots of the treatment groups closely overlapped at all time points (). When the continuous LTOT duration variable was used (n=2,249), the SHR was similar (SHR: 0.99; 95% CI: 0.98–1.01). Adjusted cause-specific hospitalizations due to respiratory disease was similar between the 2 treatment groups (SHR: 1.00; 95% CI: 0.86–1.17), as was the rate of hospitalizations due to nonrespiratory cause (SHR: 0.92; 95% CI: 0.75–1.14) ().
Table 2 Results from Fine–Gray regression of LTOT duration and hospitalizations
Figure 1 Cumulative incidence of first hospitalization in LTOT daily duration 24 h/d vs 15 h/d.
Note: Results from Fine–Gray analyses adjusted for baseline age, sex, oxygen dose (L/min), PaO2 (air), baseline PaCO2 (air), FEV1, WHO performance status, body mass index, treatment with oral glucocorticoids, and comorbid conditions including anxiety, renal failure and number of cardiovascular diagnoses.
Abbreviations: FEV1, forced expiratory volume in one second; LTOT, long-term oxygen therapy; PaCO2, arterial blood gas tension of carbon dioxide; PaO2, arterial blood gas tension of oxygen; SHR, subdistribution hazard ratio; WHO, World Health Organization.
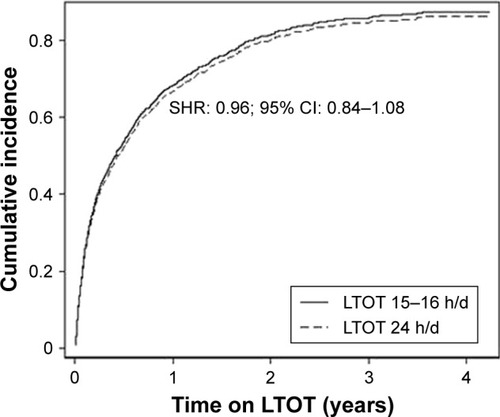
Figure 2 All-cause and cause-specific hospitalizations in LTOT daily duration 24 h/d vs 15 h/d.
Abbreviations: FEV1, forced expiratory volume in one second; LTOT, long-term oxygen therapy; PaCO2, arterial blood gas tension of carbon dioxide; PaO2, arterial blood gas tension of oxygen; SHR, subdistribution hazard ratio; WHO, World Health Organization.
Discussion
Main findings
The primary finding of this study is that the rate of hospitalization due to any cause or due to respiratory disease is equal with LTOT 24 h/d compared with 15–16 h/d in patients with COPD. Although the group with LTOT 24 h/d had statistically significantly lower baseline PaO2, potentially indicating more severe disease, the analyses adjusted for PaO2 and subsequently no independent difference in the associations with number of hospitalizations was found.
Although LTOT has been reported to decrease the risk for hospitalizationCitation1,Citation2 and death,Citation7 the optimal daily duration for LTOT is still unclear. The current recommendation to use LTOT 15 h/d and preferably higher is based on pooled results from the MRC and NOTT studies where LTOT 15 h/d and 24 h/d, respectively, were both associated with increased survival compared with no or with only nocturnal oxygen.Citation1,Citation2 However, our recently published work with the Swedish registry data indicates that there may indeed be no survival benefit from using LTOT continuously compared with LTOT 15–16 h/d.Citation4 In the present study, time to first hospitalization was analyzed as primary outcome instead of time to death. Our results showing that the rate of hospitalizations was equal in patients with 24 h/d and 15–16 h/d is consistent with the previous finding of similar mortality risk in these groups.Citation4 The present findings reflect real-world data and thus have high external validity, as patients starting LTOT today are older, have more comorbid conditions, and include more women compared with the MRC and NOTT studies.Citation1,Citation2
As for the other results of the Fine–Gray regression model, several cardiovascular diagnoses, maintenance treatment with oral glucocorticoids and lower PaCO2 were all associated with a higher risk for hospitalization. Cardiovascular comorbidity has been previously shown to influence risk for hospitalization in patients with COPD,Citation20,Citation21 and our association of oral steroids with an increased risk for hospitalization is consistent with a corresponding report of higher mortality risk in COPD and maintenance treatment with oral steroids. As for the unexpected association of higher PaCO2 with a lower risk for hospitalization, we speculate that a possible explanation may be due to some patients having coexisting overlap syndrome, where PaCO2 is higher but home ventilator therapy prevents hospitalizations. However, we do not have data on number of home ventilators in our study.
Strengths and limitations
The major strengths of this study is that it is a multicenter, national study with a large study population and that the Swedish register data used for patient characteristics and outcome variables are well reliable.Citation13,Citation14,Citation16 A major limitation is that we have no objective data on actual daily oxygen utilization. Therefore, we cannot exclude the possibility that patients prescribed LTOT 24 h/d utilized oxygen for fewer hours per day in real life or that patients prescribed 15–16 h/d used oxygen more. If this was the case, this could explain why no differences in hospitalization were found. However, our findings reflect effectiveness of prescribed oxygen durations in clinical practice, and a previous study has indicated that patients with significant hypoxemia as in our study population are adherent to prescribed LTOT duration.Citation22 Another limitation is that the Swedevox data reports prescribed oxygen duration at baseline, and we cannot exclude that patients are either increasing or decreasing their prescribed dose of oxygen. In addition, there may be a selection bias due to doctor’s decisions, where factors like comorbid pulmonary hypertension and functional status may influence the choice of oxygen duration. Finally, the observational design of the study means that the results may be biased, as the groups are not randomized. Although we tried to deal with this using a multivariate model with adjustment for potential confounders, our hypothesis that LTOT 24 h/d does not lower the risk for mortality or hospitalizations compared with LTOT 15–16 h/d needs to be examined in a randomized controlled trial.
Clinical implications
The results of this study support that there are no benefits of prescribing LTOT 24 h/d compared with 15–16 h/d for prevention of hospitalizations. This finding, together with our previous study where no differences in survival was shown for LTOT 24 vs 15–16 h/d, could be of great clinical importance for the small but resource-intensive group of patients with hypoxemia due to COPD. LTOT is associated with considerable costs and side effects, and may lead to social isolation.Citation12,Citation23,Citation24 In addition, low-flow oxygen therapy has been shown to be associated with oxidative stress and inflammation, which potentially could contribute to increased morbidity and negative health effects.Citation25,Citation26 Subsequently, it is important to establish that LTOT 24 h/d is an unnecessary burden to patients with oxygen-dependent COPD.
Conclusion
LTOT prescribed 24 h/d was not associated with decreased hospital admissions compared with LTOT 15–16 h/d in patients with oxygen-dependent COPD. The results need to be further examined in a randomized controlled trial.
Acknowledgments
We thank Swedevox for supplying data for the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- Nocturnal Oxygen Therapy Trial GroupContinuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trialAnn Intern Med19809333913986776858
- Medical Research Council Working PartyLong term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working PartyLancet1981182226816866110912
- AnthonisenNRPrognosis in chronic obstructive pulmonary disease: results from multicenter clinical trialsAm Rev Respir Dis19891403 Pt 2S95S992675713
- AhmadiZSundhJBornefalk-HermanssonAEkströmMLong-term oxygen therapy 24 vs 15 h/d and mortality in chronic obstructive pulmonary diseasePLoS One2016119e016329327649490
- CoquartJBLe RouzicORacilGWallaertBGrosboisJMReal-life feasibility and effectiveness of home-based pulmonary rehabilitation in chronic obstructive pulmonary disease requiring medical equipmentInt J Chron Obstruct Pulmon Dis2017123549355629263659
- Garcia-AymerichJMonsóEMarradesRMRisk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation. EFRAM studyAm J Respir Crit Care Med200116461002100711587986
- RingbaekTJViskumKLangePDoes long-term oxygen therapy reduce hospitalisation in hypoxaemic chronic obstructive pulmonary disease?Eur Respir J2002201384212166578
- AlbertRKAuDHBlackfordALLong-Term Oxygen Treatment Trial Research GroupA randomized trial of long-term oxygen for COPD with moderate desaturationN Engl J Med2016375171617162727783918
- TurnerAMSenSSteeleyCEvaluation of oxygen prescription in relation to hospital admission rate in patients with chronic obstructive pulmonary diseaseBMC Pulm Med20141412725096821
- RingbaekTMartinezGLangePThe long-term effect of ambulatory oxygen in normoxaemic COPD patients: a randomised studyChron Respir Dis2013102778423431028
- RingLDanielsonEPatients’ experiences of long-term oxygen therapyJ Adv Nurs19972623373449292368
- DislerRTGreenALuckettTExperience of advanced chronic obstructive pulmonary disease: metasynthesis of qualitative researchJ Pain Symptom Manage20144861182119924780181
- EkströmMAhmadiZLarssonHA nationwide structure for valid long-term oxygen therapy: 29-year prospective data in SwedenInt J Chron Obstruct Pulmon Dis2017123159316929133978
- LudvigssonJFAnderssonEEkbomAExternal review and validation of the Swedish national inpatient registerBMC Public Health20111145021658213
- WettermarkBHammarNForedCMThe new Swedish Prescribed Drug Register – opportunities for pharmacoepidemiological research and experience from the first 6 monthsPharmacoepidemiol Drug Saf200716772673516897791
- BrookeHLTalbäckMHörnbladJThe Swedish cause of death registerEur J Epidemiol201732976577328983736
- EkströmMPHermanssonABStrömKEEffects of cardiovascular drugs on mortality in severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187771572023328521
- EkströmMPBornefalk-HermanssonAAbernethyAPCurrowDCSafety of benzodiazepines and opioids in very severe respiratory disease: national prospective studyBMJ2014348g44524482539
- AhmadiZBornefalk-HermanssonAFranklinKAMidgrenBEkströmMPHypo- and hypercapnia predict mortality in oxygen-dependent chronic obstructive pulmonary disease: a population-based prospective studyRespir Res2014153024625018
- CurkendallSMDeLuiseCJonesJKCardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patientsAnn Epidemiol2006161637016039877
- ManninoDMThornDSwensenAHolguinFPrevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPDEur Respir J200832496296918579551
- KatsenosSConstantopoulosSHLong-term oxygen therapy in COPD: factors affecting and ways of improving patient compliancePulm Med2011201132536221941649
- CroxtonTLBaileyWCLong-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop reportAm J Respir Crit Care Med2006174437337816614349
- StollerJKPanosRJKrachmanSDohertyDEMakeBLong-term Oxygen Treatment Trial Research GroupOxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trialChest2010138117918720605816
- Foschino BarbaroMPServiddioGRestaOOxygen therapy at low flow causes oxidative stress in chronic obstructive pulmonary disease: prevention by N-acetyl cysteineFree Radic Res200539101111111816298736
- HolguinFFolchEReddSCManninoDMComorbidity and mortality in COPD-related hospitalizations in the United States, 1979–2001Chest200512842005201116236848
