Abstract
Purpose
To assess the comparative efficacy of short-acting muscarinic antagonists (SAMAs), long-acting muscarinic antagonists (LAMAs), LAMA in combination with long-acting beta-agonists (LABAs; LAMA/LABAs) and inhaled corticosteroids (ICS) in combination with LABA (ICS/LABAs) for the maintenance treatment of COPD.
Materials and methods
We systematically reviewed 74 randomized controlled trials (74,832 participants) published up to 15 November 2017, which compared any of the interventions (SAMA [ipratropium], LAMA [aclidinium, glycopyrronium, tiotropium, umeclidinium], LAMA/LABA [aclidinium/formoterol, indacaterol/glycopyrronium, tiotropium/olodaterol, umeclidinium/vilanterol] and ICS/LABA [fluticasone/vilanterol, budesonide/formoterol, salmeterol/fluticasone]) with each other or with placebo. A random-effects network meta-analysis combining direct and indirect evidence was conducted to examine the change from baseline in trough FEV1, transition dyspnea index, St George’s Respiratory Questionnaire and frequency of adverse events at weeks 12 and 24.
Results
Inconsistency models were not statistically significant for all outcomes. LAMAs, LAMA/LABAs and ICS/LABAs led to a significantly greater improvement in trough FEV1 compared with placebo and SAMA monotherapy at weeks 12 and 24. All LAMA/LABAs, except aclidinium/formoterol, were statistically significantly better than LAMA monotherapy and ICS/LABAs in improving trough FEV1. Among the LAMAs, umeclidinium showed statistically significant improvement in trough FEV1 at week 12 compared to tiotropium and glycopyrronium, but the results were not clinically significant. LAMA/LABAs had the highest probabilities of being ranked the best agents in FEV1 improvement. Similar trends were observed for the transition dyspnea index and St George’s Respiratory Questionnaire outcomes. There were no significant differences in the incidences of adverse events among all treatment options.
Conclusion
LAMA/LABA showed the greatest improvement in trough FEV1 at weeks 12 and 24 compared with the other inhaled drug classes, while SAMA showed the least improvement. There were no significant differences among the LAMAs and LAMA/LABAs within their respective classes.
Introduction
COPD is a chronic disorder characterized by fixed airway obstruction with accompanying respiratory symptoms such as persistent and progressive breathlessness, chronic productive cough and limited exercise capacity. It is predominantly caused by smoking; however, other factors, particularly occupational exposures, may also contribute to the development of COPD. The impairment of lung function is usually progressive and is not fully reversible. Exacerbations often occur, where there is a rapid and sustained worsening of symptoms beyond normal day-to-day variations. COPD is a global health problem that causes substantial morbidity and mortality. It is the fourth leading cause of death worldwide.Citation1
Current disease management guidelines developed by GOLD recommend maintenance therapy with either a long-acting muscarinic antagonist (LAMA) or a long-acting beta agonist (LABA) in patients with moderate or severe COPD (Groups B–D) when short-acting muscarinic antagonists (SAMAs) fail to control symptoms and exacerbation rates.Citation2 Patients who have persistent symptoms or exacerbations should be treated with a combination of LAMA and LABA (LAMA/LABA) or inhaled corticosteroids (ICS) and LABA (ICS/LABA). To our knowledge, there is no published systematic review that compares all treatment options. The aim of this network meta-analysis was to comprehensively compare the efficacy and safety of the individual agents under the various therapeutic classes of inhalers commonly used in the treatment of COPD, namely SAMAs, LAMAs, LAMA/LABA fixed-dose combinations (FDCs) and ICS/LABA FDCs. LABA monotherapy was not included in this analysis as it is infrequently used compared to the other classes of inhalers in Singapore.
Materials and methods
This review followed the PRISMA guidelines.
Search strategy
A systematic search of PubMed and Embase was conducted up to 15 November 2017. The search strategy employed a combination of medical subject headings and text words related to the drug classes of interest, the term “COPD” and their synonyms (Table S1). Reference lists from published systematic reviews were hand-searched for additional publications. The searches were limited to English language.
Study selection
Randomized, parallel-group, controlled design studies of ≥12 weeks’ duration, which compared LAMA/LABA FDCs (aclidinium/formoterol 400/12 mcg twice a day [AclForm], indacaterol/glycopyrronium 110/50 mcg once a day [IndaGlyco], tiotropium/olodaterol 5/5 mcg once a day [TioOlo], umeclidinium/vilanterol 62.5/25 mcg once a day [UmecVil]), LAMAs (aclidinium 400 mcg once a day [Acl], glycopyrronium 50 mcg once a day [Glyco], tiotropium 18 mcg [Tio18] or 5 mcg [Tio5] once a day, umeclidinium 62.5 mcg once a day [Umec]), ICS/LABA FDC (fluticasone/salmeterol 250/50 mcg [SFC250] or 500/50 mcg [SFC500] twice a day, fluticasone/vilanterol 100/25 mcg once a day [FFVI], budesonide/formoterol 320/9 mcg twice a day [BudeForm]) and SAMA (ipratropium 40 mcg four times a day [Ipra]) with each other or with placebo were selected if they included adults with stable, moderate-to-very severe COPD. The eligible study treatments were restricted to all combinations at their licensed doses which were available at the time of review. Studies were required to report at least one of the following clinical and health status endpoints: trough FEV1, transitional dyspnea index (TDI), St George’s Respiratory Questionnaire (SGRQ) and safety (frequency of adverse events [AEs]). There was considerable heterogeneity in the definition of exacerbation outcomes across the studies, which limited their ability to be pooled in a network. Most of the earlier studies defined exacerbation by the symptoms (eg, at least 3 days of increased sputum production), while more recent studies defined exacerbation by the treatment received (eg, requiring corticosteroids or hospital admission).
Data extraction and risk of bias assessment
Two authors (MIAA and LET) independently reviewed the search results and assessed the eligibility of the studies for selection. Any disagreements were resolved by discussion to achieve consensus. The data extraction was performed independently using a standard template and checked for discrepancies. Specific data points of interest that were only presented in graphs were extracted using WebPlotDigitizer.Citation3 Risk of bias was assessed using the Cochrane Collaboration Risk of Bias tool.Citation4 Domains assessed were random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data and selective outcome reporting. Biases were reported as high or low or unclear. Assumption checking for homogeneity, similarity and consistency was also conducted.
Data synthesis and analysis
A frequentist random-effects network meta-analysis was performed using the “network” routine within the mvmeta package in Stata 15 statistical software (StataCorp, College Station, TX, USA).Citation5 Network meta-analysis allows for simultaneous analysis of direct comparisons of interventions (head-to-head) within randomized controlled trials and indirect comparisons across trials based on a common comparator, provided that the studies included are comparable in terms of treatment effect modifiers. The synthesis of direct and indirect evidence produces a more precise and refined estimate of treatment effectiveness by maximizing the use of available data for all treatments within the network. Direct pairwise comparisons were also conducted using the metan package in Stata.Citation6
The primary outcome was trough FEV1 (in mL); secondary outcomes included were TDI, SGRQ and AEs. For the continuous outcomes (FEV1, SGRQ and TDI), the mean difference (MD) in the change from baseline values between the two arms was used in the analyses. The minimal clinically important difference (MCID) for FEV1 is 100 mL.Citation7,Citation8 The proportions of patients who attained the MCID in TDI (TDI responders, a ≥1 unit increase in TDI)Citation9 and SGRQ (SGRQ responders, a ≥4 unit decrease in SGRQ score)Citation10 were also analyzed. Observation time points of 12 and 24 weeks were chosen as they were the two consistently reported time points across the studies. A result was considered significant if the 95% CI did not include 0 or one for the continuous and dichotomous outcomes, respectively. The Surface Under the Cumulative RAnking (SUCRA) curve was obtained to determine the relative probability of a treatment being the best option for each outcome measure.Citation11
Possible network inconsistency was assessed using the design-by-treatment model approach described by White.Citation5 This approach provided a global test for inconsistency, with a P-value <0.05 indicating violation of the consistency assumption in the network.
Results
Search and selection results
The electronic database search identified 1,611 citations, of which 1,485 were excluded on abstract review (Figure S1). A further 50 were excluded after reviewing the full-text articles, leaving 74 studies reported by 75 articles included in the final selection.
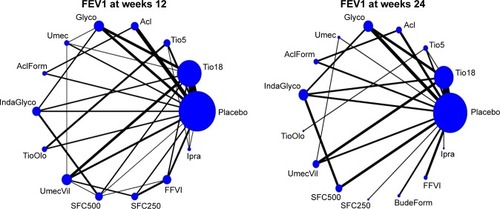
The 74 studies selected for inclusion were published between 2000 and 2017. The study characteristics are presented in . Thirty-nine studies (53%) were placebo controlled and 35 were active controlled. Overall, there were more published studies for tiotropium 18 mcg compared to the other agents, given it was the first LAMA licensed for COPD. Most studies (67 studies; 91%) had large sample sizes with >200 participants. All studies included patients aged at least 35 years, with a smoking history of at least 10 packs per year. Six studies only included patients with a prior history of exacerbations, while five studies only included patients without any exacerbation history. The remaining 63 studies did not specify exacerbation history in their inclusion/exclusion criteria. The most commonly reported primary outcome across the studies was lung function (trough FEV1); limited data for other patient-related outcomes (TDI and SGRQ) were also available. Data from the intention-to-treat and full analysis set for all trials were extracted. The network plots for trough FEV1 at weeks 12 and 24 are shown in .
Table 1 Study characteristics of the included trials in NMA
Figure 1 Evidence network of available trials showing direct comparisons of agents with respect to lung function (trough FEV1) at weeks 12 and 24.
Abbreviations: Acl, aclidinium; AclForm, aclidinium/formoterol; BudeForm, budesonide/formoterol; FFVI, fluticasone/vilanterol; Glyco, glycopyrronium; IndaGlyco, indacaterol/glycopyrronium; Ipra, ipratropium; SFC250, fluticasone/salmeterol 250/50 mcg; SFC500, fluticasone/salmeterol 500/50 mcg; Tio5, tiotropium 5 mcg; Tio18, tiotropium 18 mcg; TioOlo, tiotropium/olodaterol; Umec, umeclidinium; UmecVil, umeclidinium/vilanterol.
A total of 74,832 patients were included in the 74 studies. The key patient characteristics and assessment of risk of bias for each study are presented in . Mean ages ranged from 61 to 73 years; proportion of males and current smokers ranged from 48% to 99% and from 22% to 88%, respectively. The mean FEV1 predicted at baseline ranged from 35% to 78%. Majority of the studies were assessed to have low or unclear risk of bias. Inconsistency models were not significant for all outcomes, implying that the consistency assumption was not violated.
Table 2 Baseline characteristics and risk of bias of the included trials in NMA
Efficacy
Trough FEV1 change from baseline
Trough FEV1 results were reported in 59 studies at week 12 and in 39 studies at week 24. All LAMAs, LAMA/LABAs and ICS/LABAs led to significantly greater improvement in trough FEV1 compared to SAMA and placebo at weeks 12 and 24 ( and ). While some of the comparisons among LAMAs, LAMA/LABAs and ICS/LABAs showed statistical significance, the results were generally not clinically significant with respect to an MCID of 100 mL. For example, among the LAMAs, Umec led to statistically significant improvement in trough FEV1 at week 12 compared to Tio18 (mean difference [MD] of 37 mL, 95% CI 13–62 mL) and Glyco (MD 31 mL, 95% CI 6–57 mL). However, there were no significant differences in trough FEV1 for all LAMA vs LAMA comparisons at week 24. Among the LAMA/LABAs, there were no significant differences between IndaGlyco, TioOlo and UmecVil at weeks 12 and 24. IndaGlyco and UmecVil showed statistically significant improvement in FEV1 when compared to AclForm at both weeks 12 and 24. The MDs ranged from 43 to 59 mL (point estimates). Statistically significant improvement at weeks 12 and 24 was also seen for IndaGlyco, TioOlo and UmecVil compared to all LAMAs. The MDs ranged from 48 to 88 mL (point estimates). On the other hand, AclForm exhibited no significant difference compared to any LAMAs. Among the ICS/LABAs, the results were mixed with some showing significant differences between the agents (FFVI vs SFC250 at week 12, MD 40 mL; FFVI vs BudeForm at week 24, MD 58 mL; BudeForm vs SFC250 at week 24, MD −82 mL). When compared to the LAMAs, ICS/LABAs had a similar effect on FEV1 at week 12, except SFC250 vs Umec (MD −52 mL, 95% CI −85 to −18 mL, favoring Umec) and FFVI vs Tio18 (MD 25 mL, 95% CI 4–47 mL, favoring FFVI). At week 24, ICS/LABAs were generally comparable to the LAMAs, except BudeForm vs Glyco (MD −40 mL, 95% CI −77 to 3 mL, favoring Glyco) and Tio18 (MD −35 mL, 95% CI −69 to −1 mL favoring Tio18). When compared to the LAMA/LABAs, ICS/LABAs conferred significantly less improvement at weeks 12 and 24. The MDs in improvements in FEV1 between the ICS/LABAs and LAMA/LABAs were smaller with AclForm vs ICS/LABAs than with the other three LAMA/LABAs (IndaGlyco, TioOlo and UmecVil).
Table 3 Treatment effects on FEV1 at week 12 – NMA results: combining direct and indirect evidence (lower triangle) and direct evidence (upper triangle)
Table 4 Treatment effects on FEV1 at week 24 – NMA results: combining direct and indirect evidence (lower triangle) and direct evidence (upper triangle)
Transition dyspnea index
TDI scores were reported in 28 studies at week 12 and in 19 studies at week 24. Significantly greater improvements in TDI scores were observed with all LAMAs, LAMA/LABAs and ICS/LABAs compared to SAMA and placebo at weeks 12 and 24 (Tables S2 and S3). There were no significant differences between the LAMA/LABAs except UmecVil vs TioOlo at week 12 (MD −0.51, 95% CI −0.94 to −0.07, favoring TioOlo). The difference was not considered to be clinically significant (MCID ≥1 point increase). There were no significant differences in TDI scores between all LAMA–LAMA comparisons at weeks 12 and 24. There were, however, some statistically significant improvements in TDI scores for LAMA/LABAs compared to LAMAs. Nonetheless, the magnitudes of difference in TDI scores were all lower than the MCID of ≥1 unit for these comparisons. Similarly, there were some statistically significant (but not clinically significant) differences when LAMA/LABAs were compared to ICS/LABAs. There were no significant differences in TDI scores when LAMAs were compared to ICS/LABAs at both time points.
In terms of the proportion of TDI score responders (who achieved a minimum of 1-point improvement in TDI score), results were reported in 15 studies at week 12 and in 16 studies at week 24. Study participants treated with LAMAs, LAMA/LABAs or ICS/LABAs were more likely to achieve improvement in TDI score than those receiving placebo or SAMA (Tables S4 and S5). There was some evidence to show that LAMA/LABA treatment had higher odds of achieving TDI score improvement compared to LAMAs (UmecVil vs Tio18 at week 12: OR 1.49, 95% CI 1.16–1.93; IndaGlyco vs Tio18 at week 24: OR 1.45, 95% CI 1.11–1.89; AclForm vs Tio18 at week 24: OR 1.37, 95% CI 1.04–1.80). There were no significant differences in TDI score improvement when ICS/LABAs were compared with LAMAs or LAMA/LABAs.
St George’s Respiratory Questionnaire
Health-related quality-of-life (HRQoL) benefits as measured by SGRQ scores were reported in 34 studies at week 12 and in 29 studies at week 24. All LAMAs, LAMA/LABAs and ICS/LABAs showed statistically significant improvement in SGRQ score compared to placebo at week 12; however, the point estimates did not achieve clinical significance (MCID ≥4-point decrease) for SFC250, Acl, Glyco, Tio5 and Tio18 (Table S6). At week 24, only some of the LAMAs and LAMA/LABAs showed statistically significant improvements in HRQoL vs placebo (Table S7), but none of the results reached clinical significance. A similar trend was seen when LAMAs, LAMA/LABAs and ICS/LABAs were compared to SAMA. Within each class, the LAMAs, LAMA/LABAs and ICS/LABAs led to similar HRQoL improvements at weeks 12 and 24. While there were some statistically significant differences between LAMA/LABAs and LAMAs, these differences were not clinically significant.
In terms of the proportion of SGRQ score responders (achieving at least a 4-point reduction in SGRQ), results were reported in 21 studies at week 12 and in 19 studies at week 24. At both time points, all LAMAs, LAMA/LABAs and ICS/LABAs led to a significantly higher proportion of study participants achieving an improvement in SGRQ score compared to placebo (Tables S8 and S9). Relative to SAMA, only IndaGlyco and TioOlo showed a statistically significant difference in SGRQ responders at week 12 (Ipra vs IndaGlyco: OR 0.61, 95% CI 0.40–0.94; Ipra vs TioOlo: OR 0.53, 95% CI 0.33–0.85). There were no significant differences within the LAMA, LAMA/LABA and ICS/LABA classes at weeks 12 and 24 for SGRQ responders; however, some evidence was available to suggest that the LAMA/LABAs led to a higher proportion of SGRQ responder compared to the LAMAs at both weeks 12 and 24.
Adverse events
Incidences of AEs were reported in 17 studies at week 12 and in 27 studies at week 24. There were no significant differences in the proportion of patients who experienced AEs for any comparison at week 12 (Table S10). At week 24, a significantly higher proportion of patients receiving SFC500 had AEs compared to those receiving IndaGlyco (SFC500 vs IndaGlyco: OR 1.34, 95% CI 1.04–1.72; Table S11). This translated to a Number Needed to Harm of 14. No other significant differences with respect to AEs were found in other comparisons.
Ranking of treatments
In general, the LAMA/LABAs ranked the highest among the different drug classes for lung function improvement (FEV1) at weeks 12 and 24, while placebo and SAMA ranked the lowest. The SUCRA values for LAMA/LABAs ranged from 64.5% to 97.6% (). The trend remained constant for all outcomes, with LAMA/LABAs having the highest SUCRA scores.
Table 5 SUCRA values for all interventions for each outcome
Discussion
Our network meta-analysis is the first to utilize a frequentist framework to comprehensively compare the effectiveness of SAMAs, LAMAs, LAMA/LABAs and ICS/LABAs using published randomized controlled studies. A frequentist framework allowed us to make statistical inference/comparisons based on significance testing using P-values. With regards to lung function, our results showed that LAMAs, LAMA/LABAs and ICS/LABAs led to a greater improvement in trough FEV1 compared with placebo and SAMA monotherapy. All LAMA/LABAs except aclidinium/formoterol were significantly better than LAMA monotherapy in improving lung function. Limited evidence also suggested LAMA/LABAs led to greater improvements than ICS/LABAs. Of note, there was markedly more evidence available for lung function than other patient-relevant outcomes. Similar trends were, nonetheless, observed with respect to improvements in TDI and SGRQ scores, although not all results were statistically significant. Improvements with FEV1 have been correlated with improvements in quality of life as demonstrated in previous analyses.Citation12 A recent study by Sion et al (2017) reported similar findings that LAMA/LABAs combinations were better than Tio alone or placebo.Citation88
Our results did not show any clinically significant differences among the different LAMAs and LAMA/LABAs within their classes, for all outcomes. These results were congruent with other published network meta-analyses which compared outcomes within the drug classes. Cope et al,Citation89 Karabis et alCitation90 and Ismaila et alCitation91 evaluated the comparative efficacy among the LAMA agents through a Bayesian framework and found no differences among them. Similarly, Schlueter et alCitation92 and Huisman et alCitation93 evaluated the comparative efficacy among LAMA/LABAs using the Bayesian approach and found no differences among all agents. Our analysis, which employs a frequentist framework and uses a network with more comprehensive treatment options (SAMA, LAMA, LAMA/LABA and ICS/LABA) for stable COPD, adds further confidence to these findings and expands the existing evidence base.
In considering the results, we need to be mindful of the limitations of the analysis. FEV1 is the only outcome that is consistently reported across the trials. Given TDI and SGRQ outcomes can be reported as either total score or proportion of responders, this resulted in many studies not reporting both types of outcome. Therefore, there was uncertainty in our analysis of TDI and SGRQ outcomes, with the results reflected in wide CIs.
In addition, some included studies were open label (Bateman et alCitation51 and Kerwin et alCitation13), and hence were associated with a high risk of bias in terms of lack of blinding. Incomplete outcome data in some studies also may have increased uncertainty around some results. Small study bias was considered unlikely, given that most included trials had a sample size of at least 100 patients and each arm of all included comparisons had at least 50 patients. Most of the included studies were of a short duration with only 16 studies, out of the 74, reporting outcomes beyond the 24-week time point. Therefore, only the 12- and 24-week time points were selected for evaluation.
When performing network meta-analysis, patients from all pair-wise meta-analysis have not been randomized to different trials and randomization would, therefore, not hold across the set of trials used for the analysis. Thus, it is important to assess imbalance in patient characteristics and effect modifiers across trials to determine the face validity of the analysis. To ensure the assumptions of homogeneity and transitivity are met, the distribution of potential effect modifiers, such as gender distribution, mean age, and proportion of smokers, was assessed and found to be similar across the direct comparisons in the network. However, other effect modifiers, including mean COPD duration and proportion with exacerbation history, were not reported in the majority of the trials, which limited our ability to determine if our assumptions were met with respect to these characteristics. However, despite this limitation, it is unlikely that our results would be substantially biased given the consistency of results demonstrated between the network and direct comparison meta-analyses.
To address the potential influence of certain treatment effect modifiers, network meta-regression would have been appropriate to explore the impact of covariates on all of the data and allow for the simultaneous consideration of continuous and categorical covariates. However, we did not perform a meta-regression mainly due to the fact that the variation in FEV1 was too small, and also due to the limited number of studies available in parts of the network. Model diagnostics and adequacy are difficult to assess. Even if the network meta-regression was performed, individual patient data would be necessary to avoid ecological bias and to gain greater statistical power to detect differences in treatment effects between the effect modifiers.
It is worth noting that although we have reported the ranking of all treatments using SUCRA curves, large differences in ranking probabilities between two treatments do not necessarily mean significant difference in relative treatment effect. To achieve a more objective assessment, the magnitude of absolute benefit should be accompanied with ranking information in order to minimize potential biased interpretation.
Finally, our approach was based on individual treatments (eg, Acl, Glyco, Tio18, Tio5, Umec) instead of drug classes (eg, LAMAs) as it facilitated comparisons both within and across classes. However, this led to multiple comparisons involving all treatments from each class and, thus, difficulty in drawing conclusions at a therapeutic class level. The number of studies available for each individual treatment was also small, which may have resulted in low statistical power.
Conclusion
LAMA/LABA showed greatest improvement in lung function at weeks 12 and 24 compared with the other inhaled drug classes, while SAMA showed the least improvement. There were no significant differences among the LAMAs and LAMA/LABAs within their respective classes. Results from our analysis may play a role in assisting clinicians make evidence-based treatment decisions and also in advising policymakers on the most effective treatments when making subsidy decisions. Other factors, including cost-effectiveness and patient preferences, may also be taken into account when determining the most optimal treatments for patients with stable COPD.
Acknowledgments
David Bin-Chia Wu left the Agency for Care Effectiveness, Ministry of Health, Singapore during the course of manuscript development.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health OrganizationThe top 10 causes of death [updated January 2017] Available from: http://www.who.int/gho/mortality_burden_disease/causes_death/top_10/en/Accessed 17 September, 2018
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management and prevention of COPD2018 Available from: http://goldcopd.orgAccessed 17 September, 2018
- RohatgiAWebPlotDigitizer Version 4.1 [updated 8 January 2018] Available from: https://automeris.io/WebPlotDigitizer/. Accessed 17 September, 2018
- HigginsJPAltmanDGGotzschePCJuniPMoherDOxmanADThe Cochrane Collaboration’s tool for assessing risk of bias in randomised trialsBMJ2011343d592822008217
- WhiteIRMultivariate random-effects meta-regression: updates to mvmetaStata J201111255270
- HarrisRJBradburnMJDeeksJJHarbordRMAltmanDGSterneJAMetan: fixed and random-effects meta-analysisStata J200881328
- CazzolaMMacNeeWFau MartinezFJOutcomes for COPD pharmacological trials: from lung function to biomarkers2008312416469
- DonohueJFMinimal clinically important differences in COPD lung functionCOPD20052111112417136971
- MahlerDAWitekTJThe MCID of the transition dyspnea index is a total score of one unitCOPD2005219910317136969
- AlmaHde JongCJelusicDHealth status instruments for patients with COPD in pulmonary rehabilitation: defining a minimal clinically important differenceNPJ Prim Care Respir Med2016261604127597571
- ChaimaniAHigginsJPMavridisDSpyridonosPSalantiGGraphical tools for network meta-analysis in STATAPLoS One2013810e7665424098547
- WestwoodMBourbeauJJonesPWCerulliACapkun-NiggliGWorthyGRelationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic reviewRespir Res2011124021477298
- KerwinEMD’UrzoADGelbAFLakkisHGarciaGil ECaractaCFACCORD I study investigatorsEfficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I)COPD2012929010122320148
- RennardSIScanlonPDFergusonGTACCORD COPD II: a randomized clinical trial to evaluate the 12-week efficacy and safety of twice-daily aclidinium bromide in chronic obstructive pulmonary disease patientsClin Drug Investig20133312893904
- JonesPWSinghDBatemanEDEfficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN studyEur Respir J201240483083622441743
- LeeSHLeeJYooKHEfficacy and safety of aclidinium bromide in patients with COPD: a Phase 3 randomized clinical trial in a Korean populationRespirology20152081222122826370136
- D’UrzoAFergusonGTvan NoordJAEfficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trialRespir Res20111215622151296
- WangCSunTHuangYEfficacy and safety of once-daily glycopyrronium in predominantly Chinese patients with moderate-to-severe chronic obstructive pulmonary disease: the GLOW7 studyInt J Chron Obstruct Pulmon Dis201510576825609940
- ChapmanKRBeehKMBeierJA blinded evaluation of the efficacy and safety of glycopyrronium, a once-daily long-acting musca-rinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 studyBMC Pulm Med2014144424438744
- KerwinEHébertJGallagherNEfficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 studyEur Respir J20124051106111423060624
- AmbrosinoNFoglioKBalzanoGPaggiaroPLLessiPKestenSTiotropium and exercise training in COPD patients: effects on dyspnea and exercise toleranceInt J Chron Obstruct Pulmon Dis20083477178019281092
- BrusascoVHodderRMiravitllesMKorduckiLTowseLKestenSHealth outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPDThorax200358539940412728159
- CasaburiRBriggsDDDonohueJFSerbyCWMenjogeSSWitekTJThe spirometric efficacy of once-daily dosing with tiotropium in stable COPD: a 13-week multicenter trial. The US Tiotropium Study GroupChest200011851294130211083677
- CasaburiRMahlerDAJonesPWA long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J200219221722411866001
- ChanCKMaltaisFSigouinCHaddonJMFord GTSAFE Study GroupA randomized controlled trial to assess the efficacy of tiotropium in Canadian patients with chronic obstructive pulmonary diseaseCan Respir J200714846547218060091
- CovelliHBhattacharyaSCassinoCConoscentiCKestenSAbsence of electrocardiographic findings and improved function with once-daily tiotropium in patients with chronic obstructive pulmonary diseasePharmacotherapy200525121708171816305289
- DonohueJFFogartyCLotvallJOnce-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropiumAm J Respir Crit Care Med2010182215516220463178
- JohanssonGLindbergARombergKNordströmLGerkenFRoquetABronchodilator efficacy of tiotropium in patients with mild to moderate COPDPrim Care Respir J200817316917518536860
- MoitaJBárbaraCCardosoJTiotropium improves FEV1 in patients with COPD irrespective of smoking statusPulm Pharmacol Ther200821114615117693107
- NiewoehnerDERiceKCoteCPrevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trialAnn Intern Med2005143531732616144890
- FreemanDLeeAPriceDEfficacy and safety of tiotropium in COPD patients in primary care – the SPiRiva Usual CarE (SPRUCE) studyRespir Res200784517605774
- TroostersTSciurbaFCDecramerMTiotropium in patients with moderate COPD naive to maintenance therapy: a randomised placebo-controlled trialNPJ Prim Care Respir Med2014241400324841833
- TashkinDPCelliBSennSBurkhartDKestenSMenjogeSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med2008359151543155418836213
- CelliBDecramerMKestenSLiuDMehraSTashkinDPMortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20091801094895519729663
- VerkindreCBartFAguilaniuBThe effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary diseaseRespiration200673442042716484769
- ZhouYZhongNSLiXTiotropium in early-stage chronic obstructive pulmonary diseaseN Engl J Med20173771092393528877027
- VinckenWvan NoordJAGreefhorstAPBantjeTAKestenSKorduckiLImproved health outcomes in patients with COPD during 1 yr’s treatment with tiotropiumEur Respir J200219220921611871363
- van NoordJABantjeTAElandMEKorduckiLCornelissenPJA randomised controlled comparison of tiotropium nd ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study GroupThorax200055428929410722768
- BatemanESinghDSmithDEfficacy and safety of tiotropium Respimat SMI in COPD in two 1-year randomized studiesInt J Chron Obstruct Pulmon Dis2010519720820714373
- BatemanEDTashkinDSiafakasNA one-year trial of tiotropium Respimat plus usual therapy in COPD patientsRespir Med2010104101460147220620037
- VoshaarTLapidusRMaleki-YazdiRA randomized study of tiotropium Respimat Soft Mist inhaler vs. ipratropium pMDI in COPDRespir Med20081021324117996436
- WiseRAAnzuetoACalverleyPThe Tiotropium Safety and Performance in Respimat Trial (TIOSPIR), a large scale, randomized, controlled, parallel-group trial-design and rationaleRespir Res2013144023547660
- WiseRAAnzuetoACottonDDahlRDevinsTDisseBTiotropium Respimat inhaler and the risk of death in COPDN Engl J Med2013369161491150123992515
- AnzuetoAWiseRCalverleyPThe Tiotropium Safety and Performance in Respimat® (TIOSPIR®) Trial: spirometry outcomesRespir Res20151610726369563
- TrivediRRichardNMehtaRChurchAUmeclidinium in patients with COPD: a randomised, placebo-controlled studyEur Respir J2014431728123949963
- FeldmanGMaltaisFKhindriSA randomized, blinded study to evaluate the efficacy and safety of umeclidinium 62.5 μg compared with tiotropium 18 μg in patients with COPDInt J Chron Obstruct Pulmon Dis20161171973027103795
- RheaultTKhindriSVahdati-BolouriMChurchAFahyWAA randomised, open-label study of umeclidinium versus glycopyrronium in patients with COPDERJ Open Res201622
- D’UrzoADRennardSIKerwinEMMergelVLeselbaumARCaractaCFAUGMENT COPD study investigatorsEfficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD studyRespir Res20141512325756831
- SinghDJonesPWBatemanEDEfficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised studyBMC Pulm Med20141417825404569
- VogelmeierCPaggiaroPLDorcaJEfficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a Phase 3 COPD studyEur Respir J20164841030103927492833
- BatemanEDFergusonGTBarnesNDual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE studyEur Respir J20134261484149423722616
- DahlRChapmanKRRudolfMSafety and efficacy of dual bronchodilation with QVA149 in COPD patients: the ENLIGHTEN studyRespir Med2013107101558156723867808
- VogelmeierCFBatemanEDPallanteJEfficacy and safety of once-daily QVA149 compared with twice-daily salmeterol–fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group studyLancet Respir Med201311516024321804
- WedzichaJABanerjiDChapmanKRVestboJRocheNAyersRTIndacaterol–glycopyrronium versus salmeterol–fluticasone for COPDN Engl J Med2016374232222223427181606
- ZhongNWangCZhouXZhangNHumphriesMWangLLANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPDInt J Chron Obstruct Pulmon Dis2015101015102626082625
- WedzichaJADecramerMFickerJHAnalysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group studyLancet Respir Med20131319920924429126
- SinghDFergusonGTBolitschekJTiotropium + olodaterol shows clinically meaningful improvements in quality of lifeRespir Med2015109101312131926320402
- BuhlRMaltaisFAbrahamsRTiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4)Eur Respir J201545496997925573406
- BuhlRMagderSBothnerULong-term general and cardiovascular safety of tiotropium/olodaterol in patients with moderate to very severe chronic obstructive pulmonary diseaseRespir Med2017122586627993292
- KerwinEMKalbergCJGalkinDVUmeclidinium/vilanterol as step-up therapy from tiotropium in patients with moderate COPD: a randomized, parallel-group, 12-week studyInt J Chron Obstruct Pulmon Dis20171274575528280319
- SilerTMDonaldACO’DellDChurchAFahyWAA randomized, parallel-group study to evaluate the efficacy of umeclidinium/vilanterol62.5/25 μg on health-related quality of life in patients with COPDInt J Chron Obstruct Pulmon Dis20161197197927274218
- ZhengJZhongNNewlandsAChurchAGohAHEfficacy and safety of once-daily inhaled umeclidinium/vilanterol in Asian patients with COPD: results from a randomized, placebo-controlled studyInt J Chron Obstruct Pulmon Dis2015101753176726366068
- DonohueJFMaleki-YazdiMRKilbrideSMehtaRKalbergCChurchAEfficacy and safety of once-daily umeclidinium/vilanterol62.5/25 mcg in COPDRespir Med2013107101538154623830094
- DecramerMAnzuetoAKerwinEEfficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trialsLancet Respir Med20142647248624835833
- Maleki-YazdiMRKaelinTRichardNZvarichMChurchAEfficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24-week, randomized, controlled trialRespir Med2014108121752176025458157
- DonohueJFWorsleySZhuCQHardakerLChurchAImprovements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbationsRespir Med2015109787088126006754
- SinghDWorsleySZhuCQHardakerLChurchAUmeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trialBMC Pulm Med2015159126286141
- TashkinDPRennardSIMartinPEfficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trialDrugs200868141975200018778120
- RennardSITashkinDPMcelhattanJEfficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trialDrugs200969554956519368417
- BhattSPDransfieldMTCockcroftJRA randomized trial of once-daily fluticasone furoate/vilanterol or vilanterol versus placebo to determine effects on arterial stiffness in COPDInt J Chron Obstruct Pulmon Dis20171235136528176907
- KerwinEMScott-WilsonCSanfordLA randomised trial of fluticasone furoate/vilanterol (50/25 μg; 100/25 μg) on lung function in COPDRespir Med2013107456056923352226
- MartinezFJBosciaJFeldmanGFluticasone furoate/vilanterol (100/25; 200/25 μg) improves lung function in COPD: a randomised trialRespir Med2013107455055923332861
- CovelliHPekBSchenkenbergerIScott-WilsonCEmmettACrimCEfficacy and safety of fluticasone furoate/vilanterol or tiotropium in subjects with COPD at cardiovascular riskInt J Chron Obstruct Pulmon Dis201611112
- PepinJLCockcroftJRMidwinterDSharmaSRubinDBAndreasSLong-acting bronchodilators and arterial stiffness in patients with COPD: a comparison of fluticasone furoate/vilanterol with tiotropiumChest201414661521153025058845
- DransfieldMTFeldmanGKorenblatPEfficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patientsRespir Med201410881171117924998880
- AgustíAde TeresaLde BackerWA comparison of the efficacy and safety of once-daily fluticasone furoate/vilanterol with twice-daily fluticasone propionate/salmeterol in moderate to very severe COPDEur Respir J201443376377224114969
- AsaiKKobayashiAMakiharaYJohnsonMAnti-inflammatory effects of salmeterol/fluticasone propionate 50/250 mcg combination therapy in Japanese patients with chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis20151080381125945045
- HananiaNADarkenPHorstmanDThe efficacy and safety of fluticasone propionate (250 microg)/salmeterol (50 microg) combined in the Diskus inhaler for the treatment of COPDChest2003124383484312970006
- MahlerDAWirePHorstmanDEffectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200216681084109112379552
- CalverleyPMAndersonJACelliBSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med2007356877578917314337
- JonesPWAndersonJACalverleyPMHealth status in the TORCH study of COPD: treatment efficacy and other determinants of changeRespir Res2011127121627828
- JenkinsCRJonesPWCalverleyPMEfficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH studyRespir Res2009105919566934
- ZhengJPYangLWuYMThe efficacy and safety of combination salmeterol (50 microg)/fluticasone propionate (500 microg) inhalation twice daily via accuhaler in Chinese patients with COPDChest200713261756176317951625
- CazzolaMAndòFSantusPA pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe-to-very severe COPDPulm Pharmacol Ther200720555656116914336
- PerngDWTaoCWSuKCTsaiCCLiuLYLeeYCAnti-inflammatory effects of salmeterol/fluticasone, tiotropium/fluticasone or tiotropium in COPDEur Respir J200933477878419129278
- DahlRGreefhorstLANowakDInhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2001164577878411549532
- TaylorJKotchARiceKIpratropium bromide hydrofluoroalkane inhalation aerosol is safe and effective in patients with COPDChest200112041253126111591569
- SionKYHuismanELPunekarYSNayaIIsmailaASA network meta-analysis of long-acting muscarinic antagonist (LAMA) and long-acting β2-agonist (LABA) combinations in COPDPulmonary Therapy201732297316
- CopeSDonohueJFJansenJPComparative efficacy of long-acting bronchodilators for COPD-a network meta-analysisRespiratory research201314110024093477
- KarabisALindnerLMocarskiMHuismanEGreeningAComparative efficacy of aclidinium versus glycopyrronium and tiotropium, as maintenance treatment of moderate to severe COPD patients: a systematic review and network meta-analysisInt J Chron Obstruct Pulmon Dis2013840524043936
- IsmailaASHuismanELPunekarYSKarabisAComparative efficacy of long-acting muscarinic antagonist monotherapies in COPD: a systematic review and network meta-analysisInt J Chron Obstruct Pulmon Dis201510249526604738
- SchlueterMGonzalez-RojasNBaldwinMGroenkeLVossFReasonTComparative efficacy of fixed-dose combinations of long-acting muscarinic antagonists and long-acting β2-agonists: a systematic review and network meta-analysisTher Adv Respir Dis20161028910426746383
- HuismanELCockleSMIsmailaASKarabisAPunekarYSComparative efficacy of combination bronchodilator therapies in COPD: a network meta-analysisInt J Chron Obstruct Pulmon Dis2015101863
