Abstract
Introduction
In patients with COPD, severe physical inactivity (SPI, which is defined as total daily energy expenditure/resting energy expenditure; physical activity level [PAL] ratio, <1.4) is associated with increased morbidity and mortality. Pulmonary rehabilitation (PR) increases physical capacity in COPD, but the impact on SPI is unknown. In this study, we aimed at elucidating the prevalence of SPI in COPD patients attending standard PR, the impact of PR on SPI prevalence, and the relationship between SPI and time spent in moderate physical activity thus whether American College of Sports Medicine (ACSM) recommendations are clinically useful in excluding SPI in COPD.
Methods
This is a prospective non-interventional pilot study on patients with COPD completing PR, consenting to wear an accelerometer (Sensewear© Armband) for a week before and after completing PR to assess changes in energy expenditure, time spent in physical activity, and number of daily steps. Low level of daily physical activity was not an inclusion criterion.
Results
In total, 57 patients completed the study and 31 (54%) had SPI at baseline. In patients with SPI, baseline median FEV1 was 48 (range, 28–86) % of predicted and GOLD B, n=11 (35%)/GOLD D, n=20 (65%). Surprisingly, 31 of SPI patients (97%) spent ≥150 minutes/week in moderate physical activity. After rehabilitation, 24 (78%) did not change activity level and were persistently SPI. We observed no differences at baseline between patient responding (n=7) vs not responding (n=24) to PR. Responders increased number of daily steps and time spent in lighter but not moderate physical activity during rehabilitation.
Conclusion
In this pilot study, SPI was prevalent, and PR had limited impact. Contraintui-tively, most patients with SPI complied with general recommendations of weekly hours spent in moderate physical activity. Our study highlights that increasing time spent in light activity rather than improving time spent in moderate activity is important in COPD patients with chronic dyspnea.
Introduction
COPD is associated with reduced levels of daily physical activity.Citation1 The chronic airflow limitation results in exercise-limiting dyspnea and fatigue, and in particular, COPD patients are at risk for severe physical inactivity (SPI), which is defined as a total daily energy expenditure/resting energy expenditure ratio <1.4.Citation2–Citation4 SPI is prevalent in the majority of COPD patients in GOLD group DCitation5 and is highly related to both morbidity and mortality.Citation6–Citation8 However, SPI assessment requires measurement of resting energy expenditure by indirect calorimetry or an accelerometer (eg, SenseWear© Armband; BodyMedia, Pittsburgh, PA, USA) because no equation for calculated resting energy expenditure has shown acceptable accuracy in COPD.Citation9 According to the American College of Sports Medicine (ACSM), healthy subjects are recommended to engage in moderate physical activity for 30 minutes a day – at least 5 days a week – to reduce the risk of chronic diseases and disabilities.Citation10 The same recommendations are applicable for older adults according to WHO.Citation11 In COPD, it is not known whether complying with the ACSM goal precludes SPI.
Pulmonary rehabilitation (PR) is a validated intervention in COPD, and exercise is the cornerstone of the program.Citation12 Even though high intensity and duration of physical activity are negatively correlated to morbidity and mortality in COPD,Citation2,Citation5,Citation6,Citation13–Citation15 it is not known whether the standard ACSM recommendation is a relevant success criterion for PR in COPD.Citation6,Citation7,Citation16–Citation19 Recent research has suggested that lighter activities are more efficient in avoiding inactivity including SPI than heavier physical activities.Citation3,Citation4,Citation15,Citation20,Citation21
In the present study, we aimed at elucidating the prevalence of SPI in COPD patients attending standard PR, the impact of PR on the prevalence of SPI, and the relationship between SPI and time spent in moderate physical activity, thus it is unknown if the ASCM recommendations is clinically useful in excluding SPI in COPD.
Methods
Design
This prospective, observational cohort study was designed as a pilot study and conducted in 2013–2014 in PR centers at Naestved Hospital and in the communities of Naestved, Faxe, and Vordingborg centers (Region Zealand, Denmark). Patients were enrolled on the first day of the rehabilitation program (Visit 1) and had a follow-up visit in the last week of the rehabilitation course (Visit 2).
Inclusion criteria
Inclusion criteria were physician-diagnosed COPD confirmed by spirometry (GOLD classes B–D),Citation12 referral for PR, ability to give informed consent, no other life-threatening illnesses, no history of asthma, and no acute exacerbation within the last 4 weeks prior to inclusion.Citation22
Written and oral informed consent was obtained. This study was approved by the Danish National Ethical Committee and complied with the Declaration of Helsinki. The study is registered with ClinicalTrials.gov (number 34431).
Study procedures
The rehabilitation program consisted of exercise training, education in management of disease and pharmacology, nutrition, and help with smoking cessation. The duration of the program ranged from 7 to 12 weeks, 2 hours twice a week according to the guidelines from the Danish Health Authority.Citation23 The exercise training consisted of 1 hour of supervised training twice a week with an equal distribution between endurance and resistance training.Citation23 Spirometry (MicroLoop© spirometer; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) measurement was performed at both visits according to the guidelines of the American Thoracic Society (ATS) and the European Respiratory Society (ERS).Citation24 Patient-reported outcome measures were obtained at both visits. Background data were obtained at Visit 1: gender, weight, height, and exacerbations (acute exacerbation in COPD [AECOPD]) comorbidities and medication.
Primary and secondary outcomes
Primary outcome
The primary outcome of this study was the prevalence of SPI before and after PR.
Secondary outcomes
The secondary outcome of this study was minutes spent in light respective moderate physical activity, number of patients spending ≥150 minutes/week in light (≥2 Metabolic Equivalent of Task [MET]) respective moderate (≥3 MET) physical activity, number of daily steps, and patient-reported outcome measures (COPD assessment test [CAT] and mMRC) before and after PR.
Physical activity assessment
Subjects were instructed to wear an activity monitor (SenseWear Armband) continuously for 7 days after each study visit. The use of SenseWear in patients with COPD has previously been validated.Citation29,Citation30 According to the manufacturer’s recommendation, the activity monitor is worn on the back of the upper right arm at the level of the triceps. This monitor assesses acceleration in two planes by using a bi-axial accelerometer which measures and stores skin temperature, near body temperature, heat flux and galvanic skin resistance. Activity is estimated in MET, a measure of energy expenditure (1 MET=1 kcal/kg/h). Output from the SenseWear includes the following:
Total energy expenditure (TEE): number of calories burnt every day.
Resting metabolic rate (RMR): the rate at which the body burns energy when it is at complete rest.
Physical activity level (PAL): TEE divided by the RMR. • Minutes spent at light (≥2 MET) and moderate (≥3 MET) physical activity.
Daily number of steps.
Data from patients wearing the monitor >2 days and >90% of daily use were included.Citation25
Patient-reported outcome measures
CAT consisted of a self-reported, well-validated 5-point Likert scale, eight-item questionnaire with four respiratory items and four general health items (score range, 0–40).Citation26 mMRC scale consisted of a self-reported, well-validated, 5-point Likert scale, one-item questionnaire (score range, 0–4). A score ≥2 is considered “severe dyspnea” and is an inclusion criterion for PR in Denmark.Citation12,Citation18
Statistical analyses
Statistical analyses were performed using STATA 14.1. Missing data were omitted from analyses.
Continuous data are presented as a median (range) and categorical data as incidence (%). Differences were assessed using the chi-squared test or Wilcoxon signed-rank test. Statistical significance was set at P<0.05.
Results
Patients
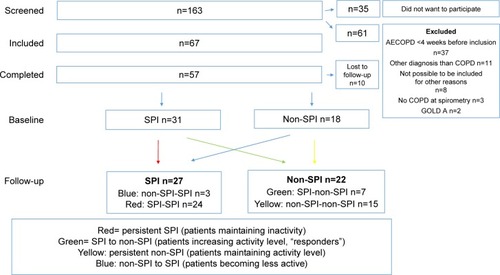
Between January 15, 2013, and February 10, 2014, 163 patients were consecutively screened, and 67 patients were included (). Totally, 57 patients completed the trial (community-based rehabilitation: n=43, 75%; hospital-based rehabilitation: n=14, 25%) and were included in the analysis (female: 53%; age, 68 years [range, 50–80 years]). Baseline median FEV1 was 50 (range, 22–96) % of predicted, and patients were divided between GOLD class B (n=28) respective D (n=29). Median body mass index (BMI) was 27 kg/m2 (range, 17–51 kg/mCitation2), and median self-reported number of moderate to severe AECOPD in preceding 12 months was 1 (0–7; ). Patients completing the study and patients dropping out did not differ significantly in baseline characteristics. Eight of the patients (14%) had missing SenseWear data at either baseline or follow-up. shows that 74% complied with the ASCM’s recommendation for physical activity at baseline and that PR increased time spent in light but not moderate physical activity. Nine patients (16%) used a walker, and they did not differ significantly regarding daily steps or minutes/week spent in ≥3 MET as opposed to patients not using walking aids. We registered the following comorbidities: 1) ischemic heart disease, n=3 (5%); 2) heart failure, n=12 (21%); 3) osteoporosis, n=5 (9%); 4) arthritis or joint pain, n=32 (56%); 5) anxiety, n=18 (32%); 6) depression, n=9 (16%); 7) stroke or other neurologic disease, n=5 (9%); and 8) diabetes, n=4 (7%).
Table 1 Group characteristics (n=57)
Table 2 Impact of pulmonary rehabilitation on time spent in physical activity and number of patients being active for ≥150 minutes/ week measured using activity monitor (SenseWear©) during 1 week
Patients with SPI at baseline
Totally, 31 (54%) patients had SPI at baseline, yet 96% of patients with baseline SPI spent ≥150 minutes/week in moderate physical activity (≥3 MET), thus complying with ASCM recommendation for the general population (). Only seven patients (22%) responded to PR by increasing PAL to ≥1.4 (“responders”; ). Responders significantly improved number of daily steps and time spent in lighter but not moderate physical activity compared to patients with persistent SPI. Analyzing differences between responders and patients with persistent SPI revealed no significant baseline differences ().
Table 3 Subgroup analyses of patients with SPI at baseline (n=31): differences at baseline and follow-up between patients remaining severely inactive (SPI) and patients not being severely inactive (non-SPI) at follow-up (responders)
Discussion
This pilot study showed that SPI is prevalent in patients referred for PR and that rehabilitation improved objectively measured physical activity in a minority of these patients. To our knowledge, this is the first study to address these issues.
It is well established that self-reported level of physical activity is poorly associated with objectively measured physical activity.Citation27 Surprisingly, we found that 96% of patients with SPI spent ≥150 minutes/week in objectively measured moderate physical activity. Thus, the general recommendations for physical activity by ACSMCitation10 seem not to be clinically meaningful in COPD patients with chronic dyspnea (mMRC, ≥2). Our data support that increasing lighter rather than moderate or hard physical activity is more efficient in reducing sedentary lifestyle.Citation3,Citation4,Citation16,Citation20,Citation21 However, the paradoxical finding of sedentary lifestyle (SPI) and sufficient amounts of moderate physical activity might have other explanations. As SPI is defined as a low ratio between total daily energy expenditure and resting energy expenditure, SPI might be a result of low total daily energy expenditure and/or increased resting energy expenditure. Several studies have found that moderate to severe COPD is associated with increased resting energy expenditure, lower fat-free body mass, and increased protein turnover.Citation4,Citation5,Citation28 In addition, more profound structural and metabolic changes in peripheral and respiratory muscles seen in COPD patients result in increased energy expenditure (kcal/kg/h=MET) at lower levels of exercise.Citation29 This is even more pronounced with low BMI, as energy expenditure is weight dependent; however, Kao et alCitation28 found that low fat-free mass but not low BMI was associated with increased energy expenditure. We found no differences in baseline BMI in responders vs patients with persistent SPI (), but did not include measurements of fat-free mass. We found no differences in resting energy expenditure between responders and patients with persistent SPI, but the statistical power was low due to the limited sample size; thus, we cannot rule out that COPD patients with SPI benefiting from PR is a distinct subgroup.
The PAL and time spent in moderate physical activity are two different ways to measure physical activity, and both are important predictors of morbidity and mortality.Citation6–Citation8,Citation10 Although the impact of PR on quality of life (QoL), exercise capacity, and dyspnea is well established, less evidence exists on the impact on time spent in moderate daily physical activity, although some studies have focused on daily steps and walking speed.Citation22,Citation30–Citation37
Limitations
There are several limitations in this study, which needs consideration. First, the present study is a pilot study and thus vastly underpowered to draw solid conclusion on statistically insignificant findings. However, this observational study included COPD patients admitted daily for PR and was not preselected according to self-reported or measured levels of physical activity. Second, PR was delivered at four sites with different duration despite involving mandatory elements of training and education twice a week according to the Danish guidelines.Citation23 Our data sample was too small to adjust for this heterogeneity. Yet, the recommended duration of PR varies among guidelines (4–12 weeks American Association of Cardiovascular and PR; ≥8 weeks ATS/ERS; not specified by ASCM), whereas these guidelines agree on sessions at least three times weekly.Citation38 Thus, we might have observed a smaller impact of rehabilitation by following the Danish guidelines. It will be interesting to ascertain SPI prevalence as well as impact of rehabilitation in larger centers with more prevalent sessions.
Third, the accelerometer used (SenseWear) has – despite being well validated in COPDCitation39 – several limitations. It is worn on the upper right arm and is not usable in water. Thus, measurements of energy expenditure during swimming or activities with fixed arms (eg, cycling, walking aids) are absent or underestimated. In our cohort, a minority used a walker. Furthermore, to ensure representative activity data, we excluded data if SenseWear was worn for <48 hours or if on-body time was <90%. In addition to the challenge of recollecting physical activity, Pitta et alCitation40 reported adherence difficulties on using an accelerometer in 19% of COPD subjects including body placement and technical issues, such as battery problems. In our study, the missing SenseWear data were 14% both due to exclusion criteria as mentioned above and complete lack of obtained data. Little is known about the day-to-day variations in objectively measured physical activity in moderate to severe COPD patients, but it is considerable in healthy adults.Citation41 It has been documented that PALs increase during the week wearing an accelerometer, probably due to the novelty-induced increased awareness of healthy living.Citation41,Citation42 Fourth, we did not adjust for comorbidities such as osteoporosis, low back pain, diabetes, poor nutrition, cognitive dysfunction, heart disease, rheumatoid diseases, anxiety, or depression, which are all independently associated with decreased physical activity.Citation38,Citation40,Citation43–Citation46 Even though comorbidities are present in 70% of COPD patients and known to interfere with the outcome of PR, an improvement is still seen.Citation47,Citation48 However, the impact of exercise training provided during rehabilitation is not COPD specific, and all patients volunteered to participate in the rehabilitation program and the study. This selection probably selected the patients most eager to improve their levels of physical activity.
Conclusion
Our present pilot study suggests that objectively measured SPI is common in patients referred for PR, which only improves daily physical activity in a minority of subjects despite wearing an accelerometer in a clinical trial. SPI at baseline was not precluded by being moderately physically active for ≥150 minutes/week. As SPI is significantly associated with morbidity and mortality, future studies should address SPI-focused intervention strategies and elucidate factors associated with failure to improve physical activity in COPD patients receiving PR.
Acknowledgments
The study was supported by the Research Unit at Naestved Hospital and through a one-year scholarship from Southern University of Denmark. Financial contributions to equipment and laboratory testing were provided by Maersk Foundation, Meyers scholarship, and the local Research Foundation at Naestved Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
- Van RemoortelHHornikxMDemeyerHDaily physical activity in subjects with newly diagnosed COPDThorax2013681096296323604460
- Cindy NgLWMackneyJJenkinsSHillKDoes exercise training change physical activity in people with COPD? A systematic review and meta-analysisChron Respir Dis201291172622194629
- ManiniTMEverhartJEPatelKVDaily activity energy expenditure and mortality among older adultsJAMA2006296217117916835422
- WatzHWaschkiBMeyerTMagnussenHPhysical activity in patients with COPDEur Respir J200933226227219010994
- WatzHWaschkiBBoehmeCClaussenMMeyerTMagnussenHExtrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional studyAm J Respir Crit Care Med2008177774375118048807
- Garcia-AymerichJLangePBenetMSchnohrPAntóJMRegular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort studyThorax200661977277816738033
- WaschkiBKirstenAHolzOPhysical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort studyChest2011140233134221273294
- Gimeno-SantosEFreiASteurer-SteyCDeterminants and outcomes of physical activity in patients with COPD: a systematic reviewThorax201469873173924558112
- RaoZYWuXTWangMYHuWComparison between measured and predicted resting energy expenditure in mechanically ventilated patients with COPDAsia Pac J Clin Nutr201221333834622705422
- HaskellWLLeeIMPateRRPhysical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart AssociationMed Sci Sports Exerc20073981423143417762377
- WHO [homepage on the Internet]Physical Activity and Older Adults Recommended levels of physical activity for adults aged 65 and above2018 Available from: http://www.who.int/dietphysicalactivity/fact-sheet_olderadults/en/Accessed September 26, 2018
- GOLDGOLD 2017 Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease2017 Available from: https://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/Accessed October 4, 2018
- Garcia-AymerichJLangePBenetMSchnohrPAntóJMRegular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort studyAm J Respir Crit Care Med2007175545846317158282
- KandaMMinakataYMatsunagaKValidation of the triaxial accelerometer for the evaluation of physical activity in Japanese patients with COPDIntern Med201251436937522333371
- BahadoriKFitzgeraldJMRisk factors of hospitalization and readmission of patients with COPD exacerbation – systematic reviewInt J Chron Obstruct Pulmon Dis20072324125118229562
- PittaFTroostersTSpruitMAProbstVSDecramerMGosselinkRCharacteristics of physical activities in daily life in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2005171997297715665324
- TroostersTSciurbaFBattagliaSPhysical inactivity in patients with COPD, a controlled multi-center pilot-studyRespir Med201010471005101120167463
- VorrinkSNKortHSTroostersTLammersJWLevel of daily physical activity in individuals with COPD compared with healthy controlsRespir Res2011123321426563
- HartmanJEBoezenHMZuidemaMJde GreefMHTen HackenNHPhysical activity recommendations in patients with chronic obstructive pulmonary diseaseRespiration20148829210024851826
- LeeYJHungWLThe relationship between exercise participation and well-being of the retired elderlyAging Ment Health201115787388121547748
- SparlingPBHowardBJDunstanDWOwenNRecommendations for physical activity in older adultsBMJ2015350h10025608694
- CelliBRDecramerMWedzichaJAAn Official American Thoracic Society/European Respiratory Society Statement: Research questions in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20151917e4e2725830527
- SundhedsstyrelsenNational klinisk retningslinje for rehabilitering af patienter med KOL; 2014 Available from: https://www.sst.dk/da/udgivelser/2018/nkr-rehabilitering-af-patienter-med-kolAccessed October 4, 2018 Danish
- MillerMRHankinsonJBrusascoVStandardisation of spirometryEur Respir J200526231933816055882
- DemeyerHBurtinCvan RemoortelHStandardizing the analysis of physical activity in patients with COPD following a pulmonary rehabilitation programChest2014146231832724603844
- GuptaNPintoLMMoroganABourbeauJThe COPD assessment test: a systematic reviewEur Respir J201444487388424993906
- ThyregodMBodtgerUCoherence between self-reported and objectively measured physical activity in patients with chronic obstructive lung disease: a systematic reviewInt J Chron Obstruct Pulmon Dis2016112931293827932873
- KaoCCHsuJWBandiVHananiaNAKheradmandFJahoorFResting energy expenditure and protein turnover are increased in patients with severe chronic obstructive pulmonary diseaseMetabolism201160101449145521550084
- BarreiroEGeaJMolecular and biological pathways of skeletal muscle dysfunction in chronic obstructive pulmonary diseaseChron Respir Dis201613329731127056059
- DemeyerHBurtinCHornikxMThe Minimal Important Difference in Physical Activity in Patients with COPDPLoS One2016114e015458727124297
- SaundersTCampbellNJasonTObjectively Measured Steps/ Day in Patients With Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-AnalysisJ Phys Act Health201613111275128327334811
- SewellLSinghSJWilliamsJECollierRMorganMDHow long should outpatient pulmonary rehabilitation be? A randomised controlled trial of 4 weeks versus 7 weeksThorax200661976777116449270
- CoronadoMJanssensJPde MuraltBTerrierPSchutzYFittingJWWalking activity measured by accelerometry during respiratory rehabilitationJ Cardiopulm Rehabil200323535736414512781
- DallasMIMcCuskerCHaggertyMCRochesterCLZuwallackRNortheast Pulmonary Rehabilitation C. Using pedometers to monitor walking activity in outcome assessment for pulmonary rehabilitationChron Respir Dis2009621722419858351
- PittaFTroostersTProbstVSLangerDDecramerMGosselinkRAre patients with COPD more active after pulmonary rehabilitation?Chest2008134227328018403667
- EganCDeeringBMBlakeCShort term and long term effects of pulmonary rehabilitation on physical activity in COPDRespir Med2012106121671167923063203
- MccarthyBCaseyDDevaneDMurphyKMurphyELacasseYPulmonary rehabilitation for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20152CD003793
- GarveyCBaylesMPHammLFPulmonary Rehabilitation Exercise Prescription in Chronic Obstructive Pulmonary Disease: Review of Selected Guidelines: AN OFFICIAL STATEMENT FROM THE AMERICAN ASSOCIATION OF CARDIOVASCULAR AND PULMONARY REHABILITATIONJ Cardiopulm Rehabil Prev2016362758326906147
- GoreSBlackwoodJGuyetteMAlsalaheenBValidity and Reliability of Accelerometers in Patients With COPDJ Cardiopulm Rehabil Prev201838314715829120966
- PittaFTroostersTProbstVSSpruitMADecramerMGosselinkRQuantifying physical activity in daily life with questionnaires and motion sensors in COPDEur Respir J20062751040105516707399
- PedersenESDanquahIHPetersenCBTolstrupJSIntra-individual variability in day-to-day and month-to-month measurements of physical activity and sedentary behaviour at work and in leisure-time among Danish adultsBMC Public Health2016161122227914468
- VoigtLBaumannSUllrichAWeymarFJohnUUlbrichtSThe effect of mere measurement from a cardiovascular examination program on physical activity and sedentary time in an adult populationBMC Sports Sci Med Rehabil2018101129410786
- No authors listedAmerican College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adultsMed Sci Sports Exerc19983069759919624661
- PittaFTroostersTSpruitMADecramerMGosselinkRActivity monitoring for assessment of physical activities in daily life in patients with chronic obstructive pulmonary diseaseArch Phys Med Rehabil200586101979198516213242
- SteeleBGHoltLBelzaBFerrisSLakshminaryanSBuchnerDMQuantitating physical activity in COPD using a triaxial accelerometerChest200011751359136710807823
- BrownJPMartinezCHChronic obstructive pulmonary disease comorbiditiesCurr Opin Pulm Med201622211311826814720
- HornikxMVan RemoortelHDemeyerHThe influence of comorbidities on outcomes of pulmonary rehabilitation programs in patients with COPD: a systematic reviewBiomed Res Int201314614824490146
- MesquitaRVanfleterenLEFranssenFMObjectively identified comorbidities in COPD: impact on pulmonary rehabilitation outcomesEur Respir J201546254554826113670