Abstract
Background
Endocan is thought to be a novel inflammatory marker that is associated with a variety of inflammatory diseases. However, its role in the pathogenesis of COPD remains unclear. This study aims to explore the potential role of endocan in COPD.
Methods
In total, 27 healthy volunteers, 55 COPD patients and 36 acute exacerbation of chronic obstructive pulmonary disease (AECOPD) patients were included in the study. Basic demographic characteristics, clinical features and blood samples were collected. Magnetic luminex screening assays were used to detect the concentration of endocan, Fas and Fas ligand (Fas-L) in plasma. Differences between groups were compared using an Independent sample t-test, Welch’s t-test, chi-squared test and Wilcoxon rank sum test. The correlations of plasma endocan with lung function parameters, Fas and Fas-L were analyzed by Pearson’s partial correlation test (adjusted for age, gender, body mass index and smoking history) and multiple linear regression.
Results
Plasma endocan levels in COPD patients were significantly higher than those in healthy volunteers (509.7±18.25 pg/mL vs 434.8±18.98 pg/mL (P=0.0124)), and AECOPD patients had the highest levels of endocan (524.7±27.18 pg/mL). Correlation analysis showed that circulating endocan had a negative correlation to FEV1/FVC, FEV1/predictive and FVC (adjusted r=−0.213, P=0.03; adjusted r=−0.209, P=0.034; and adjusted r=−0.300, P=0.002, respectively), and had a positive correlation to Fas (adjusted r=0.280, P=0.004).
Conclusion
Our study shows that elevated circulating endocan levels are associated with reduced lung ventilation function in COPD and AECOPD patients. In addition, endocan may influence apoptosis in COPD, suggesting that endocan may play a role in COPD pathogenesis.
Keywords:
Introduction
COPD, a chronic disease that is characterized by airflow limitation and affects the airway and lung parenchyma, is a global disease closely related to smoking and is expected to become the fifth leading cause of disability.Citation1,Citation2 Acute exacerbation of COPD is very common and seriously affects quality of life and is the main cause of death in COPD patients.Citation3,Citation4 Pulmonary vascular endothelial cell damage and vascular inflammation are involved in the pathogenesis of COPD. Pulmonary inflammation can also spread to the systemic circulation and can increase the occurrence of cardiovascular events.Citation5,Citation6 Endothelial cells play an important role in acute inflammation related to COPD exacerbation.Citation7–Citation9 However, it is not yet clear what role endothelial cells play in COPD and what factors are involved.
Endocan, formerly known as endothelial cell-specific molecule 1 (ESM-1), is an endothelial-associated proteoglycan that is secreted mainly by activated endothelial cells. It is preferentially expressed in vascular endothelial cells in the lung and kidney, and its overexpression can be seen in some neonatal and proliferating tissues as well as some tumors.Citation10,Citation11
Recent studies have shown that endocan is associated with endothelial dysfunction and regulates inflammation in patients with inflammatory diseases, such as sepsis and chronic kidney disease.Citation12,Citation13 Thus, endocan is considered as a novel inflammation-related factor.Citation14,Citation15 The interaction of endocan with lymphocyte function-associated antigen-1 inhibits leukocytes from binding to the vascular endothelium.Citation10,Citation16 Multiple cytokines, including TNF-A, interleukin-1, microbial lipopolysaccharide and angiogenic factors can upregulate endocan expression, while interferon-γ seems to inhibit it.Citation17,Citation18 Endocan can relieve the apoptosis of lung epithelial cell in acute lung injury (ALI) by releasing over-activated mitochondrial unfolded protein response; endocan also promotes the differentiation and apoptosis of gastric adenocarcinoma cell by activating caspase- and p53-dependent apoptotic pathway.Citation19,Citation20
Therefore, we proposed that endocan may participate in the inflammatory activity and abnormal apoptosis of COPD. In this study, we aimed to evaluate the plasma levels of endocan in healthy subjects and patients with COPD, and to compare the correlation between endocan and changes in lung function and apoptosis.
Methods
Inclusion and exclusion criteria
The research protocol complied with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the West China Hospital of Sichuan University of the People’s Republic of China. All subjects provided written informed consent. From January to December 2016, the healthy volunteers, COPD patients and those with exacerbation of COPD were recruited at the hospital outpatient and inpatient departments of West China Hospital of Sichuan University. All patients were previously diagnosed with COPD based on GOLD documents. All subjects were asked about their medical history in detail, and chest CTs from the most recent year were reviewed. Exclusion criteria were as follows: 1) suffering from diseases other than COPD or acute exacerbation of chronic obstructive pulmonary disease (AECOPD), 2) unable or unwilling to cooperate with a doctor, 3) unable or unwilling to perform lung function tests, and 4) suffering from pulmonary diseases (acute respiratory distress syndrome [ARDS], asthma and lung cancer) or other diseases known to affect plasma endocan (including tumors, atherosclerosis and chronic kidney disease).
Collection of demographic and clinical characteristics
Basic information such as name, gender, ethnicity, age and smoking history was collected for all subjects. All the subjects underwent lung function test, administered by professionals, in the pulmonary function room, and the function data were collected. To avoid bias, the researchers who conducted spirometry testing did not know the patient’s enrollment beforehand.
Measurement of endocan, Fas and Fas ligand
Blood samples were collected from subjects the morning after enrollment. All subjects fasted from 9:00 pm the night before. Venous blood was collected from the median cubital vein and plasma was separated and stored immediately at −80°C until analysis. The endocan, Fas and Fas ligand (Fas-L) levels in the plasma were measured using the Magnetic Luminex Screening Assay (R&D Systems, Minneapolis, MN, USA) on a Bio-Plex 200 (Bio-Rad, Hercules, CA, USA) in the Division of Laboratory of West China Hospital. The detection sensitivity of endocan, Fas and Fas-L were 1.08 pg/mL, 3.23 pg/mL and 1.23 pg/mL, respectively.
Statistical analysis
All data were analyzed using SPSS 22.0 for Windows (IBM, Chicago, IL, USA) and figures were created using GraphPad Prism 6.01 for Windows (GraphPad Software Inc, La Jolla, CA, USA). For quantitative data, Shapiro–Wilk tests were used to determine whether they obey the normal distribution. Differences between groups were analyzed by independent sample t-test, t’ test, chi-squared test or Wilcoxon rank sum test. The correlations between plasma biomarkers and lung function parameters were analyzed using the Pearson’s partial correlation test to correct for age, gender, body mass index (BMI) and smoking history. Multiple linear regression analysis was conducted to confirm the aforementioned relationships. All data are presented as mean ± SD, and P-values <0.05 were considered statistically significant.
Results
Subject characteristics
In total, 118 subjects including 27 healthy controls, 55 COPD patients and 36 AECOPD patients with available lung function parameters and endocan levels were enrolled in the study. Among the 27 healthy controls, 55 COPD patients and 36 AECOPD patients, 8, 43 and 20 had a history of smoking, respectively. We applied the Global Initiative for Chronic Obstructive Lung Disease (GOLD) to grade airflow limitation in patients. In total, 19 subjects were classified as GOLD 1, 40 as GOLD 2, 23 as GOLD 3 and 9 as GOLD 4. There were no statistical differences in gender, BMI or number of current smokers and previous smokers between the three groups; however, healthy controls were younger than the COPD and AECOPD groups (P<0.05). Compared with healthy controls, FVC, FEV1, FEV1/Predictive FEV1 (FEV1/Pre) and FEV1/FVC were significantly lower in COPD and AECOPD patients (P<0.05). shows the characteristics of these three groups.
Table 1 Characteristics of healthy volunteers and patients
Increased plasma endocan levels in patients with COPD and AECOPD
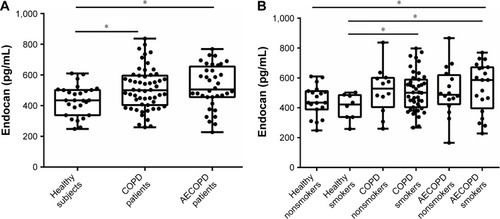
Our analyses showed that endocan levels in COPD patients (509.7±18.25 pg/mL) and AECOPD patients (524.7±27.18 pg/mL) were higher than those in healthy controls (434.8±18.98 pg/mL) (P=0.0124 and P=0.0139, respectively, ). No statistical difference was found between COPD patients and AECOPD patients. The average endocan level was 447.0±23.58 pg/mL (n=19) for healthy nonsmokers, 405.8±30.82 pg/mL (n=8) for healthy smokers, 517.2±45.03 pg/mL (n=12) for COPD nonsmokers, 507.6±19.97 pg/mL (n=43) for COPD smokers, 509.1±40.78 pg/mL (n=16) for AECOPD nonsmokers and 537.2±37.16 pg/mL (n=20) for AECOPD smokers. Endocan levels in COPD and AECOPD smokers were higher than those in healthy smokers (P=0.0405 and P=0.0449, respectively, ). AECOPD smokers had significantly elevated endocan levels when compared with healthy nonsmokers (P=0.049, ).
Figure 1 Plasma endocan levels in healthy, COPD and AECOPD groups.
Abbreviation: AECOPD, acute exacerbation of chronic obstructive pulmonary disease.
Correlations between endocan and pulmonary function parameters, Fas and Fas-L
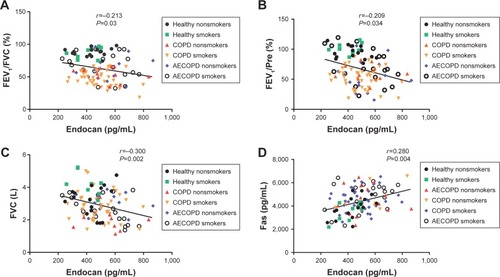
After adjusting for age, gender, BMI and smoking history, we found that plasma endocan in all healthy people and COPD patients was negatively correlated with FEV1/FVC (adjusted r=−0.251, P=0.036, ), FEV1/Pre (adjusted r=−0.244, P=0.042, ) and FVC (adjusted r=−0.263, P=0.028, ). We also found that endocan levels were positively correlated with Fas (adjusted r=0.318, P=0.007, ); however, there was no obvious correlation between endocan and Fas-L (adjusted r=0.101, P=0.404). In addition, endocan in all subjects was correlated with FEV1/FVC (adjusted r=−0.213, P=0.03, ), FEV1/Pre (adjusted r=−0.209, P=0.034, ), FVC (adjusted r=−0.300, P=0.002, ) and Fas (adjusted r=0.280, P=0.04, ), and the correlation between endocan with Fas-L was not found (adjusted r=0.146, P=0.140).
Figure 2 Correlations between plasma levels of endocan with FEV1/FVC, FEV1/Pre, FVC and Fas in healthy and COPD groups.
Notes: After adjusting for age, gender, BMI and smoking history, endocan was negatively correlated with FEV1/FVC (adjusted r=−0.251, P=0.036) (A); FEV1/Pre (adjusted r=−0.244, P=0.042) (B); FVC (adjusted r=−0.263, P=0.028) (C); and positively correlated with Fas (adjusted r=0.318, P=0.007) (D).
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; BMI, body mass index; pre, predicted.
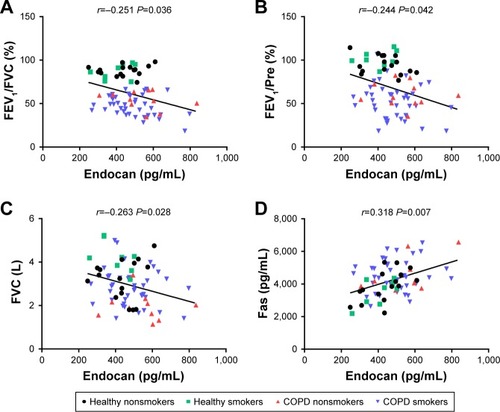
Figure 3 Correlations between plasma levels of endocan with FEV1/FVC, FEV1/Pre, FVC and Fas in healthy, COPD and AECOPD groups.
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; BMI, body mass index; pre, predicted.
Multiple linear regression analysis
Multiple linear regression analysis showed that age, BMI, gender, smoking history, FEV1/FVC, Fas and Fas-L were the independent parameters associated with endocan (), and age, FEV1/FVC, Fas and Fas-L were statistically significant in this model (P<0.05).
Table 2 Multiple linear regression analysis for circulating endocan levels
Discussion
This study was the first to explore the role of endocan in COPD. We found that endocan levels in COPD and AECOPD patients were significantly higher than those in healthy controls. Endocan level was negatively correlated with FEV1/FVC, FEV1/Pre and FVC, and was positively correlated with Fas, suggesting that endocan may be a useful biomarker for COPD.
We found that the endocan levels of COPD patients were significantly higher than those of healthy controls. While changes in pulmonary vascular structure and function are involved in the pathophysiological changes of COPD,Citation21 the specific mechanism underlying the changes remains unclear. Endocan is a soluble dermatan sulfate proteoglycan produced by activation of the endothelium. It regulates cell proliferation, differentiation, migration and adhesion through various means and is a novel inflammatory marker.Citation18 Endocan can influence the development of ALI and ARDS and may play an important role in predicting the prognosis of ARDS patients.Citation22–Citation24 Our work shows that endocan may also play a role in the inflammatory response of COPD.
In addition, our results show that endocan levels are negatively correlated with FEV1/FVC, FEV1/Pre and FVC. Endocan is mainly expressed in the pulmonary microcirculation where it plays an important role in the maintenance of endothelial homeostasis and is closely related to inflammation.Citation17 Elevation of COPD airway inflammatory factors may be associated with endothelial dysfunction, and endothelial damage is proportional to the severity of bronchial obstruction.Citation32,Citation33 Our study demonstrates that endocan links airway obstruction with endothelial system changes. Thus, endocan can be used as a simple indicator of FEV1/FVC, FEV1/Pre and FVC in patients with COPD or AECOPD, especially under circumstances in which lung function tests cannot be performed.
Endocan was also positively correlated with Fas. Fas is a type I transmembrane glycoprotein belonging to the tumor necrosis factor receptor and the nerve growth factor receptor superfamily. When Fas binds to the Fas-L, it recruits several adapter proteins and forms the death-inducing signaling complex, eventually activating caspase-3, 6, 7 and inducing apoptosis.Citation20,Citation34,Citation35 Previous studies have shown that endocan can be involved in cell apoptosis through mitochondrial unfolded protein response and caspase- and p53-dependent apoptotic pathway.Citation19,Citation20 Abnormal structural cell apoptosis in COPD leads to destruction of terminal airways, alveolar walls and pulmonary capillary beds, and is an important mechanism involved in the development of COPD.Citation36 Therefore, we speculated that endocan may be involved in abnormal cell apoptosis in COPD, with abnormal apoptosis leading to damage and remodeling of the lung structure, which in turn affects ventilatory function in COPD.
There are several limitations to this study to be noted. First, because of the strict inclusion and exclusion criteria, we recruited only 118 subjects. The limited number of patients may affect the P-value of the correlation analysis and may result in false-negative correlations between endocan and other pulmonary function parameters. Endocan levels in AECOPD patients were higher than those in COPD patients; however, there was no statistical significance. Perhaps, there will be more discoveries as we expand the sample size. Second, the subjects included in the healthy control group were younger than those in COPD and AECOPD groups. However, we adjusted for age during the analysis and there are no studies showing that age has an important relationship with endocan expression. Ultimately, our study only shows that endocan may be related to COPD, but the specific mechanism remains unknown. Future studies should expand the sample size and focus on the mechanism by which endocan affects COPD.
Conclusion
Overall, our findings indicate that endocan may be associated with a decrease in ventilatory function in COPD and may be related to apoptosis of structural pulmonary cells, revealing that endocan may play an important role in the pathogenesis of COPD. This work provides new insights into COPD pathogenesis.
Author contributions
Luqi Dai and Junyun He designed this research, and all the authors contributed toward subject recruitment, data collection, statistical analysis, and drafting and critically revising the manuscript and all authors gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by grants from the National Key Research and Development Program in China (numbers 2016YFC1303600 and 2016YFC1304500), the National Natural Science Foundation of China (numbers 81470236 and 81670038), and the Sichuan Science and Technology Support Program (number 2015SZ0151). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- BarnesPJInflammatory mechanisms in patients with chronic obstructive pulmonary diseaseJ Allergy Clin Immunol20161381162727373322
- McDonoughJEYuanRSuzukiMSmall-airway obstruction and emphysema in chronic obstructive pulmonary diseaseN Engl J Med2011365171567157522029978
- HillasGPerlikosFTzanakisNAcute exacerbation of COPD: is it the “stroke of the lungs”?Int J Chron Obstruct Pulmon Dis2016111579158627471380
- WedzichaJADonaldsonGCExacerbations of chronic obstructive pulmonary diseaseRespir Care200348121204121314651761
- ManSFvan EedenSSinDDVascular risk in chronic obstructive pulmonary disease: role of inflammation and other mediatorsCan J Cardiol201228665366122902150
- SampsonasFAntonacopoulouASpathasDPositive association between two polymorphic sites (+134 insA/delA and G198T) of the endothelin-1 gene and chronic obstructive pulmonary disease. A case-control studyRespir Med2010104111412019640695
- PolosaRMalerbaMCacciolaRREffect of acute exacerbations on circulating endothelial, clotting and fibrinolytic markers in COPD patientsIntern Emerg Med20138756757421660493
- PolatliMCakirACildagOBolamanAZYeniseyCYeniceriogluYMicroalbuminuria, von Willebrand factor and fibrinogen levels as markers of the severity in COPD exacerbationJ Thromb Thrombolysis20082629710217622488
- RolandMBhowmikASapsfordRJSputum and plasma endothelin-1 levels in exacerbations of chronic obstructive pulmonary diseaseThorax2001561303511120901
- DeleheddeMDevenynsLMaurageCAVivèsRREndocan in cancers: a lesson from a circulating dermatan sulfate proteoglycanInt J Cell Biol2013201370502723606845
- KechagiaMPapassotiriouIGourgoulianisKIEndocan and the respiratory system: a reviewInt J Chron Obstruct Pulmon Dis2016113179318728003744
- PaulyDHamedSBehnesMEndothelial cell-specific molecule-1/endocan: Diagnostic and prognostic value in patients suffering from severe sepsis and septic shockJ Crit Care2016311687526489483
- YilmazMISiriopolDSaglamMPlasma endocan levels associate with inflammation, vascular abnormalities, cardiovascular events, and survival in chronic kidney diseaseKidney Int20148661213122024988065
- CoxLAvan EijkLTRamakersBPInflammation-induced increases in plasma endocan levels are associated with endothelial dysfunction in humans in vivoShock201543432232625565643
- LeeWKuSKKimSWBaeJSEndocan elicits severe vascular inflammatory responses in vitro and in vivoJ Cell Physiol2014229562063024446198
- SarrazinSLyonMDeakinJACharacterization and binding activity of the chondroitin/dermatan sulfate chain from Endocan, a soluble endothelial proteoglycanGlycobiology201020111380138820581009
- LassallePMoletSJaninAESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokinesJ Biol Chem19962713420458204648702785
- KaliAShettyKSEndocan: a novel circulating proteoglycanIndian J Pharmacol201446657958325538326
- ZhangXZhuangRWuHA novel role of endocan in alleviating LPS-induced acute lung injuryLife Sci2018202899729627442
- SumeiZShaolongCXiangWYinliangQQingZYuanWEndocan reduces the malign grade of gastric cancer cells by regulating associated protein expressionTumour Biol20163711149151492127644250
- PeinadoVIPizarroSBarberàJAPulmonary vascular involvement in COPDChest2008134480881418842913
- MikkelsenMEShahCVScherpereelALower serum endocan levels are associated with the development of acute lung injury after major traumaJ Crit Care2012275522.e11522.e17
- TangLZhaoYWangDEndocan levels in peripheral blood predict outcomes of acute respiratory distress syndromeMediators Inflamm2014201462518062518925132734
- ZhangXZhuangRWuHA novel role of endocan in alleviating LPS-induced acute lung injuryLife Sci2018202899729627442
- BokovPDelclauxCInterpretation and use of routine pulmonary function tests: Spirometry, static lung volumes, lung diffusion, arterial blood gas, methacholine challenge test and 6-minute walk testRev Med Interne201637210011026657268
- de TorresJPCasanovaCMontejo de GarciniAAguirre-JaimeACelliBRGender and respiratory factors associated with dyspnea in chronic obstructive pulmonary diseaseRespir Res200781817341300
- O’DonnellDEWebbKABreathlessness in patients with severe chronic airflow limitation. Physiologic correlationsChest199210238248311516410
- MahutBChevalier-BidaudBPlantierLDiffusing capacity for carbon monoxide is linked to ventilatory demand in patients with chronic obstructive pulmonary diseaseCOPD201291162122292594
- ZavorskyGSvan der LeeICan the measurement of pulmonary diffusing capacity for nitric oxide replace the measurement of pulmonary diffusing capacity for carbon monoxide?Respir Physiol Neurobiol201724191627884796
- BaltaSMikhailidisDPDemirkolSOzturkCCelikTIyisovAEndocan: A novel inflammatory indicator in cardiovascular disease?Atherosclerosis2015243133934326448266
- SarrazinSAdamELyonMEndocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapyBiochim Biophys Acta200617651253716168566
- MoroLPedoneCScarlataSMalafarinaVFimognariFAntonelli-IncalziREndothelial dysfunction in chronic obstructive pulmonary diseaseAngiology200859335736418388072
- HashimotoMTanakaHAbeSQuantitative analysis of bronchial wall vascularity in the medium and small airways of patients with asthma and COPDChest2005127396597215764783
- StonemanVEBennettMRRole of Fas/Fas-L in vascular cell apoptosisJ Cardiovasc Pharmacol200953210010819188840
- YoneharaSIshiiAYoneharaMA cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factorJ Exp Med19891695174717562469768
- HoughtonAMMechanistic links between COPD and lung cancerNat Rev Cancer201313423324523467302
