Abstract
COPD is characterized by chronic bronchitis, chronic airway obstruction, and emphysema, leading to a progressive and irreversible decline in lung function. Inflammation is central for the development of COPD. Chronic inflammation in COPD mainly involves the infiltration of neutrophils, macrophages, lymphocytes, and other inflammatory cells into the small airways. The contribution of resident airway structural cells to the inflammatory process is also important in COPD. Airway remodeling consists of detrimental changes in structural tissues and cells including airway wall thickening, epithelial metaplasia, goblet cell hypertrophy, and smooth muscle hyperplasia. Persistent airway inflammation might contribute to airway remodeling and small airway obstruction. However, the underlying mechanisms remain unclear. In this review, we will provide an overview of recent insights into the role of major immunoinflammatory cells in COPD airway remodeling.
Keywords:
Introduction
COPD is characterized by chronic bronchitis, chronic airway obstruction, airway remodeling, and emphysema, leading to a progressive and irreversible decline in lung function.Citation1 Inflammation is central for COPD development and the release of inflammatory mediators and destructive enzymes by inflammatory cells particularly infiltrating immune cells, which is implicated in the progressive destruction of the lung in COPD.Citation2,Citation3 However, the role of resident structural cells in this process should not be discounted.
Remodeling has been described in central airways, distal airways, and lung parenchyma. It is a process of structural changes involving hyperplasia of airway epithelial cells, thickening of the reticular basement membrane (RBM), deposition of collagen, peribronchial fibrosis, airway epithelial-to-mesenchymal transition, and bronchial smooth muscle cell hyperplasia.Citation4 In COPD, remodeling of the parenchyma contributes to emphysema, while small airway remodeling largely results in airway obstruction. These changes cause the airflow limitation seen in COPD patients. However, the underlying mechanisms remain unclear.
The chronic inflammation in COPD involves the infiltration of the major inflammatory cells including neutrophils, monocytes/macrophages, and lymphocytes into the airway and lung tissue, and these can be detected in bronchoalveolar fluid and induced sputum.Citation5 It is generally acknowledged that persistent chronic inflammation may contribute to not only bronchial remodeling but also parenchyma remodeling to some extent.Citation6,Citation7 In this review, we will highlight the recent studies that have provided additional insight into the role of these major inflammatory cells in COPD airway remodeling.
Neutrophils
Neutrophils are key inflammatory cells in the pathogenesis of COPD, with sputum and blood neutrophilia being a characteristic feature of all COPD patients. They have also been reported as a marker of COPD severity.Citation8,Citation9 An observational study found that patients with higher sputum neutrophil percentages had a higher dyspnea score across different severities of COPD.Citation10
Neutrophils are recruited to the airways of COPD patients and secrete several serine proteases including neutrophil elastase (NE), matrix metalloproteinase (MMP), as well as myeloperoxidase (MPO) all of which contribute to alveolar destruction.Citation11,Citation12 In addition, some neutrophil-derived chemokines such as IL-1 and CXCL8/IL-8 are proven to be involved in tissue injury and remodeling in a mouse model.Citation13
MMPs are a family of zinc-dependent proteases that can be secreted by stromal cells, neutrophils, and macrophages. They are commonly classified according to the substrates they degrade. The majority of MMPs implicated in emphysema pathogenesis include the collagenase MMP-1, the gelatinase MMP-9, and the metalloelastase MMP-12.Citation14 Among those, the gelatinase MMP-9 is synthesized by mature neutrophils and is mainly stored in intracellular granules of neutrophils and is secreted extracellular after activation.Citation15
MMP-9 activity is countered by the tissue inhibitors of metalloproteinases, and any changes in the activity of this enzyme will alter this balance.Citation13 Most studies have shown increased MMPs in bronchoalveolar lavage fluid (BALF) and plasma of emphysema patients and contribute to airway obstruction by destroying the structural components of extracellular matrix (ECM).Citation16,Citation17 Moreover, as MMP-9 is a known target of Wnt/β-catenin signaling, it has been proved to be induced by transforming growth factor-β (TGF-β) + poly(I:C) treatment through the β-catenin pathway.Citation18 In animal models of COPD, it demonstrated that dominant-negative MafB suppressed porcine pancreatic elastase-induced emphysema by downregulating MMPs.Citation19 Considering the significant role of MMP-9 in the above studies, it may be worthwhile exploring its role in the function of different primary cells from patients with disease.
NE is a neutrophil-derived serine proteinase that has proven to be involved in tissue damage and remodeling,Citation20 and further a study found that mice deficiency in NE resulted in the protection of mice against emphysema after cigarette smoke (CS) exposure.Citation21 The underlying mechanism(s) may largely depend on the fact that NE has a similar ability as MMPs in causing tissue damage by degrading the structural components of ECM.Citation22 Moreover, NE can cooperate with MMPs and amplify the effect of ECM degradation.Citation23 In addition to matrix degradation, NE can also promote peri-bronchial fibrosis by enhancing fibroblast proliferation.Citation24 Moreover, NE is a potent stimulant of mucus secretion from submucosal glands and goblet cells, which are involved in airway obstruction.Citation25 The combined effect of NE on matrix degradation, fibroblast proliferation, and mucus metaplasia might accelerate small airway obstruction in disease.
MPO is a product of both neutrophils and macrophages and mainly stored in the primary granules of neutrophils. It is an inflammatory mediator that is upregulated during the inflammatory response and can also accelerate the inflammatory response.Citation26 3-Chlorotyrosine expression is strongly associated with MPO activity in the sputum of COPD patients, suggesting that it might act as a biomarker for MPO-mediated tissue damage in COPD pathogenesis.Citation27 MPO inhibitors prevent the development of emphysema and remodeling of small airways in an animal model of COPD.Citation28 These studies indicated detrimental effects of MPO in the pathogenesis of airway remodeling. However, a study of myocardial infarction in MPO knockout mice observed increased expression of MMPs.Citation29 This finding suggests a possible protective role of MPO in airway remodeling.
Neutrophil extracellular traps (NETs) are released by activated neutrophils and decorated with histones and enzymes such as NE and MPO that ensnare bacteria; however, excessive formation of NETs may contribute to organ damage.Citation30 Large amounts of NETs were observed in the airways of COPD patients and associated with disease severity and exacerbation frequency.Citation31
The high-mobility group box 1 (HMGB1) is a protein released by necrosis of neutrophil cells that warn and activate inflammation. COPD patients express high HMGB1 levels both in sputum and in plasma.Citation32 HMGB1 has a marked effect on epithelial cell repair and restitution via activation of toll-like receptor 4 (TLR4) and/or receptor for advanced glycation end (RAGE) signaling, which may explain, in least in part, the mechanism of airway remodeling.Citation33
Macrophages
Macrophages are mononuclear leukocyte-derived inflammatory cells the numbers of which are increased in the airways, BALF, alveolar areas, and in induced sputum of COPD patients and correlated with the inflammatory response and alveolar wall destruction in COPD.Citation34 They produce a host of inflammatory mediators implicated in COPD such as IL-1β, tumor necrosis factor-α (TNF-α), IL-8, monocyte chemoattractant protein-1 (MCP-1), reactive oxygen species, and MMPs.Citation35
IL-8 is a member of the CXC chemokine family and is secreted by macrophages, epithelial cells, and even endothelial cells. It is a major inflammatory factor in the sputum and BALF in COPD patients. IL-8 can enhance the expression of MUC5AC directly or indirectly by inducing the secretion of NE from neutrophils, leading to mucin overproduction and airway obstruction.Citation36 And the inhibition of IL-8 by azithro-mycin in stable neutrophilic COPD adults showed a reduced severe exacerbation rate.Citation37 Further studies demonstrated that knockout of CXCR2, a receptor of IL-8, protected mice from cigarette smoke-induced lung inflammation and DNA damage in COPD pathogenesis.Citation38
IL-1β and TNF-α cytokines are proinflammatory cytokines that are mainly secreted by macrophages. Both IL-1β and TNF-α receptor knockout mice are protected from developing emphysema and small airway remodeling when compared with wild-type mice in response to CS.Citation39 However, in a randomized phase II study, MEDI8968, an anti-IL-1R1 antibody, did not protect COPD patients from lung function decline.Citation40 The different outcomes that attribute to the trials are based on measures of lung function, sputum inflammatory cells, or exacerbation rates, which may not reflect the remodeling effect of these drugs. In addition, persistent production of IL-1β upregulates the expression of neutrophilic cytokines and MMPs including MMP-9 and MMP-12 in mice and results in airway inflammation and alveolar enlargement.Citation41,Citation42 Alveolar and airway wall remodeling occurs in SPC-TNF-α mice with increased expression of elastin-degrading enzymes and consequent matrix remodeling.Citation43 This effect was attributed to TNF-α-induced stimulation of MMPs and NE and the activation of CD8+ T lymphocytes all of which contribute to the destruction of lung tissues.
Different TGF-β isoforms exist, including TGF-β1, TGF-β2, and TGF-β3. Among them, TGF-β1 is implicated in the progression of COPD pathogenesis.Citation44,Citation45 In relation to COPD, TGF-β induces the secretion of ECM, proliferation of smooth muscle cells, and transition of epithelial-to-mesenchymal phenotype.Citation46 Exposure to CS increases the production of TGF-β from epithelial cells and inflammatory cells, and this was linked to CS-induced lung injury and airway remodeling in COPD.Citation47 This might partially provide a mechanism for CS-induced small airway obstruction.
Macrophages are just as an important source of MMPs as neutrophils. Activation of macrophages in lungs plays a critical role in MMP production that contributes to alveolar wall destruction. Researchers found that the loss of tyrosine phosphatase 2 in mice, a macrophage activation regulator, resulted in TGF-β activation, thereby upregulating MMP-12 expression in macrophages, leading to progressive emphysema-like injury in the mice lungs.Citation48 Moreover, recently a study suggested that dehydration of airway surface can also activate macrophages to produce MMP-12 and trigger MMP-12-dependent emphysema independent of CS.Citation49 These findings might provide some clues to explore non-CS-induced emphysema.
MCP-1 is a monocyte chemokine that can be produced by macrophages, epithelial cells, smooth muscle cells, and even endothelial cells.Citation50 Although some studies showed that the protein level of MCP-1 was not correlated with the number of macrophages, intratracheal instillation of MCP-1 in macrophage elastase knockout mice resulted in an increased macrophage infiltration into the airway.Citation51 The ability of MCP-1 to accumulate macrophages might help to accelerate the process of airway obstruction.
Autophagy plays a critical role in the development of many inflammatory cells such as macrophages, neutrophils, and lymphocytes, which play critical roles in the development and pathogenesis of COPD inflammation.Citation52 In vivo studies in mice showed that miR-34/449 overexpression leads to decreased ovalbumin-induced airway remodeling by suppressing autophagy-related airway inflammation and fibrosis.Citation53
Overall, macrophages are major inflammatory cells in COPD lung. They are directly involved in the process of airway remodeling by secreting enzymes and inflammatory factors that act directly and indirectly on airway structural cells to modulate epithelial and stromal cell function.
Mast cells
Mast cells are multifunctional immune cells composed of two subsets: mucosal mast cell (MCT) and connective tissue mast cell (MCTC).Citation54 Mast cells have been implicated in asthma for many years where, in addition to the release of lipid mediators and other bronchoconstrictor agents, they promote airway remodeling.Citation55 However, they have been poorly studied in COPD.
Increased numbers of mast cells have been reported in COPD patients with centrilobular emphysema, where they are mainly distributed in the bronchial mucosa, parenchyma, and even smooth muscle.Citation56 The population of mast cells within the lung may also change with disease, with increased MCTC and decreased MCT reported in COPD. Moreover, the increased number of MCTC positively correlated with airway remodeling and poorer lung function.Citation57,Citation58 In addition, perivascular mast cell density is positively correlated with increased angiogenesis in the RBM of COPD airways where they are proposed to contribute to airway remodeling.Citation59,Citation60 Furthermore, IL-17A, which has been reported to be upregulated in COPD,Citation61 can stimulate mast cells to secrete the proangiogenic mediators, basic fibroblast growth factor and vascular endothelial growth factor (VEGF), which both drive vascular remodeling.Citation62
However, analysis of the distribution of tryptase and chymase staining in mast cells indicates that mast cells are positively correlated with lung function,Citation63 and this was particularly the case with chymase-positive mast cells.Citation64 Results from the latter research may be confounded by the presence of pulmonary hypertension. In addition, although mast cells appear to play a role in COPD, the molecular mechanism(s) by which they act are unknown. Mast cells are a rich source of inflammatory cytokines, proteases, VEGF, and mast cell-specific mediators such as histamine and cysteinyl leukotrienes.Citation65 Further investigations into the mechanisms of mast cells involved in the development of airway remodeling are required.
Lymphocytes
The adaptive immune system is activated in COPD, with infiltration of T-cells, B-cells, T-helper type 17 (Th17) cells along with a decrease in regulatory T-cells within the airways.Citation66,Citation67 Mice lacking either B-cells or T-cells fail to elicit airway remodeling illustrating the importance of the adaptive immune response in airway remodeling.Citation68
T-lymphocytes are increased in the lung parenchyma and airways of smokers when compared with never smokers whether they develop COPD or not. There is a greater increase in CD8+ cells compared with CD4+ cells.Citation69 The increased CD8+ cells in the peripheral airways of smokers with COPD have been related to smoking-induced airway limitation.Citation70,Citation71 In addition, T-cells can cause lung tissue destruction directly by T-cell-induced cytotoxicity or indirectly by activating macrophages.Citation72,Citation73 These data suggest that increased CD8+ cells in COPD act as a bridge between smoking and airway obstruction.
CD8+ cells can be divided into TC1 cells and TC2 cells according to the cytokines they secrete. Isolation of CD4+ and CD8+ cells from COPD BALF indicates that CD8+ TC2 cells, which mainly produce IL-4 and IL-5 cytokines, were significantly increased in COPD lungs and might promote tissue damage and the development of emphysema during exacerbations.Citation74
The number of B-cells within lymphoid follicles is greatly increased in advanced stages of COPD patients.Citation75 CXCL13 is a B-cell attractant or chemokine,Citation76 and the reduction of CXCL13 expression attenuated CS-induced BALF inflammatory cell numbers and partially protected alveolar walls from destruction but had no effect on the development of airway remodeling.Citation77
Th17 cells are the major source of the cytokine IL-17.Citation78 IL-17 can enhance airway smooth muscle contraction and proliferation, and Th17-deficient mice are protected from airway remodeling after chronic allergen challenge in an animal model of asthma.Citation79
Innate lymphoid cells
Innate lymphoid cells (ILCs) are a new class of immune cells that can be classified into three groups (ILC1, ILC2, and ILC3) according to their phenotype and function. ILCs are widely expressed in many tissues such as skin, mucosal membranes, and lung tissues.Citation80
Most studies examining ILC expression and function in airway disease have focused on asthma. ILC3s play a role in driving neutrophilic inflammation, and the number of natural cytotoxicity receptor (NCR−) expressing ILC3 cells was increased in COPD lung.Citation81 ILC3s can activate TGF-β, which is a key mediator for tissue and mucosal repair.Citation82 More recent evidence indicates the enhanced presence of primed NRP1+ ILC3s, which produce high amounts of cytokines in the lungs of smokers with and without COPD where they may play a role in angiogenesis and/or the initiation of lymphoid follicles.Citation83 This result implies that ILC3s may participate in the process of airway remodeling in COPD.
ILC1 frequency has also been reported to be increased in COPD and to correlate with disease severity and susceptibility to exacerbations. This may reflect the functional plasticity of ILC2 cells and an attenuation of antiviral immunity.Citation84 Most recently, the combination of microCT analysis, histology, and gene expression profiling indicated that signatures for ILC1s, but not ILC2s or ILC3s, were associated with centrilobular emphysema. This suggests that the alveolar destruction observed in COPD is driven by a Th1 response activated by infiltrating ILC1s.Citation85
Overall, there are few studies that focus on the relationship between ILCs and airway remodeling of COPD. Considering the role of the immune response in COPD pathogenesis, further elucidation of the functional role of ILCs subsets in COPD and its correlation with other inflammatory cells is essential.
Conclusions
COPD is a chronic inflammatory disease involving the infiltration of various inflammatory cells including neutrophils, macrophages, lymphocytes, mast cells, and ILCs.Citation3 The infiltration of inflammatory cells can contribute to the detrimental changes observed in structural cells such as airway epithelial cells, stromal cells, and parenchyma cells. The effects of these inflammatory cells on remodeling are attributed to direct or indirect release of factors such as inflammatory cytokines, proteases, and growth factors (). However, definitive proof of their role will require controlled clinical studies targeting specific cell types and/or remodeling factors with airway remodeling as a defined outcome. Currently, we do not have good biomarkers of remodeling, and imaging techniques are not yet sensitive to directly visualize airway remodeling changes.
Figure 1 Role of inflammatory cells in airway remodeling in COPD.
Abbreviations: ILC, innate lymphoid cell; MCP, monocyte chemoattractant protein; MMPs, matrix metalloproteinases; MPO, myeloperoxidase; NE, neutrophil elastase; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
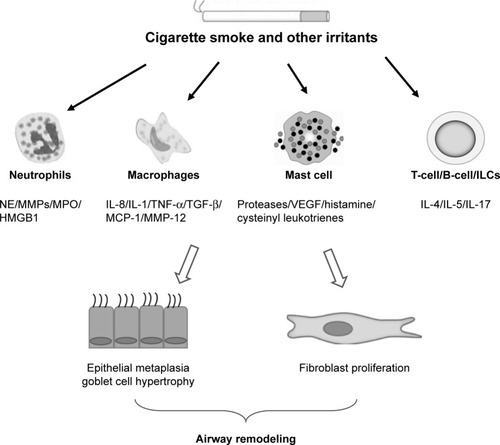
The mechanisms of airway remodeling are poorly studied in COPD compared with asthma. Airway remodeling is characterized by the changes in tissue, cellular, and molecular components, thereby contributing to pathological changes to the epithelium, airway smooth muscle, vessels, and ECM.Citation86 Inflammation in the airways of COPD is largely attributed to smoking and usually enhanced by bacterial and viral infection although this may also be present in exsmokers.Citation87 Experimental studies show that CS exposure can directly lead to the changes in structural cells seen in the lung tissue and small airways as a result of inflammatory response and oxidative stress.Citation88 It is notable that smoking cessation does not prevent the progression of chronic inflammation and oxidative stress and that these are associated with persistent tissue destruction and remodeling.Citation89
We posit that in COPD lungs, inflammatory cells infiltrate into the bronchial mucosa and lung parenchyma. They affect airway destruction and remodeling by directly secreting enzymes and inflammatory cytokines or by indirectly regulating other cellular functions. Some of the above factors can promote airway destruction and remodeling, whereas other factors may protect from tissue damage and reconstruction. Overall, inflammatory cells influence the structural cell destruction, hyperplasia of smooth muscle cells, metaplasia of goblet cells, and subepithelial fibrosis seen in COPD.
It is not completely known how different inflammatory mediators function in the process of airway remodeling and how remodeling contributes to the decrease in lung function. Present treatments can at best only partially reduce the inflammatory response and barely prevent or reverse airway remodeling. Given the fact that inflammatory cells induce such significant effects on airway remodeling in COPD pathogenesis, it is imperative to explore the mechanisms of airway remodeling in COPD and delineate new therapeutic avenues.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grants 81070025 and 81470237), the Jiangsu Health Promotion Project, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (Grant JX10231802). Research by IMA is also supported by the NIHR Biomedical Research Center at Imperial College London.
Disclosure
IMA is supported by the MRC (G1001367/1) and the Wellcome Trust (093080/Z/10/Z). The authors report no conflicts of interest in this work.
References
- VestboJHurdSSAgustíAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- PolosukhinVVRichmondBWDuR-HSecretory IgA deficiency in individual small airways is associated with persistent inflammation and remodelingAm J Respir Crit Care Med201719581010102127911098
- RichmondBWDuRHHanWBacterial-derived neutrophilic inflammation drives lung remodeling in a mouse model of COPDAm J Respir Cell Mol Biol201858673674429314863
- HirotaNMartinJGMechanisms of airway remodelingChest201314431026103224008953
- HoggJCTimensWThe pathology of chronic obstructive pulmonary diseaseAnnu Rev Pathol2009443545918954287
- SohalSSWardCDanialWWood-BakerRWaltersEHRecent advances in understanding inflammation and remodeling in the airways in chronic obstructive pulmonary diseaseExpert Rev Respir Med20137327528823734649
- BousquetJJefferyPKBusseWWJohnsonMVignolaAMAsthma. From bronchoconstriction to airways inflammation and remodelingAm J Respir Crit Care Med200016151720174510806180
- SinghDEdwardsLTal-SingerRRennardSSputum neutrophils as a biomarker in COPD: findings from the ECLIPSE studyRespir Res20101117720550701
- MendyAFornoENiyonsengaTGasanaJBlood biomarkers as predictors of long-term mortality in COPDClin Respir J20181251891189929227024
- BartoliMLCostaFMalagrinòLSputum inflammatory cells in COPD patients classified according to GOLD 2011 guidelinesEur Respir J201647397898026846824
- BardoelBWKennyEFSollbergerGZychlinskyAThe balancing act of neutrophilsCell Host Microbe201415552653624832448
- WangYJiaMYanXIncreased neutrophil gelatinase-associated lipocalin (NGAL) promotes airway remodelling in chronic obstructive pulmonary diseaseClin Sci2017131111147115928381600
- BaekKJChoJYRosenthalPAlexanderLENizetVBroideDHHypoxia potentiates allergen induction of HIF-1α, chemokines, airway inflammation, TGF-β1, and airway remodeling in a mouse modelClin Immunol20131471273723499929
- HendrixAYKheradmandFThe role of matrix metalloproteinases in development, repair, and destruction of the lungsProg Mol Biol Transl Sci201714812928662821
- MeijerMRijkersGTvan OverveldFJNeutrophils and emerging targets for treatment in chronic obstructive pulmonary diseaseExpert Rev Clin Immunol20139111055106824168412
- D’ArmientoJMGoldklangMPHardiganAAIncreased matrix metalloproteinase (MMPs) levels do not predict disease severity or progression in emphysemaPLoS One201382e5635223441181
- OstridgeKWilliamsNKimVRelationship between pulmonary matrix metalloproteinases and quantitative CT markers of small airways disease and emphysema in COPDThorax201671212613226645414
- RoyerP-JHenrioKPainMTLR3 promotes MMP-9 production in primary human airway epithelial cells through Wnt/β-catenin signalingRespir Res201718120829237464
- AidaYShibataYAbeSInhibition of elastase-pulmonary emphysema in dominant-negative MafB transgenic miceInt J Biol Sci201410888289425170302
- PolverinoERosales-MayorEDaleGEDembowskyKTorresAThe role of neutrophil elastase inhibitors in lung diseasesChest2017152224926228442313
- GuyotNWartelleJMalleretLUnopposed cathepsin G, neutrophil elastase, and proteinase 3 cause severe lung damage and emphysemaAm J Pathol201418482197221024929239
- LermanIHammesSRNeutrophil elastase in the tumor microenvironmentSteroids20181339610129155217
- GuoYMaLZhangFSunRLiTNeutrophil elastase ameliorates matrix metalloproteinase-9 to promote lipopolysaccharide-induced acute lung injury in mice 1Acta Cirurgica Brasileira201631638238827355745
- GregoryADKlimentCRMetzHENeutrophil elastase promotes myofibroblast differentiation in lung fibrosisJ Leukoc Biol201598214315225743626
- AraiNKondoMIzumoTTamaokiJNagaiAInhibition of neutrophil elastase-induced goblet cell metaplasia by tiotropium in miceEur Respir J20103551164117119897560
- NauseefWMBiosynthesis of human myeloperoxidaseArch Biochem Biophys20186421929408362
- O’DonnellCNewboldPWhitePThongBStoneHStockleyRA3-Chlorotyrosine in sputum of COPD patients: relationship with airway inflammationCOPD20107641141721166629
- ChurgAMarshallCVSinDDLate intervention with a myeloperoxidase inhibitor stops progression of experimental chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20121851344321997333
- AskariATBrennanMLZhouXMyeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarctionJ Exp Med2003197561562412615902
- QiuS-LZhangHTangQ-YaNeutrophil extracellular traps induced by cigarette smoke activate plasmacytoid dendritic cellsThorax201772121084109328720648
- DickerAJCrichtonMLPumphreyEGNeutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary diseaseJ Allergy Clin Immunol2018141111712728506850
- GangemiSCasciaroMTrapaniGAssociation between HMGB1 and COPD: a systematic reviewMediators Inflamm20152015818
- OjoOORyuMHJhaAUnruhHHalaykoAJHigh-mobility group box 1 promotes extracellular matrix synthesis and wound repair in human bronchial epithelial cellsAm J Physiol Lung Cell Mol Physiol201530911L1354L136626432865
- AroraSDevKAgarwalBDasPSyedMAMacrophages: their role, activation and polarization in pulmonary diseasesImmunobiology20182234–538339629146235
- BarnesPJCellular and molecular mechanisms of chronic obstructive pulmonary diseaseClin Chest Med2014351718624507838
- JundiKGreeneCMTranscription of interleukin-8: how altered regulation can affect cystic fibrosis lung diseaseBiomolecules2015531386139826140537
- SimpsonJLPowellHBainesKJThe effect of azithromycin in adults with stable neutrophilic COPD: a double blind randomised, placebo controlled trialPLoS One201498e10560925148049
- LernerCALeiWSundarIKRahmanIGenetic ablation of CXCR2 protects against cigarette smoke-induced lung inflammation and injuryFront Pharmacol2016739127826243
- ChurgAZhouSWangXWangRWrightJLThe role of interleukin-1beta in murine cigarette smoke-induced emphysema and small airway remodelingAm J Respir Cell Mol Biol200940448249018931327
- CalverleyPMASethiSDawsonMA randomised, placebo-controlled trial of anti–interleukin-1 receptor 1 monoclonal antibody MEDI8968 in chronic obstructive pulmonary diseaseRespir Res201718115328793896
- LappalainenUWhitsettJAWertSETichelaarJWBryKInterleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lungAm J Respir Cell Mol Biol200532431131815668323
- JiMWangYLiXQianZUp-regulation of ICAM-1mRNA and IL-1βmRNA in lung tissues of a rat model of COPDInt J Clin Exp Med2015811219562196326885167
- ReynaertNEurlingsIMerckenEInvolvement of JNK in TNFα driven remodellingEur Respir J201546A5058
- di StefanoASangiorgiCGnemmiITGF-beta signaling pathways in different compartments of the lower airways of patients with stable COPDCheast20181534851862
- MichaeloudesCKuoC-HHajiGMetabolic re-patterning in COPD airway smooth muscle cellsEur Respir J2017505170020229191950
- GohySTHupinCFregimilickaCImprinting of the COPD airway epithelium for dedifferentiation and mesenchymal transitionEur Respir J20154551258127225745049
- HoangLLNguyenYPAspeéRTemporal and spatial expression of TGF-β following airway remodeling to tobacco smoke in ratsAm J Respir Cell Mol Biol201654687288126637070
- XuJTaoBGuoXMacrophage-restricted Shp2 tyrosine phosphatase acts as a Rheostat for MMP12 through TGF-β activation in the prevention of age-related emphysema in miceJ Immunol201719972323233228814604
- TrojanekJBCobos-CorreaADiemerSAirway mucus obstruction triggers macrophage activation and matrix metalloproteinase 12-dependent emphysemaAm J Respir Cell Mol Biol201451570972024828142
- YoshimuraTThe chemokine MCP-1 (CCL2) in the host interaction with cancer: a foe or ally?Cell Mol Immunol201815433534529375123
- HautamakiRDKobayashiDKSeniorRMShapiroSDRequirement for macrophage elastase for cigarette smoke-induced emphysema in miceScience19972775334200220049302297
- QianMFangXWangXAutophagy and inflammationClin Transl Med2017612428748360
- YinHZhangSSunYMicroRNA-34/449 targets IGFBP-3 and attenuates airway remodeling by suppressing Nur77-mediated autophagyCell Death Dis201788e299828796252
- ErjefältJSMast cells in human airways: the culprit?Eur Respir Rev20142313329930725176966
- CruseGBraddingPMast cells in airway diseases and interstitial lung diseaseEur J Pharmacol201677812513825959386
- VirkHArthurGBraddingPMast cells and their activation in lung diseaseTransl Res2016174607626845625
- AnderssonCKMoriMBjermerLLöfdahlCGErjefältJSAlterations in lung mast cell populations in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2010181320621719926870
- BallarinABazzanEZentenoRHMast cell infiltration discriminates between histopathological phenotypes of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2012186323323922679009
- SoltaniAEweYPLimZSMast cells in COPD airways: relationship to bronchodilator responsiveness and angiogenesisEur Respir J20123961361136722034650
- LiHYangTNingQCigarette smoke extract–treated mast cells promote alveolar macrophage infiltration and polarization in experimental chronic obstructive pulmonary diseaseInhal Toxicol2015271482283126671198
- RoosABSandénCMoriMBjermerLStampfliMRErjefältJSIL-17A is elevated in end-stage chronic obstructive pulmonary disease and contributes to cigarette smoke–induced lymphoid neogenesisAm J Respir Crit Care Med2015191111232124125844618
- RoosABMoriMGuraHKIncreased IL-17RA and IL-17RC in end-stage COPD and the contribution to mast cell secretion of FGF-2 and VEGFRespir Res20171814828298222
- GosmanMMPostmaDSVonkJMAssociation of mast cells with lung function in chronic obstructive pulmonary diseaseRespir Res2008916418783610
- KosanovicDDahalBKPetersDMHistological characterization of mast cell chymase in patients with pulmonary hypertension and chronic obstructive pulmonary diseasePulm Circ20144112813625006428
- MortazEFolkertsGRedegeldFMast cells and COPDPulm Pharmacol Ther201124436737221463700
- ShaykhievRCrystalRGInnate immunity and chronic obstructive pulmonary disease: a mini-reviewGerontology201359648148924008598
- BrusselleGGJoosGFBrackeKRNew insights into the immunology of chronic obstructive pulmonary diseaseThe Lancet2011378979510151026
- AuroraABBalukPZhangDImmune complex-dependent remodeling of the airway vasculature in response to a chronic bacterial infectionJ Immunol2005175106319632616272283
- MikkoMForsslundHCuiLIncreased intraepithelial (CD103+) CD8+ T cells in the airways of smokers with and without chronic obstructive pulmonary diseaseImmunobiology2013218222523122652413
- RavensbergAJSlatsAMvan WeteringSCD8+ T cells characterize early smoking-related airway pathology in patients with asthmaRespir Med2013107795996623639272
- KimW-DChiH-SChoeK-HA possible role for CD8+ and non-CD8+ cell granzyme B in early small airway wall remodelling in centrilobular emphysemaRespirology201318468869623421932
- GadgilADuncanSRRole of T-lymphocytes and pro-inflammatory mediators in the pathogenesis of chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis20083453154119281072
- MajoJGhezzoHCosioMGLymphocyte population and apoptosis in the lungs of smokers and their relation to emphysemaEur Respir J200117594695311488331
- BarczykAPierzchałaWKonOMCosioBAdcockIMBarnesPJCytokine production by bronchoalveolar lavage T lymphocytes in chronic obstructive pulmonary diseaseJ Allergy Clin Immunol200611761484149216751017
- HoggJCChuFUtokaparchSThe nature of small-airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med2004350262645265315215480
- VugaLJTedrowJRPanditKVC-X-C motif chemokine 13 (CXCL13) is a prognostic biomarker of idiopathic pulmonary fibrosisAm J Respir Crit Care Med2014189896697424628285
- BrackeKRVerhammeFMSeysLJRole of CXCL13 in cigarette smoke-induced lymphoid follicle formation and chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013188334335523742729
- DuanMCZhangJQLiangYInfiltration of IL-17-producing T cells and Treg cells in a mouse model of smoke-induced emphysemaInflammation20163941334134427150336
- TanHLRosenthalMIL-17 in lung disease: friend or foe?Thorax201368878879023604380
- RobinetteMLColonnaMInnate lymphoid cells and the MHCHLA201687151126812060
- de GroveKCProvoostSVerhammeFMCharacterization and quantification of innate lymphoid cell subsets in human lungPLoS One2016111e014596126727464
- MarashianSMMortazEJamaatiHRRole of innate lymphoid cells in lung diseaseIran J Allergy Asthma Immunol201514434636026547702
- ShikhagaieMMBjörklund ÅsaKMjösbergJNeuropilin-1 is expressed on lymphoid tissue residing LTi-like group 3 innate lymphoid cells and associated with ectopic lymphoid aggregatesCell Rep20171871761177328199847
- SilverJSKearleyJCopenhaverAMInflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungsNat Immunol201617662663527111143
- SuzukiMSzeMACampbellJDThe cellular and molecular determinants of emphysematous destruction in COPDSci Rep201771956228842670
- JefferyPKRemodeling in asthma and chronic obstructive lung diseaseAm J Respir Crit Care Med200116410 Pt 2S28S3811734464
- ChungKFAdcockIMMultifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destructionEur Respir J20083161334135618515558
- ReidelBRadicioniGClappPE-Cigarette use causes a unique innate immune response in the lung involving increased neutrophilic activation and altered mucin secretionAm J Respir Crit Care Med2018197449250129053025
- RutgersSRPostmaDSTen HackenNHOngoing airway inflammation in patients with COPD who do not currently smokeChest20001175262S
