Abstract
Purpose
The GOLD report provides a framework for classifying COPD in a way that reflects its clinical impact and allows treatment recommendations. The GOLD 2017 proposes a new classification whereby patients are grouped as A–D according to their symptoms and history of exacerbations. However, the clinical characteristics and outcomes in these patients are not well documented.
Patients and methods
In this prospective observational study, we analyzed data from the Ishinomaki COPD Network Registry. All patients with stable COPD were classified into the four groups defined by GOLD 2017. The patient demographics, clinical characteristics, number of exacerbations, and mortality rate during 1 year of follow-up were compared between the groups.
Results
Four hundred and one patients with stable COPD were identified. There were 240 patients (59.9%) in group A, 122 (30.4%) in group B, 16 (4.0%) in group C, and 23 (5.7%) in group D. Patients in groups B, C, and D had ORs of 2.95, 3.92, and 5.45, respectively, for risk of exacerbation relative to group A. Groups C and D experienced exacerbations more frequently, including exacerbations leading to hospital admission, than groups A and B (both P<0.001) during the 1-year follow-up period. Patients with a high risk of exacerbation (groups C and D) had a lower body mass index, showed more symptoms, used more respiratory medications, and had more severe airflow limitation than patients at low risk of exacerbation (groups A and B). Mortality was not different between the high-risk and low-risk groups.
Conclusion
The results of our study provide evidence that the GOLD 2017 classification identifies patients with COPD at risk of exacerbations, including those requiring hospitalization, but has a poor ability to predict mortality.
Introduction
COPD is a common, preventable, and treatable disease characterized by persistent respiratory symptoms and airflow limitation caused by airway and/or alveolar abnormalities that develop in response to significant exposure to noxious particles or gases.Citation1 Management of stable COPD had been guided by the severity of airflow limitation ().Citation2 However, patients with COPD show considerable heterogeneity in terms of their clinical presentation, response to therapy, and outcomes.Citation3–Citation5 The 2011 revision of GOLD report proposed the ABCD classification for COPD patients based on their symptoms, severity of airflow limitation, and history of exacerbations ().Citation6 This assessment system was linked to the initial management of the disease, including use of bronchodilators and inhaled corticosteroids. However, this ABCD system has some important limitations in that it performs no better than staging by spirometry for prediction of mortalityCitation7 or decline in pulmonary function.Citation8 Therefore, the ABCD classification was revised in the 2017 GOLD report, in which spirometry measurements were removed from the classification and grouping is based on symptoms and exacerbation history ().Citation1
Table 1 Overview of GOLD classification
Several studies have reported the clinical characteristics and outcomes of COPD using the GOLD 2017 classification.Citation9–Citation13 However, there were differences in the distribution of the A, B, C, and D groups between these studies. Moreover, the ability of the GOLD 2017 classification to predict clinical outcomes, including exacerbation and mortality, is not well documented.
In this prospective observational study, we aimed to investigate the distribution of clinical phenotypes of COPD in a cohort of Japanese patients according to the GOLD 2017 classification. We also evaluated the ability of the GOLD 2017 classification to predict the risk of exacerbation and mortality during 1 year of follow-up.
Patients and methods
Study design
In this study, we analyzed the data that were prospectively collected at consecutive scheduled patient visits or from patients who were newly registered in the Ishinomaki COPD Network (ICON) RegistryCitation14,Citation15 between May 2015 and August 2017.
Briefly, ICON is a regional medical liaison system that aims to provide comprehensive care to patients with COPD and is a part of a multicenter interdisciplinary collaboration between health care providers, including specialists in respiratory medicine, nurse specialists, therapists, pharmacists, and general practitioners, in Ishinomaki, Japan. Patients registered in ICON Registry receive the standard of care in general practice clinics according to the guidelines.Citation16,Citation17 The patients also undergo scheduled examinations and receive education at the Japanese Red Cross Ishinomaki Hospital (a 452-bed tertiary community hospital) every 6–12 months. Patients who experience exacerbations are first treated by their general practitioners and then referred to the Japanese Red Cross Ishinomaki Hospital if necessary. The patient education program includes training on early recognition of exacerbation and a written action plan for an exacerbation using a self-management diary. Patients are prescribed short-acting bronchodilators, but not oral corticosteroids or antibiotics, for self-administration during exacerbations.
This research is a part of an ongoing COPD cohort study registered with the UMIN Clinical Trials Registry (identifier: UMIN000017376). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Japanese Red Cross Ishinomaki Hospital (approval number: 12-14-1). All patients provided written informed consent.
Patients
Patients with stable COPD and aged ≥40 years were included in the study. Persistent airflow limitation, defined as a post-bronchodilator FEV1/FVC <0.7, was confirmed by spirometry. The exclusion criteria were chronic bronchitis or emphysema without airflow limitation, history of lung resection or tuberculosis, and exacerbation of COPD in the 4 weeks preceding collection of the data.
Clinical and physiological measurements
The sociodemographic characteristics and smoking history of each patient were recorded at baseline. Body mass index (BMI) was calculated in kg/m2. Dyspnea was evaluated using the mMRC dyspnea scale.Citation16,Citation17 COPD-related health status was assessed using the COPD Assessment Test (CAT), which is an eight-item questionnaire with possible scores ranging from 0 to 40 and higher scores indicating worse quality of life.Citation18,Citation19
Exacerbations were defined as the use of antibiotics and/or systemic corticosteroids for worsening respiratory symptoms with no evidence indicating an alternative diagnosis.Citation1 Mild exacerbations treated with short-acting bronchodilators only were not considered in the study.
All pulmonary function tests were performed while the patients were in a stable condition and by a well-trained technician following the guidelines.Citation20 The severity of airflow limitation was classified as follows: GOLD 1, FEV1≥80% predicted; GOLD 2, 50% ≤ FEV1,80% predicted; GOLD 3, 30% ≤ FEV1<50% predicted; and GOLD 4, FEV1≤30% predicted.Citation1 The ABCD grouping was performed in accordance with GOLD 2011, GOLD 2013, and GOLD 2017 ().
Longitudinal assessment
The exacerbation rate during the 1-year follow-up was evaluated at scheduled annual visits by direct patient interview, self-management diaries completed by the patient or caregiver, referral letters from general practitioners, and review of medical records. Life status was assessed during the follow-up period.
Statistical analyses
The data are shown as median (interquartile range) or mean ± SD unless otherwise specified. The baseline characteristics of the subjects in the four GOLD groups were compared using one-way ANOVA or Kruskal–Wallis test. Differences between two groups were assessed using Student’s t-test or Mann–Whitney U test. Fisher’s exact test was used to determine the association between categorical variables. The ORs of exacerbations and mortality were estimated by logistic regression analysis. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).Citation21 P-values <0.05 were considered statistically significant.
Results
Distribution of ABCD groups in the study patients
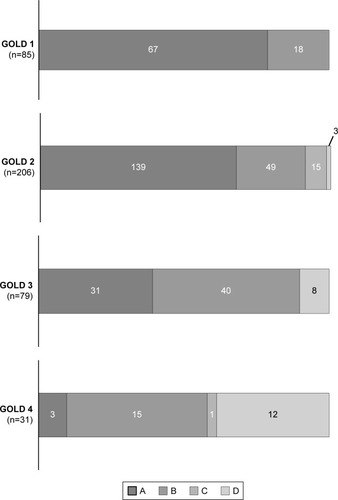
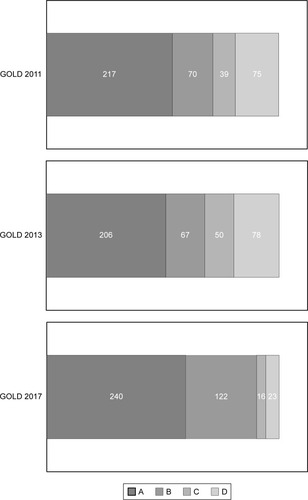
Four hundred and one patients with stable COPD (362 men, 39 women; median age 75 years) were identified. The distribution of groups A, B, C, and D according to GOLD 2011, GOLD 2013, and GOLD 2017 is shown in . According to the GOLD 2017 classification, 240 patients (59.9%) were in group A, 122 (30.4%) were in group B, 16 (4.0%) were in group C, and 23 (5.7%) were in group D. Fourteen (3.5%) of the 401 patients who were at low risk (groups A and B) according to the GOLD 2011 classification were reclassified as being at high risk (groups C and D) according to the GOLD 2017 classification (from group A to C, n=11; from group B to D, n=3). Furthermore, 89 (22.2%) of those who were at high risk according to the GOLD 2011 classification were reclassified as being at low risk according to the GOLD 2017 classification (from group C to A, n=34; from group D to B, n=55).
Figure 1 Distribution of A, B, C, and D groups according to GOLD 2011, GOLD 2013, and GOLD 2017 in the study population.
According to the GOLD 2017 classification, the majority of patients with mild airflow limitation were categorized as group A (). In contrast, those with more severe airflow limitation were more evenly distributed across the four groups, although it should be noted that the number of patients was small.
Clinical phenotypes of the study patients according to the GOLD 2017 classification
The clinical characteristics of each group according to the GOLD 2017 classification are shown in .
Table 2 Characteristics of the study patients at baseline according to the ABCD classification (n=401)
The more symptomatic patients (groups B and D) were older and had a lower BMI, lower FEV1, and more severe airflow limitation than the less symptomatic patients (groups A and C; ). The more symptomatic groups tended to use more respiratory medications than the less symptomatic groups.
Table 3 Comparison of the clinical phenotype of less symptomatic patients (groups A and C) with that of more symptomatic patients (groups B and D)
Patients at a high risk of exacerbation (groups C and D) had a lower BMI, lower FEV1, and more severe airflow limitation than those at low risk of exacerbation (groups A and B; ). The high-risk patients were also more symptomatic (according to their mMRC dyspnea scale and CAT scores) and tended to use more respiratory medications than the low-risk patients. There was no significant difference in mean patient age, proportion of men, or proportion of patients with a smoking history between the groups.
Table 4 Comparison of the clinical phenotype of patients at low risk (groups A and B) with that of patients at high risk (groups C and D)
Outcomes according to ABCD classification
Three hundred and ninety-six (98.8%) of the 401 patients completed the 1-year follow-up. The frequency of exacerbations during this time was 0.16±0.46 events per person per year and that of severe exacerbations leading to hospital admission was 0.098±0.35 events per person per year. No exacerbations were observed in 346 (87.3%) of 396 patients, and only 10 (2.5%) had frequent (two or more) exacerbations per year.
According to the GOLD 2017 classification, the frequency of exacerbations was 6.4% (15/235) in group A, 18.9% (23/122) in group B, 25.0% (4/16) in group C, and 34.8% (8/23) in group D during the study period. Patients in groups B, C, and D had ORs of 2.95 (95% CI 1.60–5.45), 3.92 (95% CI 1.47–10.4), and 5.45 (95% CI 2.59–11.5), respectively, for risk of exacerbations relative to group A (). The frequency of severe exacerbations requiring hospitalization was 1.7% (4/235) in group A, 13.9% (17/122) in group B, 12.5% (2/16) in group C, and 30.4% (7/23) in group D. Patients in groups B, C, and D had ORs of 8.19 (95% CI 2.82–23.8), 7.34 (95% CI 1.45–37.1), and 17.9 (95% CI 5.65–56.5), respectively, for risk of exacerbations requiring hospitalization relative to group A (). The patients at high risk of exacerbations (groups C and D) had significantly more frequent exacerbations, including severe exacerbations leading to hospital admission, than those at low risk (groups A and B; both P<0.001; ).
Table 5 ORs of all exacerbations, exacerbations leading to hospital admission, and all-cause mortality according to the ABCD classification
Table 6 Comparison of exacerbations and mortality between low-risk patients (groups A and B) and high-risk patients (groups C and D)
According to the GOLD 2017 classification, all-cause mortality was 1.3% (3/235) in group A, 4.9% (6/122) in group B, 0% (0/16) in group C, and 4.3% (1/23) in group D during the study period. Patients in groups B and D had ORs of 3.85 (95% CI 0.98–15.1) and 3.41 (95% CI 0.37–31.4), respectively, for mortality relative to group A (). Mortality was not significantly different between the high-risk and low-risk groups (). According to the GOLD 2011 classification, all-cause mortality was 1.4% (3/212) in group A, 5.7% (4/70) in group B, 0% (0/39) in group C, and 4.0% (3/75) in group D. According to the GOLD 2013 classification, all-cause mortality was 1.5% (3/201) in group A, 6.0% (4/67) in group B, 0% (0/39) in group C, and 3.8% (3/78) in group D. Mortality was not significantly different between the A, B, C, and D groups according to the GOLD 2011 or GOLD 2013 classification (P=0.13 and P=0.11, respectively).
Discussion
We have shown the distribution of clinical phenotypes of COPD according to the GOLD 2017 ABCD classification in a cohort of Japanese patients with COPD. The distribution of patients across the four groups in our study was different from that in previous studies.Citation9–Citation13 There may be differences in the COPD phenotypes, including frequency of exacerbations, comorbidities, and causes of death, depending on race, region, and the health care system.
The participants in this study included a small proportion (approximately 10%) of patients in groups C and D, which reflects the relatively low frequency of exacerbations in the study population. In the Hokkaido COPD cohort study,Citation22 which included a different Japanese COPD cohort, Suzuki et al demonstrated that the frequency of exacerbation was 0.20 events per person per year when defined by a prescription change and 0.13 events per year per person when defined by the use of antibiotics. Furthermore, the frequency of hospitalization for an exacerbation of COPD was 0.06 events per person per year. Our present findings are consistent with those of the Hokkaido COPD cohort study.
In this study, there were differences in clinical phenotypes between patients in groups A–D. The patients tended to receive more intensive treatment than that recommended in the GOLD 2017 report, possibly because the treatment options were selected in accordance with the earlier guidelinesCitation6,Citation16 and because step-down therapy is rarely undertaken in patients whose symptoms have improved on intensive treatment.
The results of our study show that the GOLD 2017 classification identifies patients at risk of exacerbation. Exacerbations of COPD are associated with decreased lung function,Citation23 poorer health status,Citation24 and increased mortality risk.Citation23,Citation25 Therefore, assessment of stable COPD should include evaluation of the risk of exacerbation. Recently, Hurst et al reported that the history of exacerbations was the best predictor of frequent exacerbations (two or more per year).Citation26 The GOLD 2017 classification proposes that an exacerbation history (two or more exacerbations or one or more exacerbation leading to hospital admission in the previous year) is associated with an increased risk of further exacerbations. We found that groups C and D experienced more exacerbations, especially those requiring hospitalization, than groups A and B. These results indicate that the GOLD 2017 classification identifies patients at risk of moderate-to-severe exacerbations, including those requiring hospitalization, even in the COPD population with a low frequency of exacerbations.
The findings of our study suggest that patients with a lower BMI, more symptoms, and more severe airflow limitation may be at risk of exacerbations. These results are congruent with those of previous studies demonstrating an association between deteriorating airflow limitation and an increasing prevalence of exacerbationsCitation23 and hospitalization.Citation27 Low BMICitation28 and the presence of more symptomsCitation29 have been identified as possible risk factors for exacerbations. Patients with such a phenotype might be at risk of exacerbations.
The results of our study support the view that the GOLD 2017 classification has a poor ability to predict mortality and are consistent with the findings of other recent studies.Citation11,Citation13 Cabrera López et al recently demonstrated that the risk of mortality in groups B and D was higher than that in groups A and C.Citation11 In our study, the 1-year mortality in groups B and D tended to be higher than that in groups A and C, which suggests that symptoms are more important than the exacerbation history when evaluating the mortality risk. Novel prognostic factors might be considered for evaluating mortality aside from the ABCD classification.
The primary strengths of our study are its prospective observational design and inclusion of community-dwelling patients treated by general practitioners in Ishinomaki or the surrounding cities, meaning that our study population reflects the real-world COPD population in Japan. However, the study also has some potential limitations. First, the follow-up duration was only 1 year. Second, the sample size was smaller than that in the previous large-scale studies.Citation9–Citation13 A longer-term follow-up study with a larger group of patients is needed.
Conclusion
We have shown the distribution of clinical phenotypes of COPD according to the GOLD 2017 ABCD classification in a cohort of Japanese patients with COPD. The results of our study demonstrate that the GOLD 2017 classification system identifies patients at risk of exacerbation but has a poor ability to predict mortality.
Data sharing statement
Data for the individual study participants are not available. No study data will be shared, and no further documents are available.
Author contributions
SK contributed to conception and design, acquisition of data, analysis and interpretation of data, and writing of the manuscript. MH, MI, HS, MO, and SY contributed to acquisition of data, analysis and interpretation of data, and writing of the manuscript. M Yamada contributed to analysis and interpretation of data and writing of the manuscript. HA contributed to analysis and interpretation of data and drafting the manuscript. M Yanai contributed to conception and design, acquisition of data, analysis and interpretation of data, and writing of the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Natsumi Kagabu, Fumi Chiba, Keiko Miyamoto, and Kazue Morozumi from the Outpatient Clinic of the Japanese Red Cross Ishinomaki Hospital, Ishinomaki, Japan, for their help with data management. They are also grateful to the health care professionals affiliated with ICON for their kind help and cooperation with this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- VogelmeierCFCrinerGJMartinezFJGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive SummaryAm J Respir Crit Care Med2017195555758228128970
- RabeKFHurdSAnzuetoAGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2007176653255517507545
- AgustiACalverleyPMCelliBCharacterisation of COPD heterogeneity in the ECLIPSE cohortRespir Res20101112220831787
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- SpencerSEvansDJKarnerCCatesCJInhaled corticosteroids versus long-acting β2-agonists for chronic obstructive pulmonary diseaseCochrane Database Syst Rev201112CD007033
- VestboJHurdSSAgustíAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- SorianoJBLamprechtBRamírezASMortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2007 and 2011 staging systems: a pooled analysis of individual patient dataLancet Respir Med20153644345025995071
- GoossensLMLeimerIMetzdorfNBeckerKRutten-van MölkenMPDoes the 2013 GOLD classification improve the ability to predict lung function decline, exacerbations and mortality: a post-hoc analysis of the 4-year UPLIFT trialBMC Pulm Med20141416325326750
- TudoricNKoblizekVMiravitllesMGOLD 2017 on the way to a phenotypic approach? Analysis from the Phenotypes of COPD in Central and Eastern Europe (POPE) CohortEur Respir J2017494160251828446560
- MenezesAMWehrmeisterFCPerez-PadillaRThe PLATINO study: description of the distribution, stability, and mortality according to the Global Initiative for Chronic Obstructive Lung Disease classification from 2007 to 2017Int J Chron Obstruct Pulmon Dis2017121491150128553101
- Cabrera LópezCCasanova MacarioCMarín TrigoJMComparison of the 2017 and 2015 Global Initiative for Chronic Obstructive Lung Disease Reports. Impact on Grouping and OutcomesAm J Respir Crit Care Med2018197446346929099607
- KahnertKAlterPYoungDThe revised GOLD 2017 COPD categorization in relation to comorbiditiesRespir Med2018134798529413512
- GedebjergASzépligetiSKWackerhausenLHPrediction of mortality in patients with chronic obstructive pulmonary disease with the new Global Initiative for Chronic Obstructive Lung Disease 2017 classification: a cohort studyLancet Respir Med20186320421229331311
- KobayashiSHanagamaMYamandaSIshidaMYanaiMInflammatory biomarkers in asthma-COPD overlap syndromeInt J Chron Obstruct Pulmon Dis2016112117212327660429
- KobayashiSHanagamaMYanaiMIshinomaki COPD Network (ICON) InvestigatorsEarly detection of chronic obstructive pulmonary disease in primary careIntern Med201756233153315828943559
- The Japanese Respiratory SocietyGuidelines for the Diagnosis and Treatment of COPD (Chronic Obstructive Pulmonary Disease)4th edTokyoMedical Review Company, Ltd.2013
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management and prevention of COPD [updated 2017] Available from: http://www.goldcopd.org/Accessed November 17, 2016
- JonesPWHardingGBerryPWiklundIChenWHKline LeidyNDevelopment and first validation of the COPD Assessment TestEur Respir J200934364865419720809
- TsudaTSuematsuRKamoharaKDevelopment of the Japanese version of the COPD Assessment TestRespir Investig20125023439
- MillerMRHankinsonJBrusascoVStandardisation of spirometryEur Respir J200526231933816055882
- KandaYInvestigation of the freely available easy-to-use software ‘EZR’ for medical statisticsBone Marrow Transplant201348345245823208313
- SuzukiMMakitaHItoYMClinical features and determinants of COPD exacerbation in the Hokkaido COPD cohort studyEur Respir J20144351289129724232696
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- SpencerSCalverleyPMBurgePSJonesPWImpact of preventing exacerbations on deterioration of health status in COPDEur Respir J200423569870215176682
- ConnorsAFDawsonNVThomasCOutcomes following acute exacerbation of severe chronic obstructive lung diseaseAm J Respir Crit Care Med19961544 Pt 19599678887592
- HurstJRVestboJAnzuetoAEvaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) InvestigatorsSusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- MüllerovaHMaselliDJLocantoreNHospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohortChest20151474999100725356881
- LainscakMvon HaehlingSDoehnerWBody mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary diseaseJ Cachexia Sarcopenia Muscle201122818621766053
- LeeSDHuangMSKangJThe COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patientsRespir Med2014108460060824456695