Abstract
Purpose
By reanalyzing the gene expression profile GSE76925 in the Gene Expression Omnibus database using bioinformatic methods, we attempted to identify novel candidate genes promoting the development of emphysema in patients with COPD.
Patients and methods
According to the Quantitative CT data in GSE76925, patients were divided into mild emphysema group (%LAA-950<20%, n=12) and severe emphysema group (%LAA-950>50%, n=11). Differentially expressed genes (DEGs) were identified using Agilent GeneSpring GX v11.5 (corrected P-value <0.05 and |Fold Change|>1.3). Known driver genes of COPD were acquired by mining literatures and retrieving databases. Direct protein–protein interaction network (PPi) of DEGs and known driver genes was constructed by STRING.org to screen the DEGs directly interacting with driver genes. In addition, we used STRING.org to obtain the first-layer proteins interacting with DEGs’ products and constructed the indirect PPi of these interaction proteins. By merging the indirect PPi with driver genes’ PPi using Cytoscape v3.6.1, we attempted to discover potential pathways promoting emphysema’s development.
Results
All the patients had COPD with severe airflow limitation (age=62±8, FEV1%=28±12). A total of 57 DEGs (including 12 pseudogenes) and 135 known driving genes were identified. Direct PPi suggested that GPR65, GNB4, P2RY13, NPSR1, BCR, BAG4, and IMPDH2 were potential pathogenic genes. GPR65 could regulate the response of immune cells to the acidic microenvironment, and NPSR1’s expression on eosinophils was associated with asthma’s severity and IgE level. Indirect merging PPi demonstrated that the interacting network of TP53, IL8, CCR2, HSPA1A, ELANE, PIK3CA was associated with the development of emphysema. IL8, ELANE, and PIK3CA were molecules involved in the pathological mechanisms of emphysema, which also in return proved the role of TP53 in emphysema.
Conclusion
Candidate genes such as GPR65, NPSR1, and TP53 may be involved in the progression of emphysema.
Introduction
COPD, characterized by persistent respiratory symptoms and airflow limitation, is the third leading cause of mortality worldwide.Citation1 Airflow limitation is mainly due to small airway obstruction and emphysema, which have distinct physiopathologic mechanisms.Citation2,Citation3 Most patients with COPD have pathological alterations of both emphysema and small airway obstruction, while some have only one or no obvious change.Citation4 Therefore, the two pathological phenotypes are regarded as potential subtypes of COPD.Citation5
Contrary to the feature of small airway remodeling, emphysema is due to decreased deposition and excessive destruction of extracellular matrix, leading to loss of alveolar septum and attachment.Citation6,Citation7 However, many studies show that the pathogenesis and progression mechanism of emphysema are complex and heterogeneous, which need to be further elucidated.Citation6,Citation8,Citation9
As a noninvasive tool to measure morphological indices, quantitative computed tomography (QCT) is an effective approach to determine the severity of COPD and distinguishing the above subtypes.Citation10 Its assessment of emphysema has been demonstrated to be reliable, correlating well with indices of lung function, microscopic manifestations of emphysema, and clinical status of COPD patients. In addition, its assessment of small airway obstruction is also well associated with FEV1%.Citation11,Citation12
After searching the Gene Expression Omnibus (GEO) database, which is one of the largest gene expression databases in the world, we found the original gene expression profile GSE76925 with records of QCT index.Citation13 To investigate the inherent molecular mechanisms in emphysema subtype of COPD, by using several bioinformatics methods,Citation14–Citation16 we constructed the interacting network of differentially expressed genes (DEGs) in this profile and known COPD driver genes to identify novel candidate genes promoting progression of emphysema.
Materials and methods
Acquisition of microarray data
The GEO database (http://www.ncbi.nlm.nih.gov/geo, May 18, 2017) was retrieved to obtain gene expression profiles of lung tissues of COPD patients. The dataset GSE76925, the only one with QCT indices, was downloaded.Citation13 Tests for these surgically resected lung tissue samples in GSE76925 dataset were performed using the GPL10558 platform, Illumina HumanHT-12 V4.0 expression beadchip.
Group division and statistical analysis
After screening samples’ phenotype information (from GSM2040796 to GSM2040942), samples without records of percents of low attenuation areas <−950 Hounsfield unit on inspiratory CT (%LAA-950) were ruled out. Based on the value of %LAA-950, we divided the remaining samples into two groups, severe emphysema group (%LAA-950>50%, n=11) and mild emphysema group (%LAA-950<20%, n=12).
All the continuous variables were expressed as mean ± standard deviation, and t-tests were applied to make comparison between the two groups. The categorical variables were described by constituent ratio and analyzed by Pearson chi-squared test. All statistical analyses were performed using GraphPad Prism 7 (GraphPad Software Inc, La Jolla, CA, USA). A two-side P<0.05 was considered to be statistically significant.
Identification of DEGs between severe and mild emphysema groups
To explore the underlying genes, we filtered DEGs between severe and mild emphysema groups, using GeneSpring GX software v11.5 (Agilent technologies, Santa Clara, CA, USA) at the cutoff value of corrected P-value <0.05 and |Fold Change|>1.3. We annotated them with Gene Oncology by manually retrieving Gene database (http://www.ncbi.nlm.nih. gov/gene, July 16, 2017) and roughly classified them according to the section of biological process in Gene OncologyCitation17 by retrieving the Database for Annotation, Visualization and Integrated Discovery (DAVID)Citation18 v6.8 (https://david.ncifcrf. gov/, October 9, 2018).
Retrieval of COPD driver genes
There has been a variety of known COPD-related genes in Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) guideline,Citation3 peer-reviewed literatures,Citation19–Citation23 Online Mendelian Inheritance (OMIM) databaseCitation24 (https://www.ncbi.nlm.nih.gov/omim/, July 4, 2017), and Genetic Association DatabaseCitation25 (GAD, http://geneticassociationdbnih.gov/). The GOLD guideline illustrated some mainstream mecha nisms of COPD and emphysema, such as protease– antiprotease imbalance, which guided us to further search for specific genes in some canonical reviews. In addition, OMIM and GAD are open access databases, providing a comprehensive and authoritative compendium of genetic alterations associated with disease phenotypes. Based on the keywords of COPD or emphysema, we retrieved the above literatures and databases and identified driver genes of COPD.
Direct protein–protein interaction network of DEGs and known driver genes
The topological and functional analysis of protein interaction network is helpful in the identification of key genes and functional modules that participate in disease onset and progression.Citation16 In network pharmacology, merging the interaction networks of drug predicted targets and driver genes of disease is an effective and original method to identify the concrete genes or pathways by which drug affects the disease.Citation15,Citation16 Enlightened by this analytical method, we tried to analyze the interacted relationship between DEGs and accepted mechanism of COPD in order to identify more credible DEGs participating in emphysema development.
STRING v10.5Citation26 (https://www.string-db.org/, July 20, 2017), a web database recording physical and functional protein–protein interaction (PPi) information, was used to predict the interacted relationship between driver genes and DEGs. A variety of active interaction sources in STRING were included into our search strategy, such as text mining, experiment record, database record, coexpression, neighbor-hood, gene fusion, and co-occurrence. The interaction network was further visualized by CytoscapeCitation27 v3.6.1 which is an open access software aimed at annotating and visualizing biological pathways and molecular interaction networks.
Indirect PPi of DEGs and known driver genes
Weighed protein–protein interaction network analysis has been regarded as a novel approach to highlight key functional genes of complex disorders like frontotemporal dementia.Citation14 It indicates that analyzing disease-spectrum genes, also known as first-layer interacting proteins of key genes, is a greatly potential approach to validate previous findings and explore novel disease-related mechanisms.
Thus, we retrieved STRING database v10.5 to obtain the first-layer proteins associated with DEGs products and constructed an indirect PPi of these proteins. The first-layer interacting proteins were roughly classified according to the clustering annotation of Gene Oncology and Kyoto Encyclopedia of Genes and GenomesCitation28 by the Functional Annotation tool in the DAVID database. Then, the merge tool of Cytoscape software v3.6.1Citation27 was applied to merge the indirect PPi with driver genes’ PPi to discover the interconnected and intersected functional modules and target the core genes.
In addition, highly connected nodes with a great number of edges in the network are likely to be significantly functional in the disease context and defined as hub genes.Citation29 The number of each gene node’s edges in the indirect PPi network was ranked to identify hub genes with functional significance in emphysema by Cytoscape software.
Identification of candidate transcription factors
TRANSFAC® Professional databaseCitation30 is an authoritative and paid database, recording comprehensive information of transcription factor (TF), their regulated genes and binding sites prediction profiles. We performed the TF prediction of core genes by using Gene Radar tool on the GCBI website (Genminix Informatics Ltd., Shanghai, China). Based on all transcripts of each gene (Ensembl database GRCh38 version), the Gene Radar tool could acquire comprehensive TF prediction results from the TRANSFAC Professional database. In addition, Gene Radar tool could screen out high-recommended TFs by integrating the scores from the TRANSFAC database, the existence of single-nucleotide polymorphism (SNP) loci and methylation modification in TF binding sites. Therefore, we identified the candidate TFs of core genes with high recommendation grade.
Results
Baseline characteristic between severe and mild emphysema groups
As shows, all patients were former smokers and presented with severe to very severe airflow limitation according to the GOLD guideline.Citation3 Despite relatively small sample size, a significant difference of many characteristics between two groups was observed, like the ratio of FEV1/FVC and body mass index, proving the credibility of %LAA-950-dependent grouping method.
Table 1 Comparisons of baseline characteristics suggested the credibility of %LAA-950-dependent grouping method
DEGs between two groups and the list of COPD driver genes
We identified 57 DEGs including 15 upregulated genes, 30 downregulated genes, and 12 pseudogenes (unlisted) in severe emphysema group, compared with the mild emphysema group (shown in ). The Gene Oncology annotations of 45 genes were shown in Table S1.
Table 2 Forty-five DEGs were identified between severe and mild emphysema groups
According to involved pathways, 135 retrieved COPD driver genes were separately placed in extracellular matrix-associated column, oxidative stress column, inflammation column, and others column (shown in ).
Table 3 Known driver genes of COPD as grouped into four categories
Candidate genes directly interacted with driver genes
A total of 180 genes (45 DEGs +135 driver genes) were recruited to construct the network, 7 were withdrawn for failed identification of gene symbol and 147 were found to have interaction with others. Eight of the 45 DEGs were found to have interaction relationship with driver genes: G-protein coupling receptor 65 (GPR65), Neuropeptide S receptor 1 (NPSR1), purinergic receptor P2RY13, RhoGEF and GTPase activating protein (BCR), G protein subunit β4 (GNB4), BCL2-associated athanogene 4 (BAG4), inosine monophosphate dehydrogenase 2 (IMDPH2), and Hsp40 member 14 (DNAJB14; shown in ).
Figure 1 Candidate genes were screened by direct PPi of DEGs and COPD driver genes.
Abbreviations: DEGs, differentially expressed genes; PPi, protein–protein interaction.
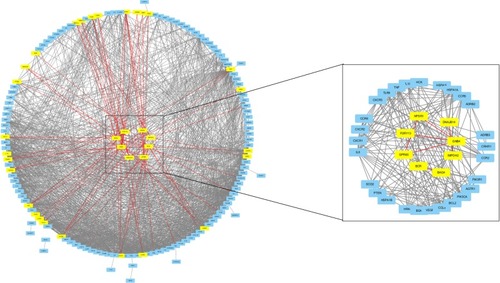
A relatively separate interacting set was composed of GPR65, NPSR1, P2RY13, and GNB4. In addition, BCR and BAG4, IMDPH2 and DNAJB14 had separately bilateral relationship. When the cutoff value of the combined interaction score was set at 0.9, we found that GNB4 and P2RY13 mostly interacted with chemokines and chemokine receptors, such as CXCR1, CCR2, CXCR2, IL8, CXCR3, CCR5, CCL5, and CCR6. BAG4 interacted with TNFα and its receptor as well as heat shock protein (HSP) family. In addition, PIK3CA and PIK3R1 may play an important role by interacting with GPR65, GNB4, BCR, NPSR1, and BAG4.
Common key genes and their TFs filtered by merging of indirect PPi and driver PPi
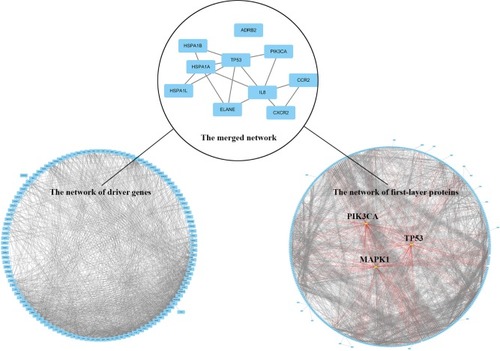
A total of 422 first-layer interacting proteins were attained by retrieving STRING database v10.5 (shown in Table S2). Among them, 375 proteins were recruited to construct the indirect PPi and the remaining proteins were withdrawn due to failed identification or isolation from interaction network. According to the number of each node’s edges in the topological network, 375 proteins in indirect PPi were ranked and the top 20 are shown in Table S3. PIK3CA, TP53, and MAPK1, the top three genes in the rank of topological nodes of indirect PPi, had separately 86, 74, and 72 interacting nodes, which showed their potentially predominant and interconnected roles in the mechanism of emphysema progression.
The merged network illustrated in shows a total of 10 genes that constituted the intersection network of the two networks: TP53, IL8, CCR2, CXCR2, PIK3CA, ELANE, HSPA1A, HSPA1B, HSPA1L, and ADRB2.
Figure 2 Common key genes are screened by merging of indirect PPi and driver PPi.
Abbreviations: DEGs, differentially expressed genes; PPi, protein–protein interaction.
TFs that could bind to promoter region of the above eight genes were retrieved and shown in Table S4. Because ADRB2 was independent from the network and none of the TFs with high recommendation score was retrieved for HSPA1B, they were omitted for presentation. What’s more, SPIB, CPBP, SATB1, ZNF333, HOXA13, KID3, SOX4, and FOXO1A were potentially meaningful TFs, which could regulate no less than half genes of the above nine genes.
Discussion
We identified eight novel candidate genes (GPR65, GNB4, P2RY13, NPSR1, BCR, BAG4, IMPDH2, and TP53) promoting the progression of emphysema by means of network analysis of DEGs and COPD driver genes.
This is the first study that QCT index was applied to classify emphysema for analyzing DEGs, and known COPD driver genes were retrieved to construct interacting networks with DEGs. Our method of direct and indirect network analysis has some merit. For analysis of DEGs, it is a difficult problem to interpret the biological role of the identified single gene in the pathogenic mechanism. Performing external and experimental validation for all DEGs is cumbersome and inefficient. Incorporating driver genes into direct network analysis with DEGs is helpful in quickly highlighting causative DEGs and excluding random DEGs caused by covariates, making the role of identified DEGs more credible. In addition, protein function is regulated not only at transcriptional level but also at posttranscription level which DNA microarray could not detect. A previous study of breast cancer demonstrated that known driver genes, with their expression profiles not changed, were still capable of interconnecting many transcriptionally dysregulated genes in the protein interacting network.Citation31 Therefore, we innovatively used the method of first-layer protein interacting network to further explore the indirect effects of DEGs and seek potential ignored genes. Furthermore, for the polygenic complex disease, a single gene is incapable of comprehensively illustrating the molecular mechanism of phenotypes. By merging the indirect PPi and driver PPi, we could efficiently extract the candidate protein networks involved in a specific disease phenotype. This method,Citation14 of which the efficacy has been confirmed in a study of frontotemporal dementia, could be applied in exploring other complex disorders or extended to other phenotypes of COPD, such as airway remodeling. By comparing difference of the critical protein interactome in different phenotypes, we could unveil the different molecular mechanisms promoting complex pathological processes, which was crucial to promote biomarker and drug discovery.
As a proton-sensing receptor, GPR65 could regulate the immune response of T cells and macrophages and induce the production of MMP3 in the acidic microenvironment.Citation32–Citation34 Asthma, another chronic airway disease with obstructive airflow limitation, was demonstrated to have local acidic microenvironment,Citation35 where eosinophil showed decreased apoptosis and increased viability in a GPR65-depedent manner.Citation36
The SNP of NPSR1 was associated with the decline of FEV1 after adjusting for covariates in normal aging population.Citation37 Moreover, DNA methylation status of NPSR1 in adult severe asthma population and childhood allergic asthma population was distinct from that of control population.Citation38 NPSR1’s expression on peripheral blood eosinophils was positively correlated with asthma’s severity and serum IgE level.Citation39
Asthma and COPD have many common traits in terms of risk factors, inflammatory responses, clinical features, and therapeutic methods.Citation3,Citation40 Furthermore, the role of eosinophils in the pathogenesis and treatment of COPD is gradually recognized.Citation3,Citation41 Therefore, we speculated that the above genes related to asthma were highly likely to be involved in the pathogenesis of COPD and emphysema.
As an extracellular ADP receptor, P2RY13 participated in purinergic signaling pathway, resulting in the apoptosis of pancreatic β-cellsCitation42 and differentiation of marrow stem cells into osteoblasts.Citation43 Since the roles of extracellular adenosine ATP and its receptor P2RX in COPD have been confirmed,Citation44,Citation45 ADP, the intermediate in purinergic metabolic pathways, may also have pathogenic effects on COPD.
In addition, common key genes identified by the indirect method matched well with the two-hit hypothesis of COPD,Citation46 especially the part of senescence and senescence-associated secretary phenotype (SASP).Citation47 Senescence is an irreversible cell state, at which a cell is deprived of its replicative capacity with cell cycle arrest.Citation48 The p53 (encoded by TP53)/p21 pathway participated in all types of senescence mechanisms, arresting cell cycle at the G1/S and G2/M check points.Citation49 SASP refers to the alteration of aging cell’s secretome toward more production of proinflammatory cytokines, including IL-8 and monocyte chemotactic protein 1 (MCP-1).Citation49 IL-8 and its receptor CXCR2, with neutrophil chemotactic ability, are just one of the most important chemokine-receptor pairs in COPD pathogenesis, as well as MCP-1 encoded by CCR2.Citation6 Moreover, phosphoinositide 3 kinase (PI3K), the product of PIK3CA, was also known as a pro-senescent kinase by inactivating HDAC-2 which is an antiaging molecule, because knockdown of HDAC-2 could induce cellular senescence by enhancing p53-dependent transcriptional responses.Citation50
Based on these evidence, we hypothesized that TP53 might play a central role in promoting progression of emphysema. Firstly, beside IL-8, CXCR2 and CCR2, elastase, the products of ELANE, and PI3K are also well-recognized COPD driver genes playing an important role in protease– antiprotease imbalance and chronic inflammation of COPD.Citation6 The involvement of these genes supports our results and in return proves the role of TP53.
Secondly, TP53 can induce cell cycle arrest, apoptosis, senescence, DNA repair, or metabolic alterations, in response to oxidative stress and DNA damage.Citation51 Some studies confirmed that TP53 was overexpressed in the emphysematous lung tissue.Citation52 A Genome-Wide Association Study for 365 patients with emphysema proved the association of TP53’s SNP with apoptotic signaling and smoking-related emphysematous changes in smoker’s lungs.Citation53 Furthermore, a RNA-sequencing study of COPD patients’ lung tissues identified the enrichment of p53/hypoxia pathway and the phenomenon of much frequent molecule’s alternative splicing in this pathway.Citation54
Thirdly, the role of TP53 in senescence might reveal its effects in COPD. Many evidences have shown the association between senescence and pathogenesis of COPD. Cellular experiments proved that alveolar epithelial and endothelial cell as well as fibroblast underwent accelerated senescence in emphysematous lung.Citation55,Citation56 Epidemiological surveys indicated that the incidence of COPD and the decline of FEV1 increased with growth of age.Citation3 Moreover, airway and parenchyma of the patients with COPD and healthy senior citizens had similar structural changes.Citation50,Citation57
There are many studies searching for key genes associated with emphysema. In a research on seeking differently expressed miRNAs of emphysema, the miR-638 was identified as an effector molecule and it could regulate accelerated senescence, which was partially consistent with our hypothesis.Citation58 However, we did not reproduce the DEGs of other studies for emphysema. On one hand, it was due to different grouping methods;Citation59 on the other hand, their samples mainly came from patients with moderate COPD (FEV1% was about 60%),Citation60,Citation61 so their results mainly explained the early mechanisms of emphysema progression.
Our study has some limitations. Firstly, the sample size is relatively small,Citation62 which is due to limited numbers of accessible datasets in GEO database. Secondly, the selection of COPD driver genes is potentially biased and incomplete so that some meaningful DEGs may be ignored. Thirdly, PPi prediction has false positives and false negatives. The web tool STRING v10.5 defines PPi by the standard of text mining, experiment record, database record, coexpression, neighborhood, gene fusion, and co-occurrence, which may have a bit of controversy. In addition, interactions proved by experiments in vitro may also have differences compared with those in vivo.
Despite these limitations, our study has put forward some novel candidate genes, and following experiments or larger databases are needed to testify the role of the above candidate genes in the mechanism of emphysema progression.
Conclusion
We have identified several novel candidate genes promoting emphysema, like GPR65, NPSR1, and TP53, which may be helpful in filling in the gap of knowledge in the field of COPD.
Acknowledgments
The authors would like to acknowledge Dr Xiao Shi who critically reviewed the article for English writing. This study was supported by the National Key Research and Development Program of China (grant Nos 2017YFC1309303 and 2017YFC1309300) and the National Natural Science Foundation of China (grant Nos 81670030 and 81470231).
Supplementary materials
Table S1 GO annotation of DEGs between severe and mild emphysema groups
Table S2 The list of first-layer interacting proteins associated with DEGs between two groups
Table S3 Top 20 topological network nodes in first-layer’s PPi
Table S4 Transcript factors of common key genes screened by indirect PPi
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Health Estimates 2016Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016GenevaWorld Health Organization2018
- HoggJCTimensWThe pathology of chronic obstructive pulmonary diseaseAnnu Rev Pathol2009443545918954287
- Global Initiative for Chronic Obstructive Pulmonary Disease: Global Strategy for the Diagnosis, Management and Prevention of COPD revised2017 Available from: https://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/Accessed April 4, 2017
- SubramanianDRGuptaSBurggrafDEmphysema- and airway-dominant COPD phenotypes defined by standardised quantitative computed tomographyEur Respir J20164819210327230444
- Ziegler-HeitbrockLFrankenbergerMHeimbeckIThe EvA study: aims and strategyEur Respir J201240482382922441733
- ChungKFAdcockIMMultifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destructionEur Respir J20083161334135618515558
- VermylenJHKalhanRRevealing the complexity of chronic obstructive pulmonary diseaseTransl Res2013162420320723932958
- GharibSAManiconeAMParksWCMatrix metalloproteinases in emphysemaMatrix Biol201873345129406250
- TuderRMPetracheIPathogenesis of chronic obstructive pulmonary diseaseJ Clin Invest201212282749275522850885
- LynchDAAl-QaisiMAQuantitative computed tomography in chronic obstructive pulmonary diseaseJ Thorac Imaging201328528429023748651
- NambuAZachJSchroederJQuantitative computed tomography measurements to evaluate airway disease in chronic obstructive pulmonary disease: relationship to physiological measurements, clinical index and visual assessment of airway diseaseEur J Radiol201685112144215127776670
- CastaldiPJSan José EstéparRMendozaCSDistinct quantitative computed tomography emphysema patterns are associated with physiology and function in smokersAm J Respir Crit Care Med201318891083109023980521
- MorrowJDZhouXLaoTFunctional interactors of three genome-wide association study genes are differentially expressed in severe chronic obstructive pulmonary disease lung tissueSci Rep201774423228287180
- FerrariRLoveringRCHardyJLewisPAManzoniCWeighted protein interaction network analysis of frontotemporal dementiaJ Proteome Res2017162999101328004582
- LiJZhaoPLiYTianYWangYSystems pharmacology-based dissection of mechanisms of Chinese medicinal formula Bufei Yishen as an effective treatment for chronic obstructive pulmonary diseaseSci Rep201551529026469778
- ZanzoniASoler-LópezMAloyPA network medicine approach to human diseaseFEBS Lett2009583111759176519269289
- HuntleyRPSawfordTMutowo-MeullenetPThe GOA database: gene Ontology annotation updates for 2015Nucleic Acids Res201543Database issueD1057D106325378336
- Huang daWShermanBTLempickiRASystematic and integrative analysis of large gene lists using DAVID bioinformatics resourcesNat Protoc200941445719131956
- MarciniakSJLomasDAGenetic susceptibilityClin Chest Med2014351293824507835
- BarnesPJThe cytokine network in chronic obstructive pulmonary diseaseAm J Respir Cell Mol Biol200941663163819717810
- McguinnessAJSapeyEOxidative stress in COPD: sources, markers, and potential mechanismsJ Clin Med201762E2128212273
- BrusselleGGJoosGFBrackeKRNew insights into the immunology of chronic obstructive pulmonary diseaseLancet201137897951015102621907865
- BurgstallerGOehrleBGerckensMThe instructive extracellular matrix of the lung: basic composition and alterations in chronic lung diseaseEur Respir J2017501160180528679607
- MckusickVAMendelian inheritance in man and its online version, OMIMAm J Hum Genet200780458860417357067
- BeckerKGBarnesKCBrightTJWangSAThe genetic association databaseNat Genet200436543143215118671
- SzklarczykDMorrisJHCookHThe STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessibleNucleic Acids Res201745D1D362D36827924014
- ShannonPMarkielAOzierOCytoscape: a software environment for integrated models of biomolecular interaction networksGenome Res200313112498250414597658
- KanehisaMFurumichiMTanabeMSatoYMorishimaKKEGG: new perspectives on genomes, pathways, diseases and drugsNucleic Acids Res201745D1D353D36127899662
- Puig-ButilleJAGimenez-XavierPViscontiAGenomic expression differences between cutaneous cells from red hair color individuals and black hair color individuals based on bioinformatic analysisOncotarget201787115891159928030792
- MatysVFrickeEGeffersRTRANSFAC: transcriptional regulation, from patterns to profilesNucleic Acids Res200331137437812520026
- ChuangHYLeeELiuYTLeeDIdekerTNetwork-based classification of breast cancer metastasisMol Syst Biol2007314017940530
- OnozawaYFujitaYKuwabaraHActivation of T cell death-associated gene 8 regulates the cytokine production of T cells and macrophages in vitroEur J Pharmacol20126831–332533122445881
- RaduCGNijagalAMcLaughlinJWangLWitteONDifferential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cellsProc Natl Acad Sci U S A200510251632163715665078
- XuHChenXHuangJIdentification of GPR65, a novel regulator of matrix metalloproteinases using high through-put screeningBiochem Biophys Res Commun201343619610323707809
- HuntJFFangKMalikREndogenous airway acidification. Implications for asthma pathophysiologyAm J Respir Crit Care Med20001613 Pt 169469910712309
- KottyanLCCollierARCaoKHEosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent mannerBlood2009114132774278219641187
- PoonAHHousemanEARyanLVariants of asthma and chronic obstructive pulmonary disease genes and lung function decline in agingJ Gerontol A Biol Sci Med Sci201469790791324253534
- ReiniusLEGrefASääfADNA methylation in the Neuropeptide S Receptor 1 (NPSR1) promoter in relation to asthma and environmental factorsPLoS One201381e5387723372674
- IlmarinenPJamesAMoilanenEEnhanced expression of neuropeptide S (NPS) receptor in eosinophils from severe asthmatics and subjects with total IgE above 100 IU/mlPeptides20145110010924239856
- SilvaGESherrillDLGuerraSBarbeeRAAsthma as a risk factor for COPD in a longitudinal studyChest20041261596515249443
- Vedel-KroghSNielsenSFLangePVestboJNordestgaardBGBlood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen General Population StudyAm J Respir Crit Care Med2016193996597426641631
- TanCSalehiASvenssonSOldeBErlingeDADP receptor P2Y(13) induce apoptosis in pancreatic beta-cellsCell Mol Life Sci201067344545319915796
- BiverGWangNGartlandARole of the P2Y13 receptor in the differentiation of bone marrow stromal cells into osteoblasts and adipocytesStem Cells201331122747275823629754
- PolosaRAdenosine-receptor subtypes: their relevance to adenosine-mediated responses in asthma and chronic obstructive pulmonary diseaseEur Respir J200220248849612212985
- PellegASchulmanESBarnesPJExtracellular adenosine 5′-triphosphate in obstructive airway diseasesChest2016150490891527568579
- AoshibaKTsujiTYamaguchiKItohMNakamuraHThe danger signal plus DNA damage two-hit hypothesis for chronic inflammation in COPDEur Respir J20134261689169523397294
- KumarMSeegerWVoswinckelRSenescence-associated secretory phenotype and its possible role in chronic obstructive pulmonary diseaseAm J Respir Cell Mol Biol201451332333325171460
- CampisiJD’Adda di FagagnaFCellular senescence: when bad things happen to good cellsNat Rev Mol Cell Biol20078972974017667954
- AbbadieCPluquetOPourtierAEpithelial cell senescence: an adaptive response to pre-carcinogenic stresses?Cell Mol Life Sci201774244471450928707011
- ItoKBarnesPJCOPD as a disease of accelerated lung agingChest2009135117318019136405
- VousdenKHPrivesCBlinded by the light: the growing complexity of p53Cell2009137341343119410540
- MorissetteMCVachon-BeaudoinGParentJChakirJMilotJIncreased p53 level, Bax/Bcl-x(L) ratio, and TRAIL receptor expression in human emphysemaAm J Respir Crit Care Med2008178324024718511705
- MizunoSIshizakiTKadowakiMp53 Signaling pathway polymorphisms associated with emphysematous changes in patients with COPDChest20171521586928315337
- KuskoRLBrothersJFTedrowJIntegrated genomics reveals convergent transcriptomic networks underlying chronic obstructive pulmonary disease and idiopathic pulmonary fibrosisAm J Respir Crit Care Med2016194894896027104832
- TsujiTAoshibaKNagaiAAlveolar cell senescence in patients with pulmonary emphysemaAm J Respir Crit Care Med2006174888689316888288
- DagouassatMGaglioloJMChruscielSThe cyclooxygenase-2-prostaglandin E2 pathway maintains senescence of chronic obstructive pulmonary disease fibroblastsAm J Respir Crit Care Med2013187770371423328527
- MercadoNItoKBarnesPJAccelerated ageing of the lung in COPD: new conceptsThorax201570548248925739910
- ChristensonSABrandsmaCACampbellJDmiR-638 regulates gene expression networks associated with emphysematous lung destructionGenome Med201351211424380442
- SpiraABeaneJPinto-PlataVGene expression profiling of human lung tissue from smokers with severe emphysemaAm J Respir Cell Mol Biol200431660161015374838
- FanerRCruzTCasserrasTNetwork analysis of lung transcriptomics reveals a distinct B-cell signature in emphysemaAm J Respir Crit Care Med2016193111242125326735770
- FrancisSMLarsenJEPaveySJExpression profiling identifies genes involved in emphysema severityRespir Res2009108119723343
- HartSNTherneauTMZhangYPolandGAKocherJPCalculating sample size estimates for RNA sequencing dataJ Comput Biol2013201297097823961961
