Abstract
Recently, two “fixed triple” single-inhaler combinations of an inhaled corticosteroid (ICS), a long-acting β2-agonist (LABA), and a long-acting muscarinic antagonist (LAMA) have become available for patients with COPD. This review presents the clinical evidence that led to the approval of these triple therapies, discusses the role of ICS in patients with COPD, and presents data on the relative efficacy of “fixed triple” (ICS/LAMA/LABA) therapy vs LAMA, ICS/LABA, and LAMA/LABA combinations, and summarizes studies in which ICS/LABAs were combined with LAMAs to form “open triple” combinations. Of the five main fixed triple studies completed so far, three evaluated the efficacy and safety of an extrafine formulation of beclometasone dipropionate, formoterol fumarate, and glycopyrronium; the other two studies evaluated fluticasone furoate, vilanterol, and umeclidinium. Overall, compared to LAMA, ICS/LABA, or LAMA/LABA, triple therapy decreased the risk of exacerbations and improved lung function and health status, with a favorable benefit-to-harm ratio. Furthermore, triple therapy showed a promising signal in terms of improved survival. The evidence suggests that triple therapy is the most effective treatment in moderate/severe symptomatic patients with COPD at risk of exacerbations, with marginal if any risk of side effects including pneumonia. Ongoing studies are examining the role of triple therapy in less severe symptomatic patients with COPD and asthma–COPD overlap.
Introduction
Current recommendations are that the pharmacological treatment of COPD should take a “personalized” approach, based around the use of either single therapy or a combination of medications with different or complementary mechanisms of action.Citation1 The role of inhaled corticosteroids (ICSs) in the maintenance treatment of COPD came under scrutiny, in part following a single study showing the benefit on exacerbation frequency of dual bronchodilation of a long-acting β2-agonist (LABA) and a long-acting muscarinic antagonist (LAMA), over a LABA plus ICS.Citation2 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) suggested that the addition of ICS to dual bronchodilation was to be reserved primarily for patients with symptomatic disease and a history of frequent exacerbations (two or more in the previous year), or one or more exacerbations leading to hospitalization.Citation1 However, recent studies in patients with moderate-to-very severe airflow limitation, most of whom had an exacerbation history, show otherwise. Large controlled trials of the two available ICS/LABA/LAMA triple combinations in a single inhaler showed significant benefits not only on exacerbations, but also on lung function, health status, rescue medication use, and importantly, potentially the risk of death compared to LAMA mono-therapy, ICS/LABA, or LAMA/LABA.Citation3–Citation9
This article critically reviews the benefits and safety profile of triple therapy in a single inhaler, and provides expert opinion on its potential role in treatment strategies of patients with COPD.
The historical perspective: bronchodilator use in COPD
Bronchodilators have occupied a central position in the pharmacological management of COPD,Citation10 because the majority of patients respond with variable degrees of bronchodilation to both β2-agonists and muscarinic receptor antagonists. In addition, all studies comparing bronchodilators with placebo showed improvement in dyspnea, probably related to bronchodilation and/or a decrease in resting lung volume and a delay in dynamic hyperinflation during situations of increased ventilator demands, such as exercise.Citation11 The deflationary effect of bronchodilators may even improve cardiac function in patients with increased resting lung volume, providing an interesting mechanism for their benefits.Citation12 The advent of tiotropium, a once daily LAMA, modified the landscape, as it was possible to achieve significant increases in trough FEV1 that was evident 24 hours after a single inhalation. This bronchodilator effect was accompanied by significant improvements in health status, dyspnea, and the rate and time to exacerbation.Citation13–Citation15
However, there were conflicting reports regarding the long-term safety of inhaled bronchodilators in COPD, primarily related to the intrinsic adrenergic effect of LABAs, and to the suppression of parasympathetic cardiac control with LAMAs.Citation15–Citation22 The completion of the 3-year TORCH trial that included a LABA monotherapy arm, and the 4-year UPLIFT trial using tiotropium proved both class of agents to have good overall safety profiles and without any cardiovascular signal.Citation23,Citation24 In addition, the larger SUMMIT trial, specifically conducted in patients at increased cardiovascular risk, showed no excess incidence of cardiovascular events or arrhythmias with a LABA compared to placebo.Citation25
The cardiovascular risk associated with combined LAMA/LABA bronchodilators has been less studied. A large network meta-analysis, including 23 trials, concluded that LAMA/LABA combination therapy had similar effects on safety outcomes compared with either monotherapy.Citation26 In contrast, an increased risk of developing heart failure was reported 1 year after changing from single to dual bronchodilator therapy in a real-world, primary care database, with no increased risk of acute myocardial infarction, stroke, or arrhythmia.Citation27 Finally, a meta-analysis comparing dual bronchodilation with monotherapy demonstrated a larger increase in FEV1 with modest difference in health status with LAMA/LABA combination compared with monotherapy, with no increase in cardiovascular risk.Citation28 However, the effect of dual bronchodilator over tiotropium alone on exacerbations remains questionable at best.Citation29
The historical perspective: ICS use in COPD
The American Thoracic Society’s (ATS) 1986 “Standards for the diagnosis and care of patients with COPD and asthma” suggested that, in asthma, both systemic and inhaled corticosteroids were to be added to bronchodilators only for the treatment and prevention of acute attacks.Citation30 In contrast, for patients with COPD, the document recommended systemic steroids only for the treatment of exacerbations, and briefly mentioned the possible use of ICS as maintenance treatment in the few patients who responded, warning against the serious adverse events associated with long-term treatment, mainly since the first ICS, beclometasone dipropionate (BDP), had been studied almost exclusively in asthma.Citation31 By the early 2000s, the first edition of the GOLD strategy document and an ATS/European Respiratory Society task-force were suggesting that ICSs could be added to bronchodilators for patients with severe COPD who are at risk of exacerbations, or in patients with mixed features of COPD and asthma.Citation32,Citation33 In fact, the Tucson epidemiology studies had shown that among patients with moderate-to-severe airflow limitation, nonsmokers with asthma and smokers with no history of asthma had very different natural histories,Citation34 while the Groningen study showed that the large bronchodilator response to ICS in severely obstructed patients was limited to those with history of asthma.Citation35 The rationale for using ICSs alone in asthma was their anti-inflammatory effects,Citation36 but it became apparent that the airway and pulmonary inflammation of asthma is quite different from that of COPD.Citation37
Four studies completed in the late 1990s investigated whether ICS could ameliorate the progression of COPD, as measured by decline in lung function.Citation38–Citation41 In three of the studies, the use of either inhaled budesonide (BUD) or triamcinolone showed no significant difference in rate of lung function decline in the patients randomized to the active medication arm compared with placebo. In contrast, in the ISOLDE study, patients receiving the ICS fluticasone propionate (FLP) had marginally improved lung function decline and better health status than those receiving placebo, with a clear effect on exacerbations, especially in patients with severe airflow limitation.Citation41,Citation42 Subsequent studies confirmed the beneficial effect of ICSs on exacerbations, especially when given in combination with LABAs.Citation43 These results, coupled with a potential signal on mortality derived from retrospective analyses of a general practice database,Citation44 and from a meta-analysis of several randomized trials,Citation45 prompted a renewed interest in ICS/LABA combinations as potential disease modifiers in COPD. This hypothesis was tested in the large TORCH trial, where the combination of the LABA salmeterol (SAL; 50 µg) and the ICS FLP (500 µg) was compared with placebo and each of the individual components on all-cause mortality over 3 years.Citation23 There was a 17% reduction in the relative risk of death between FLP/SAL and placebo, the difference just missing statistical significance (P=0.052). Since a post hoc analysis of the TORCH data suggested an effect on mortality in patients with cardiovascular disease, the even larger SUMMIT study was conducted in over 16,000 patients with moderate airflow limitation but at heightened cardiovascular risk, demonstrating a 12% reduction in the relative risk of dying with the combination of fluticasone furoate and vilanterol (FLF/VI) compared with placebo.Citation25 This difference was statistically nonsignificant (P=0.13).
However, valuable observations were made in both studies. First, a significant effect of the ICS/LABA was observed on moderate and severe exacerbations even in patients without a history of exacerbations, particularly in those with the most severe degree of airflow limitation. Second, the rates of decline in health status and lung function in both studies were decreased in patients randomized to ICS/LABA. Third, the use of ICS alone in TORCH was associated with an increased risk of death compared with the ICS/LABA combination, prompting the now accepted recommendation of not using ICS monotherapy in COPD. Finally, in TORCH, as in almost all subsequent studies using ICS containing combinations, the incidence of pneumonia was significantly increased in the ICS containing arm,Citation23,Citation46 whereas in SUMMITCitation25 and more recent studies, including some of those using triple therapy, the risk of pneumonia was minimal.Citation3,Citation5,Citation47 Other documented ICS side effects such as oral candidiasis, hoarseness and potential worsening of diabetes, osteopenia and osteoporosis, or cataract raised some questions and fueled the controversy about the correct positioning of ICS in the treatment of patients with COPD.
Clinical characteristics and markers of potential ICS response
Guidelines and expert reviews have suggested that the patients with COPD who might benefit the most from the addition of ICS to bronchodilators are those with a history of and/or concomitant asthma,Citation1,Citation48–Citation50 consistent with the results of the Groningen study.Citation35 Other clinical characteristics have also been associated with potential response to ICS, including a large bronchodilator response, history of allergies and rhinitis. However, the beneficial effect of ICS in this group of patients has never been tested in a formal randomized controlled trial (RCT). Post hoc and prespecified analyses of several RCTs have shown that the effect of ICS/LABAs on exacerbations is related to blood eosinophil counts, with higher blood eosinophil counts consistently predicting a greater effect from ICS/LABA or ICS/LABA/LAMA vs LABA, LAMA, or LAMA/LABA. RCTs comparing triple therapy vs LAMA or LAMA/LABA also confirmed greater effects of triple therapy in patients with higher eosinophil levels (≥150–200 cells/µL).Citation3,Citation6,Citation51 This is in line with a post hoc analysis of the WISDOM study and a prespecified subgroup analysis of the SUNSET study, both of which showed that ICS withdrawal caused increased exacerbations and a greater lung function decline in patients with higher blood eosinophil levels.Citation52,Citation53 We acknowledge, however, that the differences in effect between low and high eosinophil levels are numerical and not statistically significant, and that the use of blood eosinophils as a biomarker of increased risk of exacerbations and increased sensitivity to steroids is still highly debated.Citation54 Recently published analyses from the GLUCOLD study add to the complexity, since eosinophil counts in any compartment, including blood, were not associated with the response to ICS or longitudinal change in FEV1. In fact, higher biopsy eosinophil counts were associated with an increase in symptoms during 6 and 30 months of ICS treatment.Citation55
This does not negate the benefit of triple therapy for patients with eosinophil counts lower than 150 cells/µL, since in IMPACT study the prespecified analysis of the effect of triple inhaled therapy on exacerbations showed a benefit in patients with counts below that threshold, although the exacerbations reduction was lower than that observed in patients with eosinophil counts higher than 150 cells/µL.Citation6 However, a meta-analysis has recently reported an increased risk of pneumonia in patients with COPD and low eosinophil counts.Citation56 Overall, it seems that low blood eosinophil levels may be a useful indicator of a lesser response to ICS.Citation51,Citation57 This is now considered in the 2019 update of the GOLD strategy document, in which a threshold of 100 cells/µL is suggested to guide the decision not to step-up to triple therapy in patients who exacerbate when receiving LAMA/LABA therapy.Citation1 Further, the same document suggests that stepping-up to triple therapy could be of benefit to patients who exacerbate on LAMA/LABA therapy and who have blood eosinophil counts ≥100 cells/µL, with a greater magnitude of response more likely in those with higher eosinophil counts (eg, ≥300 cells/µL).Citation1
ICS/LABA vs LAMA/LABA
The previous historical developments led to the first head-to-head comparison of an ICS/LABA with a LAMA/LABA. Using exacerbations as the primary outcome, FLAME demonstrated a larger benefit from the dual bronchodilator indacaterol/glycopyrronium (IND/GLY) compared with the ICS/LABA combination of FLP/SAL, with the added benefit of higher FEVCitation1 at lower risk of pneumonia.Citation2 It is important to recognize that FLAME did not evaluate “step-up” in therapy, but a switch from one therapy to another. Furthermore, FLAME recruited patients with a history of exacerbations, suggesting that patients who were well controlled on ICS preparations were excluded, thereby biasing the selection to patients with poor response to ICS, who were thus likely to fail ICS maintenance therapy. These results, as well as the perceived risk of ICS-related side effects, led to the 2017 GOLD strategy document recommending ICS use should be reserved for selected patients with highly symptomatic COPD and a history of two or more exacerbations, or of one or more exacerbations resulting in hospitalization.Citation58
Review of triple therapy studies
Triple therapy in multiple inhalers
Most studies evaluating the efficacy of “open triple” therapy, either adding a LAMA to a fixed ICS/LABA or vice versa, not only showed improvement in lung function compared with ICS/LABA or single LAMA therapy, but also improvements in health status, rescue medication use, and the risk of exacerbations, while maintaining a good safety profile.Citation59–Citation68 In contrast, in the 1-year WISDOM trial, which was designed to test ICS withdrawal from triple therapy (with all patients receiving triple therapy for 6 weeks prior to entry, then randomized to either continue triple therapy or withdraw ICS in three steps over 12 weeks), dual bronchodilation was as effective as triple therapy in terms of exacerbation prevention.Citation69 In the patients randomized to withdraw ICS, there was a decrease of 38 mL of FEV1 at the end of WISDOM, although this stabilized over the subsequent year.Citation69 However, all these open triple studies were short in duration, underpowered or retrospective, and required the use of at least two devices, sometimes of different handling characteristics. Nonetheless, triple therapy has become increasingly popular in clinical practice worldwide,Citation70–Citation73 suggesting a continuing need to improve symptom control and reduce the risk of exacerbations and hospitalization, particularly in patients with symptomatic COPD.
Triple therapy in one inhaler
Two different ICS/LABA/LAMA combinations in a single inhaler have been studied: the first comprises beclometasone dipropionate/formoterol fumarate/glycopyrronium bromide (BDP/FF/G; TRIMBOW®, Chiesi Farmaceutici SpA) and the second includes fluticasone furoate/vilanterol/umeclidinium (FLF/VI/UMEC; TRELEGY ELLIPTA®, GlaxoSmithKline).
BDP/FF/G has been developed as an extrafine formulation (aerosol particles with mass median aerodynamic diameter <2 µm) in a pressurized metered-dose inhaler to deliver 87/5/9 µg of BDP/FF/G, two inhalations twice daily. It is indicated in the European Union (EU) for maintenance treatment in adult patients with moderate-to-severe COPD who are not adequately treated by a combination of an ICS and a LABA.
FLF/VI/UMEC has been developed as a multidose dry-powder inhaler (MDDPI) formulation to be delivered through the ELLIPTA device. Each single inhalation provides a delivered dose of 92/22/55 µg of FLF/VI/UMEC, at a recommended dose of one inhalation per day. In the EU, it is also indicated as a maintenance treatment in adult patients with moderate-to-severe COPD who are not adequately treated by a combination of an ICS and a LABA or a LABA and a LAMA. In the USA, FLF/VI/UMEC is indicated for the long-term, maintenance treatment of airflow obstruction in patients with COPD, and is also indicated to reduce exacerbations of COPD in patients with a history of exacerbations.
The BDP/FF/G program
The extrafine BDP/FF/G Phase III program included three 12-month studies (TRILOGY, TRINITY, and TRIBUTE), with a total of 2,529 patients with severe-to-very severe airflow limitation (FEV1 <50% predicted) randomized to BDP/FF/G ().Citation3–Citation5 All patients included had a high symptom burden (COPD Assessment Test [CAT] ≥10) and an exacerbation history (≥1 exacerbation in the previous year).Citation3–Citation5 All three studies excluded patients who had received triple therapy in the previous year. Overall, the studies demonstrated improvements in FEV1, health status (St George’s Respiratory Questionnaire, SGRQ) and reduction in the rate of moderate/severe exacerbations compared with ICS/LABA, LAMA monotherapy, and LAMA/LABA.
In the TRINITY study, the single inhaler BDP/FF/G combination was more effective than tiotropium alone, and was equally effective to the open triple combination of BDP/FF + tiotropium in terms of both predose FEV1 and the annual rate of moderate/severe exacerbations.Citation5 In the TRILOGY study, compared to BDP/FF, BDP/FF/G showed a prolongation of the time to first clinical important deterioration (CID) as defined by any one of the following changes: decrease ≥100 mL from baseline in FEV1, deterioration ≥4 units from baseline in SGRQ total score, deterioration ≥1 unit from baseline in Transition Dyspnea Index focal score, occurrence of a moderate/severe COPD exacerbation, or death.Citation74 Furthermore, in the subgroup of patients in TRILOGY classified as GOLD 2017 Group B (ie, those with CAT score ≥10 and one exacerbation in the previous year not leading to hospitalization or emergency room admission), BDP/FF/G reduced the rate of moderate/severe exacerbations compared to BDP/FF.Citation75 These results were consistent with those observed in TRINITY, where BDP/FF/G delayed CID compared to tiotropium, and reduced the rate of moderate/severe exacerbations in GOLD Group B patients.Citation75,Citation76 In TRIBUTE, in addition to confirming the delay of CID,Citation77 and the benefits in GOLD Group B patients,Citation78 BDP/FF/G demonstrated a favorable benefit/risk ratio compared with the dual bronchodilator combination IND/GLY;Citation3 in particular, there was a reduced rate of moderate/severe exacerbations together with comparable rates of pneumonia events, as shown in .Citation79
Figure 1 The overall benefit/risk ratio (ie, exacerbations/pneumonia) in the TRIBUTE study.
Abbreviations: BDP/FF/G, beclometasone dipropionate/formoterol fumarate/glycopyrronium; IND/GLY, indacaterol/glycopyrronium.
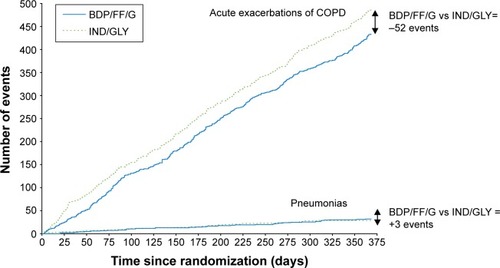
Interestingly, a pooled post hoc analysis of the three studies showed that BDP/FF/G reduced the number of fatal events by 32% compared to the non-ICS-containing reference arms (IND/GLY or tiotropium), although statistical significance was missed (P=0.096) due to the overall relatively small number of events.Citation9
The FLF/VI/UMEC program
The pivotal phase of the clinical development of FLF/VI/UMEC consisted of two main studies, FULFIL and IMPACT. Essentially, they provided consistent evidence of improvements in FEV1 and health status, and a reduction in annual exacerbation rate compared to the reference comparators ().Citation6,Citation7 In these studies, a total of 5,062 patients were randomized to FLF/VI/UMEC, all of whom had symptomatic COPD (CAT ≥10), but with the other main inclusion criteria differing slightly (). The FULFIL study compared FLF/VI/UMEC with BUD/FF MDDPI fixed combination for 24 weeks in patients with severe airflow limitation but no increased risk of exacerbations and patients with moderate airflow limitation but highly increased risk of exacerbations.Citation7 The study showed superiority of the triple combination on FEV1 and health status. A subgroup of about 24% of patients entered into an extension phase up to 52 weeks, in whom there was a reduction in the exacerbation rate with FLF/VI/UMEC compared with BUD/FF.Citation7 In the IMPACT study, FLF/VI/UMEC was compared with FLF/VI and UMEC/VI, all delivered via the ELLIPTA device.Citation6 The study showed a reduced exacerbation rate with triple therapy compared with the two dual therapies, as well as a benefit on FEV1 and health status. The large sample size of IMPACT (more than 10,000 patients randomized overall) meant that the study could demonstrate that all-cause mortality was lower with the regimens containing the ICS FLF (triple therapy and FLF/VI) than with the LAMA/LABA UMEC/VI.Citation6,Citation8 This result is consistent with the trend observed with the pooled post hoc analysis of the BDP/FF/G studies, suggesting that triple ICS-containing regimens do have an impact on mortality in patients with symptomatic COPD and an exacerbation history.
The inclusion of patients with potential concomitant asthma in the triple therapy programs
One of the criticisms of the TRIBUTE and IMPACT studies was the recruitment of patients with a history of asthma (although not current asthma) – and the consequent potential influence on the overall results of the studies. Indeed, in the editorial commentary that was published with the IMPACT study, Suissa and Drazen suggest that the inclusion of patients with a history of asthma, and more specifically the potential abrupt withdrawal of ICS in case of randomization to the LAMA/LABA arm, might have triggered exacerbations, hence exaggerating the benefit in favor of triple therapy.Citation80 However, it should be emphasized that patients included in these studies had poor lung function even after bronchodilation (especially in TRIBUTE study), so regardless of their asthma history, their COPD should be considered as the prevalent medical condition.
Pneumonia in the triple therapy programs
Since, by definition, all triple therapy regimens include ICS, the potential increased risk of infective events should be weighed against the advantages over bronchodilator therapies. The risk of pneumonia in TRILOGY was similar (3%) in the two arms, both of which contained ICS, ie, BDP/FF/G and BDP/FF.Citation4 In the TRINITY study, the incidence of pneumonia was similar in the three treatment groups (3%, 2%, and 2% for BDP/FF/G, tiotropium, and BDP/FF + tiotropium, respectively).Citation5 Similar results were seen in the TRIBUTE study, where the pneumonia incidence was not different in patients treated with either BDP/FF/G or IND/GLY (4% for both).Citation3 This result contrasts with FLAME, in which 1 year treatment with FLP/SAL was associated with a greater incidence of pneumonia (4.8%) than IND/GLY (3.2%; P=0.02).Citation2 Further, in the 6-month FULFIL trial the risk of pneumonia was higher in patients treated with FLF/UMEC/VI than BUD/FF (2.2% vs 0.8%, respectively).Citation7 Similarly, in IMPACT study patients treated with FLF/VI/UMEC had a higher incidence of pneumonia (8%) compared to UMEC/VI (5%; P<0.001) and similar incidence to that of patients treated with FLF/VI (7%; P=0.85).Citation6 This might suggest that the dose, pharmacological characteristics, such as the size or extent of the immunosuppressive effect,Citation81 or particle size of different ICS molecules, may influence the risk of ICS-associated pneumonia events; indeed, in a cohort database study, patients with obstructive lung disease who received extrafine ICS had a lower risk of pneumonia than those who received fine-particle ICS.Citation82 Interestingly, the 1-year WISDOM study showed no increased risk of pneumonia in the ICS maintenance group (tiotropium plus FLP/SAL) compared to the ICS withdrawal group (tiotropium plus SAL; 5.8% and 5.5%, respectively),Citation69 nor did the 6-month SUNSET study (1.7% and 1.1% in the maintenance [tiotropium plus FLP/SAL] vs ICS withdrawal groups [IND/GLY], respectively).Citation52 None of the cited studies showed an increased risk of respiratory tract infections other than pneumonia.
Triple therapy and survival
The most frustrating aspect of the management of COPD is the lack of solid evidence of effect of any pharmacologic treatment on mortality. As discussed above, the only two large properly designed RCTs showed a decrease in risk of death that was borderline significant,Citation23 or not statistically significant.Citation25 Both studies compared a combination of ICS/LABA with placebo and with each of the components. However, an analysis of the large UPLIFT trial suggested a potential beneficial effect on mortality of tiotropium added to usual care.Citation24
The data emerging from the triple combination studies cited above provide a glimmer of hope. Even though the studies were not designed to evaluate the effect of triple therapy on survival, in IMPACT study there was a significant survival difference in favor of the triple combination compared with the dual bronchodilator therapy and a smaller, but still important difference compared with the ICS/LABA combination.Citation6,Citation9 Most patients included in these studies had moderate, severe, and very severe airflow limitation and a history of exacerbations, many having been hospitalized for respiratory failure, with significant compromise. It is hoped that future trials designed to evaluate the effect of triple vs dual combinations with mortality as an outcome will provide an answer to this important question. Finally, it is important to note that proper management of frequently presenting concomitant chronic diseases like chronic heart failure, ischemic heart disease, stroke, diabetes, and hypertension is needed to improve overall patient outcomes.Citation83
Who should be treated with triple therapy?
As mentioned previously, BDP/FF/G and FLF/VI/UMEC are both approved in the EU as maintenance treatments in adult patients with moderate-to-severe COPD who are not adequately treated by a combination of an ICS and a LABA (with FLF/VI/UMEC also approved for patients inadequately treated with a LABA and a LAMA), whereas in the USA, FLF/VI/UMEC is approved for the long-term, maintenance treatment of airflow obstruction in patients with COPD, and is also indicated to reduce exacerbations of COPD in patients with a history of exacerbations. These slightly different indications have in common that triple therapy is recommended for patients not adequately controlled by existing inhaled therapies. Neither the European Medicines Agency nor the US Food and Drug Administration make a distinction between initiation of therapy in untreated newly diagnosed patients compared with use of triple therapy in already treated patients, as both organizations assume triple therapy is used as a step-up. Similarly, the GOLD strategy document recommends triple therapy only as a step-up from LAMA/LABA or ICS/LABA in GOLD Group D patients whose disease is not adequately controlled by LAMA/LABA or ICS/LABA.Citation1 However, due to changes over time in the GOLD definition of high exacerbation risk, the studies performed with triple therapy in a single inhaler have recruited patients who, according to the 2017 definition, would be a mixture of GOLD Group B and D.Citation3–Citation7 Thus in clinical practice, the use of triple therapy might be considered beneficial not only in GOLD Group D patients but also in patients at lower risk of exacerbations, eg, symptomatic patients with at least one moderate exacerbation. Triple therapy may even be of benefit in patients who have not exacerbated, although this will need confirming in other studies.Citation84
Concluding remarks
The most recent studies of ICS combined with LABA and LAMA in a single inhaler showed that triple therapy represents the most potent pharmacological treatment available for patients with COPD with moderate-to-very severe airflow limitation, particularly those with an exacerbation history. Compared to LAMA, ICS/LABA, or LAMA/LABA, triple therapy not only has been shown to improve lung function, health status, rescue medication use, and risk of exacerbations, but also for the first time shows a promising signal on improved survival.
Acknowledgments
The authors thank David Young of Young Medical Communications and Consulting Ltd., for providing writing support. This review was funded by Chiesi Farmaceutici SpA.
Disclosure
LV reports grants and personal fees from AstraZeneca, grants from Philips, and Fisher and Paykel, and personal fees from Chiesi, GSK, Pulmonx, Menarini, and Boehringer-Ingelheim, all outside the submitted work. LMF reports grants, personal fees, and nonfinancial support from Boehringer-Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Merck Sharp & Dohme, Takeda, AstraZeneca, Novartis, Menarini, Laboratori Guidotti, and Almirall, personal fees and nonfinancial support from Pearl Therapeutics, Mundipharma, and Boston Scientific, personal fees from Kyorin, Bayer, and Zambon, and grants from Pfizer, Dompè, Malesci, Alfasigma, and Vree Health Italia, all outside the submitted work. AP reports grants, personal fees, nonfinancial support, advisory board membership and consultancy work from Chiesi, AstraZeneca, GlaxoSmithKline, Boehringer-Ingelheim, Mundipharma, and TEVA, personal fees and nonfinancial support from Menarini, Novartis, and Zambon, and grants from Sanofi, all outside the submitted work. SP is employed by Chiesi, the sponsor of TRILOGY, TRINITY, and TRIBUTE. BC reports grants to the Division of Pulmonary and Critical Care from AstraZeneca, and that he is a consultant for GlaxoSmithKline, Boehringer-Ingelheim, AstraZeneca, Novartis, Pulmonix, Chiesi, and Menarini, all outside the submitted work. The authors report no other conflicts of interest in this work.
References
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease Available from: www.goldcopd.org. Published 2019Accessed November 19, 2018
- WedzichaJABanerjiDChapmanKRIndacaterolglycopyrronium versus salmeterol-fluticasone for COPDN Engl J Med2016374232222223427181606
- PapiAVestboJFabbriLExtrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trialLancet2018391101251076108429429593
- SinghDPapiACorradiMSingle inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trialLancet20163881004896397327598678
- VestboJPapiACorradiMSingle inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trialLancet2017389100821919192928385353
- LipsonDABarnhartFBrealeyNOnce-daily single-inhaler triple versus dual therapy in patients with COPDN Engl J Med2018378181671168029668352
- LipsonDABarnacleHBirkRFULFIL Trial: once-daily triple therapy for patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2017196443844628375647
- LipsonDABarnhartFBrealeyNReduction in all-cause mortality with single inhaler triple therapy (FF/UMEC/VI) versus dual therapy (FF/VI and UMEC/VI) in symptomatic patients with COPD: prespeci-fied analysis of the Phase III IMPACT TrialAm J Respir Crit Care Med2018197A1015
- VestboJFabbriLPapiAInhaled corticosteroid containing combinations and mortality in COPDEur Respir J20181801230
- Standards for the diagnosis and care of patients with chronic obstructive pulmonary diseaseAmerican Thoracic SocietyAm J Respir Crit Care Med19951525 Pt 2S77S1217582322
- O’DonnellDEFlügeTGerkenFEffects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPDEur Respir J200423683284015218994
- HohlfeldJMVogel-ClaussenJBillerHEffect of lung deflation with indacaterol plus glycopyrronium on ventricular filling in patients with hyperinflation and COPD (CLAIM): a double-blind, randomised, crossover, placebo-controlled, single-centre trialLancet Respir Med20186536837829477448
- CasaburiRMahlerDAJonesPWA long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J200219221722411866001
- NiewoehnerDERiceKCoteCPrevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trialAnn Intern Med2005143531732616144890
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med2008359151543155418836213
- SalpeterSROrmistonTMSalpeterEECardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysisChest200412562309232115189956
- SinghSLokeYKFurbergCDReviewASInhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysisJAMA2008300121439145018812535
- GershonACroxfordRCalzavaraACardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary diseaseJAMA Intern Med2013173131175118523689820
- WilcheskyMErnstPBrophyJMPlattRWSuissaSBronchodilator use and the risk of arrhythmia in COPD: part 1: Saskatchewan cohort studyChest2012142229830422871755
- WilcheskyMErnstPBrophyJMPlattRWSuissaSBronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohortChest2012142230531122871756
- CalverleyPMAndersonJACelliBCardiovascular events in patients with COPD: TORCH study resultsThorax201065871972520685748
- SuissaSDell’anielloSErnstPLong-acting bronchodilator initiation in COPD and the risk of adverse cardiopulmonary eventsChest20171511606727554300
- CalverleyPMAndersonJACelliBSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med2007356877578917314337
- CelliBDecramerMKestenSMortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20091801094895519729663
- VestboJAndersonJABrookRDFluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trialLancet2016387100301817182627203508
- ObaYSarvaSTDiasSEfficacy and safety of long-acting β-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysisThorax2016711152526490732
- SuissaSDell’AnielloSErnstPConcurrent use of long-acting bronchodilators in COPD and the risk of adverse cardiovascular eventsEur Respir J2017495160224528536251
- CalzettaLRoglianiPMateraMGCazzolaMA systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPDChest201614951181119626923629
- CalverleyPMAAnzuetoARCarterKTiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trialLancet Respir Med20186533734429605624
- No authors listedStandards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986Am Rev Respir Dis198713612252443605835
- BrownHMStoreyGGeorgeWHBeclomethasone dipropionate: a new steroid aerosol for the treatment of allergic asthmaBr Med J1972158005855904335298
- PauwelsRABuistASCalverleyPMAJenkinsCRHurdSSGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200116351256127611316667
- CelliBRMacNeeWATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paperEur Respir J200423693294615219010
- BurrowsBBloomJWTraverGAClineMGThe course and prognosis of different forms of chronic airways obstruction in a sample from the general populationN Engl J Med198731721130913143683459
- KerstjensHABrandPLHughesMDA comparison of bronchodilator therapy with or without inhaled corticosteroid therapy for obstructive airways disease. Dutch Chronic Non-Specific Lung Disease Study GroupN Engl J Med199232720141314191357553
- DjukanovićRWilsonJWBrittenKMEffect of an inhaled corticosteroid on airway inflammation and symptoms in asthmaAm Rev Respir Dis199214536696741546849
- FabbriLMRomagnoliMCorbettaLDifferences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2003167341842412426229
- PauwelsRALöfdahlCGLaitinenLALong-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary DiseaseN Engl J Med1999340251948195310379018
- VestboJSørensenTLangePBrixATorrePViskumKLong-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trialLancet199935391671819182310359405
- Lung Health Study Research GroupWiseRConnettJWeinmannGScanlonPSkeansMEffect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary diseaseN Engl J Med2000343261902190911136260
- BurgePSCalverleyPMJonesPWSpencerSAndersonJAMaslenTKRandomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trialBMJ200032072451297130310807619
- JonesPWWillitsLRBurgePSCalverleyPMAInhaled steroids in obstructive lung disease in Europe study investigators. Disease severity and the effect of fluticasone propionate on chronic obstructive pulmonary disease exacerbationsEur Respir J2003211687312570111
- NanniniLJPoolePMilanSJHolmesRNormansellRCombined corticosteroid and long-acting beta2-agonist in one inhaler versus placebo for chronic obstructive pulmonary diseaseCochrane Database Syst Rev201311CD003794
- SorianoJBVestboJPrideNBKiriVMadenCMaierWCSurvival in COPD patients after regular use of fluticasone propionate and salmeterol in general practiceEur Respir J200220481982512412670
- SinDDTuJVInhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2001164458058411520719
- IannellaHLunaCWatererGInhaled corticosteroids and the increased risk of pneumonia: what’s new? A 2015 updated reviewTher Adv Respir Dis201610323525526893311
- FergusonGTTashkinDPSkärbyTEffect of budesonide/formoterol pressurized metered-dose inhaler on exacerbations versus formoterol in chronic obstructive pulmonary disease: the 6-month, randomized RISE (Revealing the Impact of Symbicort in reducing Exacerbations in COPD) studyRespir Med2017132314129229103
- MiravitllesMSoler-CataluñaJJCalleMSpanish COPD Guidelines (GesEPOC) 2017. Pharmacological treatment of stable chronic obstructive pulmonary diseaseArch Bronconeumol (English Ed)2017536324335
- PostmaDSRabeKFThe asthma–COPD overlap syndromeN Engl J Med2015373131241124926398072
- GershonASCampitelliMACroxfordRCombination long-acting β-agonists and inhaled corticosteroids compared with long-acting β-agonists alone in older adults with chronic obstructive pulmonary diseaseJAMA2014312111114112125226477
- BafadhelMPavordIDRussellREKEosinophils in COPD: just another biomarker?Lancet Respir Med20175974775928601554
- ChapmanKRHurstJRFrentSMLong-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trialAm J Respir Crit Care Med2018198332933929779416
- CalverleyPMATetzlaffKVogelmeierCEosinophilia, frequent exacerbations, and steroid response in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201719691219122128306321
- RabeKFBeghéBFabbriLMPeripheral eosinophil count as a biomarker for the management of COPD: not there yetEur Respir J2017505170216529167308
- HartjesFJVonkJMFaizAPredictive value of eosinophils and neutrophils on clinical effects of ICS in COPDRespirology201823111023103129696728
- PavordIDLettisSAnzuetoABarnesNBlood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysisLancet Respir Med20164973174127460163
- PavordIDChanezPCrinerGJMepolizumab for eosinophilic chronic obstructive pulmonary diseaseN Engl J Med2017377171613162928893134
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease Available from: www.goldcopd.org. Published 2017Accessed December 21, 2016
- SinghDBrooksJHaganGCahnAO’ConnorBJSuperiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPDThorax200863759259818245142
- JungKSParkHYParkSYComparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled studyRespir Med2012106338238921975275
- HananiaNACraterGDMorrisANEmmettAHO’DellDMNiewoehnerDEBenefits of adding fluticasone propionate/salmeterol to tiotropium in moderate to severe COPDRespir Med201210619110122040533
- FrithPAThompsonPJRatnavadivelRGlycopyrronium once-daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study, a randomised controlled trialThorax201570651952725841237
- SilerTMKerwinESingletaryKBrooksJChurchAEfficacy and safety of umeclidinium added to fluticasone propionate/salmeterol in patients with COPD: results of two randomized, double-blind studiesCOPD201613111026451734
- AaronSDVandemheenKLFergussonDTiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trialAnn Intern Med2007146854555517310045
- WelteTMiravitllesMHernandezPEfficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2009180874175019644045
- LeeSDXieCMYunusFEfficacy and tolerability of budesonide/formoterol added to tiotropium compared with tiotropium alone in patients with severe or very severe COPD: a randomized, multicentre study in East AsiaRespirology201621111912726394882
- SilerTMKerwinETombsLFahyWANayaITriple therapy of umeclidinium + inhaled corticosteroids/long-acting beta2 agonists for patients with COPD: pooled results of randomized placebo-controlled trialsPulm Ther2016214358
- ShortPMWilliamsonPAElderDHJLipworthSIWSchembriSLipworthBJThe impact of tiotropium on mortality and exacerbations when added to inhaled corticosteroids and long-acting β-agonist therapy in COPDChest20121411818621799028
- MagnussenHDisseBRodriguez-RoisinRWithdrawal of inhaled glucocorticoids and exacerbations of COPDN Engl J Med2014371141285129425196117
- BrusselleGPriceDGruffydd-JonesKThe inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UKInt J Chron Obstruct Pulmon Dis20151012207221726527869
- SimeoneJCLuthraRKailaSInitiation of triple therapy maintenance treatment among patients with COPD in the USInt J Chron Obstruct Pulmon Dis201712738328053518
- MiyazakiMNakamuraHTakahashiSThe reasons for triple therapy in stable COPD patients in Japanese clinical practiceInt J Chron Obstruct Pulmon Dis2015101053105926082629
- MartinezFJRabeKFCalverleyPMADeterminants of response to roflumilast in severe COPD: pooled analysis of two randomized trialsAm J Respir Crit Care Med2018198101268127829763572
- SinghDPapiAVezzoliSCHF5993 pMDI (extrafine beclometasone dipropionate:BDP, formoterol fumarate:FF, glycopyrronium bromide:GB) reduces clinically important deteriorations (CID) in COPD: post-hoc analysis of TRILOGY studyEur Respir J201750Suppl 61PA533
- SinghDFabbriLPapiAExtrafine triple therapy reduces exacerbations in GOLD B COPD patients: post-hoc analysis of TRILOGY and TRINITYEur Respir J201750Suppl 61OA2898
- SinghDPapiAVezzoliSEffect of CHF5993 pMDI (extrafine beclometasone dipropionate:BDP, formoterol fumarate:FF, glycopyrronium bromide: GB) on clinically important deteriorations (CID) in COPD: post-hoc analysis of TRINITY studyEur Respir J201750Suppl 61PA3949
- ScuriMSinghDFabbriLMSingle inhaler extrafine triple therapy reduces clinically important deterioration (CID) in COPD compared to indacaterol/glycopyrronium: post-hoc analysis of the TRIBUTE studyAm J Respir Crit Care Med2018197A1013
- ScuriMSinghDFabbriLMSingle inhaler extrafine triple therapy improves clinical outcomes in GOLD B COPD patients: post-hoc analysis of the TRIBUTE studyAm J Respir Crit Care Med2018197A3041
- ScuriMSinghDFabbriLMRisk of pneumonia and exacerbations with single inhaler extrafine triple therapy compared to indacaterol/glycopyrronium: post-hoc analysis of the TRIBUTE studyAm J Respir Crit Care Med2018197A3030
- SuissaSDrazenJMMaking sense of triple inhaled therapy for COPDN Engl J Med2018378181723172429669218
- JansonCStratelisGMiller-LarssonAHarrisonTWLarssonKScientific rationale for the possible inhaled corticosteroid intraclass difference in the risk of pneumonia in COPDInt J Chron Obstruct Pulmon Dis2017123055306429089754
- SonnappaSMartinRIsraelERisk of pneumonia in obstructive lung disease: a real-life study comparing extra-fine and fine-particle inhaled corticosteroidsPLoS One2017126e017811228617814
- VanfleterenLUllmanAFabbriLMTime for a longer and better life for patients with COPDEur Respir J2018511170256929326323
- FergusonGTRabeKFMartinezFJTriple therapy with budes-onide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trLancet Respir Med201861074775830232048
