Abstract
Introduction
Exacerbations of COPD (ECOPDs) are important events in the course of COPD, accelerating the rate of decline in lung function and increasing the mortality risk. A growing body of evidence suggests the significance of the “frequent exacerbator” phenotype. This phenotype seems to be associated with a more severe airflow limitation, symptoms, health-related quality of life impairment, and higher mortality. However, there is no described biomarker that would help to identify this group of patients.
Patients and methods
Patients with COPD in “D” GOLD category were monitored for 3 years according to events of ECOPD. Serum samples were collected from the patients. Circulating level of plasma soluble receptor for advanced glycation end-products (sRAGE) was measured using commercially available high sensitivity kits. The receiver operating characteristic (ROC) curve analysis was used to assess the usefulness of sRAGE to identify frequent exacerbator phenotype. Log-rank test was used in the analysis of time to the subsequent exacerbation. Pearson (R) or Spearman’s rank (RS) correlation coefficients were used for correlation analysis.
Results
Nineteen patients were enrolled. The area under the ROC curve (AUROC) for sRAGE for the identification of frequent exacerbator phenotype was 0.81. Analysis identified the cutoff point as 850.407 pg/mL, characterized by a sensitivity of 0.80 (95% CI: 0.28–1.0) and specificity of 0.93 (95% CI: 0.66–1.0). Additionally, in the group with sRAGE ≤850.407 pg/mL, we observed significantly shorter time to the subsequent exacerbation: median of 32 vs 105.5 days (P=0.03). Correlation analysis revealed significant negative correlation between sRAGE and the number of exacerbations requiring hospitalization during the whole time of follow-up (RS=−0.53; P=0.02) and significant positive correlation with FEV1 expressed as the percentage of reference value (R=0.6; P=0.006).
Conclusion
sRAGE seems to be useful in the identification of frequent exacerbator phenotype. This parameter may also be used in the prediction of time to ECOPD. Our findings should be confirmed in a sufficiently powered larger sample.
Introduction
Exacerbations of COPD (ECOPDs) are key points for the natural history of COPD as they accelerate the rate of decline in lung function.Citation1–Citation3 Exacerbations requiring hospitalization are specifically important, because they are associated with significant mortality. In fact, evidence suggests that exacerbation with hypercapnia is burdened with significant in-hospital mortality.Citation4 Additionally, long-term prognosis following hospitalization for ECOPD is burdened by the average in-hospital mortality rate of 6.7%, while the average mortality rates at 3 and 6 months were reported to be 18 and 26%, respectively, and those at 5 years were reported as high as 51%.Citation5 The literature data show, that only 11.6% to 17% of COPD patients experience remittent exacerbations in 1 year.Citation6–Citation8 Nevertheless, this group induces an economic and social burden that is both substantial and increasing.Citation3 This is why there is a strong need for elaborate approaches to prevent ECOPDs and identify in advance the group of subjects who experience remittent exacerbations. To gain this goal, there is a strong need for research for new, biochemical prediction tools.
The receptor for advanced glycation end-products (RAGE) is a multiligand, pattern-recognition receptor involved in the host response to injury, infection, and inflammation.Citation9 It is an immunoglobulin superfamily protein of 35 kDa. Primarily, it plays a role of a multiligand transmembrane binder for several molecules.Citation10 RAGE localizes in various cell types, such as monocytes, macrophages, smooth muscle cells, and endothelial cells.Citation11 RAGE signaling also plays a key role in lung development and structure.Citation12 Therefore, it justifies the linkage of RAGE with the pathobiology of COPD.
Numerous studies analyzed its role in cigarette smoke-induced airway inflammation and emphysema.Citation12–Citation16 Deficiencies in soluble RAGE (sRAGE) were linked to heightened inflammation in various chronic conditions, including neutrophilic airway inflammation in asthma and COPD.Citation9 There is also an evidence for the usefulness of phenotyping of the RAGE gene, eg, the G82S polymorphism in the RAGE gene is associated with an increased risk of COPD, the GS genotype of the G82S variant is a risk factor for COPD in the Chinese population,Citation17 and genetic variants in or near RAGE have also been associated with COPD affection status and emphysema,Citation18–Citation20 while the minor allele of the rs2070600 variant was associated with protection with respect to COPD in the population of smokers.Citation21 Moreover, RAGE was considered as a potential therapeutic target for COPD.Citation22
We aimed to assess if sRAGE would be useful for the identification of patients prone to frequent ECOPDs. We also analyzed the time to the subsequent exacerbation and correlations between sRAGE and spirometry values.
Patients and methods
Study protocol was approved by Ethical Committee for Human Studies of the Medical University of Lodz. All patients provided written informed consent for the participation in the study. All study procedures were consistent with the tenets of the Declaration of Helsinki.
Patients with COPD in category D according to GOLD guidelines were enrolled to the study from July 2014 to August 2017. Patients were enrolled in the Outpatient Clinic and Department of Pneumology and Allergy, Medical University of Lodz. The main criterion was having ≥1 exacerbation(s), which required hospitalization within last 12 months and/or ≥2 exacerbations not requiring hospitalization with the need for systemic steroid therapy and/or antibiotic therapy. All patients were treated with similar therapy. The exclusion criteria comprised a history of any active malignancy, α-1-antitrypsin deficiency, HIV infection, and concomitant diagnosis of other significant respiratory diseases (eg, interstitial lung disease and parenchymal lung disease due to previous tuberculosis). Additionally, patients with exacerbations at least 8 weeks prior to sample collection were excluded from the study.
Serum samples were collected from the patients and stored at −80°C until use. Repeated freeze-thaw cycles were avoided. ELISA was used to determine the serum levels of sRAGE (BioVendor – Laboratorni medicina a.s., Brno, Czech Republic) according to the manufacturer’s recommendations.
Then, patients were monitored according to events of ECOPD by the same experienced physician (JM-D). Every such event was classified as mild (worsening of symptoms for 2 consecutive days and not treated with systemic corticosteroids and/or antibiotics), moderate (treated with systemic corticosteroids and/or antibiotics), and severe (requiring hospitalization) according to the Anthonisen criteria. Worsening of symptoms was captured in an electronic diary that alerted patients and physicians to the presence of an exacerbation. All events were confirmed by the physician. The current analysis focuses on moderate or severe exacerbations. The “frequent exacerbator” phenotype was defined by at least one hospitalization due to ECOPD or two treated at home per year in every year of prospective observation. The follow-up was terminated after 3 years in June 2018.
Continuous data were presented as the mean with SD or median with IQR, depending on the distribution of data. Variables were compared using the unpaired Student’s t-test, Welch test, or the Mann–Whitney U-test, depending on data normality and the homogeneity of variance. Categorical data were presented as absolute value with percentage and were compared using Fisher’s exact test.
The receiver operating characteristic (ROC) curve analysis was used to assess sRAGE usefulness to identify frequent exacerbator phenotype. The area under the ROC curve (AUROC) was presented as result and 95% CI, while the accuracy of the qualitative test parameters was presented as point estimates and 95% CIs. An AUROC of greater than 0.9 was considered as excellent, greater than 0.8–0.9 as very good, 0.7–0.8 as good, 0.6–0.7 as average, and below 0.6 as poor.Citation23
Log-rank test was used in the analysis of time to the subsequent exacerbation. Pearson (R) or Spearman’s rank (RS) correlation coefficients were evaluated to assess the relationships between sRAGE and spirometry values.
Data were analyzed using the R software for MacOS.Citation24
Results
A total of 19 patients were included in the study. We identified five patients (26.32%) who met the criteria of frequent exacerbator phenotype. The baseline clinical data and analysis after stratification by frequent exacerbator phenotype are presented in . As expected, frequent exacerbators experienced significantly higher number of all exacerbations during the time of follow-up: 5 (3–17) vs 1 (0.25–1.75); P=0.002. Additionally, in the nonfrequent exacerbator group, two patients were hospitalized because of ECOPD during the follow-up period (median 0) and the median of such exacerbations in the frequent exacerbator group was 2 (2–3).
Table 1 The baseline clinical data and analysis after stratification by frequent exacerbator phenotype
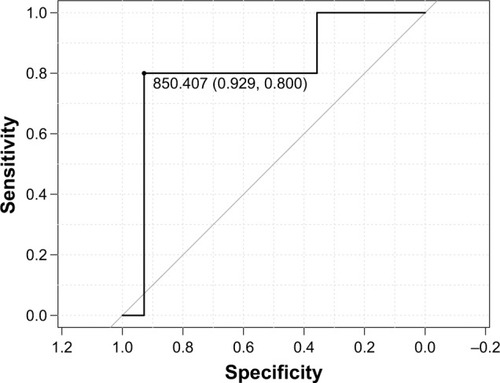
ROC analysis showed an AUROC of 0.81, which can be classified as very good accuracy according to Choi,Citation23 and identified the cutoff point as 850.407 pg/mL (), characterized by a sensitivity of 0.80 (95% CI: 0.28–1.0), specificity of 0.93 (95% CI: 0.66–1.0), positive predictive value (PPV) of 0.80 (95% CI: 0.28–1.0), and negative predictive value (NPV) of 0.93 (95% CI: 0.66–1.0). The diagnostic accuracy of the test was 0.9 (95% CI: 0.67–0.99).
Figure 1 ROC curve for sRAGE in the identification of frequent exacerbator phenotype.
Abbreviations: ROC, receiver operating characteristic; sRAGE, soluble receptor for advanced glycation end-products.
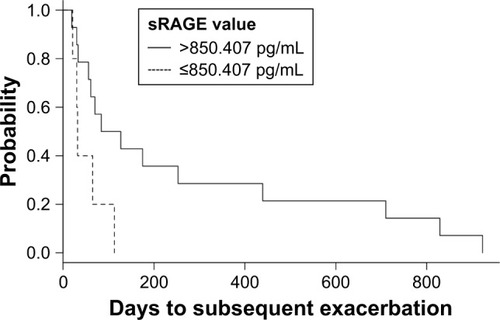
After stratification by the sRAGE cutoff point of 850.407, we observed significantly shorter time to the subsequent exacerbation (median of 105.5 days for sRAGE>850.407 pg/mL and 32 days for sRAGE ≤850.407 pg/mL) (; P-value for log-rank test =0.03).
Figure 2 Kaplan–Meier curves for the time to the subsequent exacerbation (days), stratified by the sRAGE value of 850.407 pg/mL.
Correlation analysis revealed significant negative correlation between sRAGE and the number of exacerbations requiring hospitalization during the whole time of follow-up (RS=−0.53; P=0.02) and significant positive correlation with FEV1 expressed as the percentage of reference value (R=0.6; P=0.006). We did not observe significant correlation with FVC expressed as the percentage of reference value (R=0.43; P=0.07).
Discussion
In modern treatment approaches, there is a tendency to identify relevant COPD phenotypes. It is believed that this may improve the precision, effectiveness, and safety of the treatment. Moreover, Golpe et alCitation25 reported that grouping COPD patients according to clinical phenotypes can help us to identify those with a different mortality risk.
ECOPDs account for the greatest proportion of the total COPD burden on the health care system. Additionally, from 11.6% to 17% of COPD patients experience remittent exacerbations in 1 year.Citation6–Citation8 Also, changes in GOLD guidelines tend to extract patients who experience frequent exacerbations.Citation3 Such approach enables early intensification of treatment to reduce the risk of future exacerbations in order to guide therapy.Citation3
A growing body of evidence suggests the significance of the frequent exacerbator phenotype; however, there are no unified guidelines that defne this phenotype. Also, the knowledge on this group of patients seems to be scarce. The frequent exacerbator phenotype may be defined, concordantly with our study methods, as at least one hospitalization due to ECOPD or two treated at home per year.Citation26 This phenotype seems to be associated with a more airflow limitation symptoms, health-related quality of life impairment, and higher mortality.Citation26 Gupta et alCitation27 reported the significant reduction in FEV1, diffusing capacity for carbon monoxide and transfer coefficient of the lung values in this group of patients.
There is no described biomarker that would help us to identify this group of patients. For this purpose, we propose sRAGE. ROC analysis showed very good diagnostic accuracy, and considering limitations of our pilot study, suggesting its potential usefulness. Additionally, we observed high values of accuracy of the qualitative test parameters.
Our finding is associated with potentially important clinical implications. Namely, identification of this group of patients would help us in planning more precise therapy in early stage of the disease. We believe that it will influence the natural history of the disease and, as the effect, ameliorate prognoses.
Moreover, sRAGE can also be used in the prediction of time to the subsequent exacerbation. This observation is of a great benefit for clinical practice because it would be potentially useful in indicating patients with a high risk of upcoming exacerbation and, thus, require early intensive treatment.
The parameter also correlated with FEV1, reflecting the relationship between decrease in FEV1 with increasing airway inflammation, which intensifies the progression of the disease.Citation28
Study limitations
Small sample size is the major limitation of our study and determines this as a pilot study. Therefore, results should be considered as hypothesis generating and should be confirmed in a sufficiently powered larger sample. Small sample size of our study results from careful selection of the study group. All patients were treated by the same clinically experienced physician, and they were treated with similar therapy. The study group was observed for 3 years. Such studies are long lasting, time consuming, and involving deep cooperation with physicians. Additionally, our group consisted mainly of female subjects, which does not fully reflect overall COPD population. The female patients presented better compliance with the study protocol and more frequently expressed their consent for participation in the study. Moreover, there is an evidence that female COPD patients might be more prone to severe exacerbations, might a long period of symptoms before being admitted to the hospital, have higher number of hospitalizations, and prolonged length of hospital stay.Citation29
Conclusion
sRAGE seems to be useful in the identification of frequent exacerbator phenotype. This parameter may also be used in the prediction of time to ECOPD. Our findings should be confirmed in a sufficiently powered larger sample.
Acknowledgments
The costs of this study were defrayed from regular finances of the Department of Pneumology and Allergy, Medical University of Lodz, Poland (503/1-151-03/503-11-001-17).
Disclosure
The authors report no conflicts of interest in this work.
References
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- KannerREAnthonisenNRConnettJELung Health Study Research GroupLower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health studyAm J Respir Crit Care Med2001164335836411500333
- Global Initiative for Chronic Obstructive Pulmonary DiseaseGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease Available from: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdfAccessed August 21, 2018
- ConnorsAFDawsonNVThomasCOutcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments)Am J Respir Crit Care Med19961544 Pt 19599678887592
- HoogendoornMHoogenveenRTRutten-van MölkenMPVestboJFeenstraTLCase fatality of COPD exacerbations: a meta-analysis and statistical modelling approachEur Respir J201137350851520595157
- SuissaSDell’anielloSErnstPLong-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortalityThorax2012671195796322684094
- BeehKMGlaabTStowasserSCharacterisation of exacerbation risk and exacerbator phenotypes in the POET-COPD trialRespir Res20131411624168767
- HanMKQuibreraPMCarrettaEEFrequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohortLancet Respir Med20175861962628668356
- SukkarMBWoodLGToozeMSoluble RAGE is deficient in neutrophilic asthma and COPDEur Respir J201239372172921920897
- MccanceDRDyerDGDunnJAMaillard reaction products and their relation to complications in insulin-dependent diabetes mellitusJ Clin Invest1993916247024788514859
- MukhopadhyaySMukherjeeTKBridging advanced glycation end product, receptor for advanced glycation end product and nitric oxide with hormonal replacement/estrogen therapy in healthy versus diabetic postmenopausal women: a perspectiveBiochim Biophys Acta20051745214515515890418
- YonchukJGSilvermanEKBowlerRPCirculating soluble receptor for advanced glycation end products (sRAGE) as a biomarker of emphysema and the RAGE axis in the lungAm J Respir Crit Care Med2015192778579226132989
- ChenMWangTShenYKnockout of RAGE ameliorates mainstream cigarette smoke-induced airway inflammation in miceInt Immunopharmacol20175023023528704797
- LeeHParkJRKimWJBlockade of RAGE ameliorates elastase-induced emphysema development and progression via RAGE-DAMP signalingFaseb J20173152076208928148566
- ReynoldsPRKastelerSDSchmittREHoidalJRReceptor for advanced glycation end-products signals through Ras during tobacco smoke-induced pulmonary inflammationAm J Respir Cell Mol Biol201145241141821131443
- RobinsonABJohnsonKDBennionBGReynoldsPRRAGE signaling by alveolar macrophages influences tobacco smoke-induced inflammationAm J Physiol Lung Cell Mol Physiol201230211L1192L119922505673
- LiYYangCMaGAssociation of polymorphisms of the receptor for advanced glycation end products gene with COPD in the Chinese populationDNA Cell Biol201433425125824520905
- CastaldiPJChoMHSan José EstéparRGenome-wide association identifies regulatory Loci associated with distinct local histogram emphysema patternsAm J Respir Crit Care Med2014190439940925006744
- ManichaikulAHoffmanEASmolonskaJGenome-wide study of percent emphysema on computed tomography in the general population. The Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource StudyAm J Respir Crit Care Med2014189440841824383474
- ChoMHCastaldiPJHershCPA Genome-Wide Association Study of Emphysema and Airway Quantitative Imaging PhenotypesAm J Respir Crit Care Med2015192555956926030696
- YoungRPHayBAHopkinsRJDoes RAGE protect smokers from COPD?Eur Respir J201138374374421885423
- ChenLLiuLWangTShenYCWenFQReceptor for advanced glycation end products: a new theraputic target for chronic obstructive pulmonary disease?Arch Med Res2013441757623287523
- ChoiBCSlopes of a receiver operating characteristic curve and likelihood ratios for a diagnostic testAm J Epidemiol199814811112711329850136
- Core Team RR: A Language and Environment for Statistical ComputingR Foundation for Statistical ComputingVienna, Austria2016
- GolpeRSuárez-ValorMMartín-RoblesIMortality in COPD patients according to clinical phenotypesInt J Chron Obstruct Pulmon Dis2018131433143929750029
- Le RouzicORocheNCortotABDefining the “Frequent Exacerbator” Phenotype in COPD: A Hypothesis-Free ApproachChest201815351106111529054347
- GuptaPPGovidagoudarMBYadavRAgarwalDClinical and pulmonary functions profiling of patients with chronic obstructive pulmonary disease experiencing frequent acute exacerbationsLung India2018351212629319029
- OhJYSinDDLung inflammation in COPD: why does it matter?F1000 Med Rep201242323236338
- BlumenthalJAEmeryCFSmithPJThe effects of a telehealth coping skills intervention on outcomes in chronic obstructive pulmonary disease: primary results from the INSPIRE-II studyPsychosom Med201476858159225251888
