Abstract
Nutritional problems are an important part of rehabilitation for chronic obstructive pulmonary disease (COPD) patients. COPD patients often present with malnutrition, sarcopenia, and osteoporosis with possible onset of cachexia, with an inadequate dietary intake and a poor quality of life. Moreover, diet plays a pivotal role in patients with COPD through three mechanisms: regulation of carbon dioxide produced/oxygen consumed, inflammation, and oxidative stress. A narrative review based on 99 eligible studies was performed to evaluate current evidence regarding optimum diet therapy for the management of COPD, and then a food pyramid was built accordingly. The food pyramid proposal will serve to guide energy and dietary intake in order to prevent and treat nutritionally related COPD complications and to manage progression and COPD-related symptoms. The nutrition pyramid described in our narrative review is hypothetical, even in light of several limitations of the present review; the main limitation is the fact that to date there are no randomized controlled trials in the literature clearly showing that improved nutrition, via the regulation of carbon dioxide produced/oxygen consumed, inflammation and oxidative stress, improves symptoms and/or progression of COPD. Even if this nutritional pyramid is hypothetical, we hope that it can serve the valuable purpose of helping researchers focus on the often-ignored possible connections between body composition, nutrition, and COPD.
Introduction
Nutritional problems are an important part of rehabilitation for all disabled subjects, especially in chronic obstructive pulmonary disease (COPD) patients. There are numerous mechanisms that interfere with the functioning of the respiratory system in these patients. Nutrition plays a pivotal role, for both the prevention of risk of COPD, and treatment of COPD. The literature has shown that there is an obvious link between some dietary models and the progression of this disease. Dietary patterns associated with benefits in prevention of the risk of respiratory diseases include those typical of the Mediterranean diet, while fast food intake and westernized eating habits have adverse associations.Citation1–Citation7 In particular, the excessive consumption of red meat, processed meat and sweetened drinks and the reduction of dairy product intake showed a worsening of lung function,Citation8 while a diet rich in whole grains, vegetables, fruit and fish showed a lower risk of newly diagnosed COPD.Citation5
COPD and BMI
Weight and body composition also have an impact on progression of COPD. It should be emphasized that most studies consider body mass index (BMI) rather than body composition.Citation9 Several studies have reported that low BMI is an independent risk factor for mortality in subjects with COPD,Citation10,Citation11 with a inflection point for BMI equal to 21 Kg/m2 and a mortality increase below this value.Citation9,Citation12 The prevalence of underweight patients with COPD varies, ranging between 3% and 19% with BMI ≤ 18.5 kg/m2Citation10,Citation13,Citation14 and equal to 22% if a BMI is lower than 21 kg/m2 is considered.Citation15 This prevalence also increases with the severity of the disease,Citation16 and the association with BMI is stronger in subjects with severe COPD.Citation9
A low fat free mass (FFM) should be considered a predictor of independent mortality,Citation17 and not an adaptive mechanism to reduce the metabolic rate.Citation18 This unintentional weight loss can reach 80% due to the non-satisfaction of energy and protein needs.Citation19 A frequent and involuntary weight loss inevitably leads to malnutrition, which can be established regardless of weight, with incidence estimates reaching 75%.Citation19 Very often, with the worsening of the disease, the condition of pulmonary cachexia is reached; the exact cause and mechanisms of the disease are still poorly understood, but potential factors include oxidative stress and inflammation.Citation20
Since weight reduction or a sudden weight loss negatively correlates with the progression of the disease itself, a BMI indicative of overweight or obesity could be considered as protective against COPD. Several studies have evaluated the prevalence of obesity in patients with COPD with results ranging 7.2% of the Spanish population to 54% of the resident population in Northern California, passing 14% in Northern Europe, on 18% in the Netherlands, 20% in Slovenia, 23% in Latin America, 24.6% in Canada.Citation10,Citation13–Citation15,Citation21–Citation23
In the Copenhagen City Heart Study, in a17-year of follow-up study that had a group of 2132 subjects with COPD, a low BMI value was predictive of a poor prognosis (thus greater chance of mortality). The association between BMI and survival differed significantly with the stage of COPD. In mild and moderate COPD, the lowest risk occurred in normal-weight/overweight subjects (with a U-shaped relationship), while in severe COPD mortality continued to decrease with increasing BMI. The same result was noted for deaths from COPD-related respiratory causes by Landbo et al.Citation9
Another large cohort study, which lasted 12 years, involved over 1 million South Koreans aged between 30 and 95 and showed that a higher BMI (BMI> 25 up to 30 kg/m2) clearly reduces the risk of mortality from respiratory causes in patients with COPD.Citation24 This result was confirmed in 2012, in a large meta-analysis conducted by Cao et al, which considered 21,150 participants with COPD, and the study asserted that overweight and obesity were associated with lower mortality.Citation11
In obstructive pulmonary diseases, therefore, what is called the “obesity paradox” can be present, which is more evident for subjects with severe bronchial obstruction.Citation25 More than obesity, perhaps being overweight should be highlighted, as some studies indicated that patients with COPD show a lower mortality risk in overweight patients.Citation10,Citation15 To confirm this data, Eisner and colleagues evaluated the impact of fat mass (FM) on functional limitation: a greater FM was associated with a decrease in the walk test in six minutes (from −13 meters per 1 kg of mass increase fat in men and −11 meters in women) and a poorer Short Physical Performance Battery (SPPB) summary performance score.Citation13
Thus it appears that the increase in FM, and not simply the loss of FFM, is an important precursor for the development of functional limitation and that this process occurs at an early age in COPD compared to the general population.Citation13 In addition, visceral fat has been associated with an increased cardiovascular risk in subjects with COPD.Citation26 Accordingly, nutritional status is of great importance and is a determining factor for the outcome of COPD.Citation17
Caloric Intake
There are several papers that over the years have tried to define the daily calorie expenditure in patients with COPD. In 1994 Ganzoni et alCitation27 hypothesized a daily calorie intake equal to 45 Kcal/Kg/body weight, taken up in a work in 2019 by Collins et alCitation28 which, however, does not seem to have a response in clinical practice, as overestimated. In 1997 Baarends et al effectively evaluated a total daily calorie intake equal to the Basal Energy Expenditure (BEE) multiplied by 1.7; this energy expenditure seems to be mainly due to the effort given by physical activity (intended as physical activity levels, PAL)Citation29 and not by the BEE component as previously described by other authors.Citation30,Citation31 In 2010, some authors validated a specific predictive equation for the calculation of BEE in underweight patients, which however takes into account the value of the FFM, and therefore is not easily feasible or accessible to everyone.Citation32 Lastly, in 2011, some authors evaluated the total energy expenditure (TEE) in this category of subjects, using 2 frequency questionnaires on the levels of physical activity, subsequently comparing them with two methods for calculating the TEE; in the first method, the daily caloric intake was considered equal to30 kcal/Kg/body weight, while in the second case the energy requirement was obtained by multiplying the BEE x 1.7. By comparing the results of the frequency questionnaires with the methods of calculating the TEE, it was more suitable to administer a total caloric quantity equal to 30 kcal/kg/body weight. However, the authors underline how this formula can be effective on the calculation of needs referred to the population and not to the individual subject. For this reason, it is essential to carry out a personalized and tailored nutritional evaluation.Citation33
Sarcopenia and Osteoporosis
The increase in oxidative stress in patients with cachectic COPD is negatively associated with FFM and muscle strength,Citation34 and the mediators of systemic inflammation, such as TNF-α and NF-kB, are implicated in the muscle wasting of COPD.Citation35,Citation36 The decrease in muscle mass and muscle strength are typical consequences of sarcopenia; it is estimated that the percentage of sarcopenic COPD patients ranges from 12 to 39%, based on the evaluation method, bioempedentiometry (BIA) or double-beam X densitometer (DXA, gold standard instrument for the evaluation of body composition),Citation37–Citation40 with a higher incidence in subjects with lower BMI,Citation37 with a worse BODE scoreCitation37 and with cachexia.Citation40 It also appears to be associated with some inflammatory markers such as IL-6 and TNFα.Citation39 Furthermore, in a 2007 study on the assessment of body composition in patients with COPD, a clear association was found between well-preserved muscle mass (investigated with BIA) and lower risk of functional limitation (mainly assessed with 6-minute walk test).Citation13 Additionally, subjects with COPD are more likely to suffer from osteoporosis, and osteopenia is also higher in (26% and 54% respectively) than in control groups, with an increase in prevalence and severity with increasing intensity of COPD.Citation41 The incidence of new cases of osteoporosis during the course of the disease should not be underestimated; one work assessed a 14% increase in cases after a three-year follow-up period, mainly due to new vertebral fracture findings.Citation42
This could be due to the combination of factors, including smoking or previous smoking, the use of systemic corticosteroids, vitamin D deficiency and loss of lean mass, all of which are risk factors for the incidence of osteoporotic disease. It is not only important to evaluate the BMI but also and above to evaluate all the body composition by BIA or DXA, which indicate the quantity of muscle, as a gold standard assessment, present and bone mineralometry in order to verify the presence of sarcopenia or osteoporosis. In these situations, nutrition can be useful as prevention and treatment.
In conclusion, COPD patients often present with malnutrition, sarcopenia, osteoporosis with the possibility of the onset of cachexia, with an inadequate dietary intake and a poor quality of life.Citation19 Thus, the state of nutrition and the body composition of COPD patients must be assessed in order to provide adequate nutritional counseling. In particular, attention must paid to the number of meals and their quality in order to assess the total energy intake and the contribution of macro and micronutrients, so as to set an adequate and personalized dietary plan to obtain an improvement in nutritional status.Citation19,Citation43,Citation44 According to a recent official statement by the American Thoracic Society (ATS)/European Respiratory Society (ERS), in the context of non-pharmacological therapies, the objective assessment of nutritional status must be considered as an integral part of the management of the respiratory patient, with particular attention to the analysis of the muscular compartment, both respiratory and peripheral muscles.Citation45
Given this background, the present review aimed to evaluate the existing evidence regarding optimum diet therapy for the management of inflammation, oxidative stress and respiratory gas exchanges in subjects with COPD.
Materials and Methods
The present narrative review was performed following the steps of Egger et alCitation46 as follows:
1. Configuration of a working group: three operators skilled in clinical nutrition, of whom one acting as a methodological operator and two participating as clinical operators.
2. Formulation of the revision question on the basis of considerations made in the abstract:
the state of the art on dietary management of COPD patients in order to manage the carbon dioxide produced and oxygen consumed, other than management of inflammation and oxidative stress.
3. Identification of relevant studies: a research strategy was planned on PubMed, Public MedIine run by the National Center of Biotechnology Information (NCBI) of the National Library of Medicine of Bethesda (USA), as follows:
a) definition of the key words: COPD, foods, inflammation, oxidative stress, nutrients, respiratory gas exchanges allowing the definition of the interest field of the documents to be searched, grouped in inverted commas (“ … ”) and used separately or in combination;
b) use of: the Boolean (a data type with only two possible values: true or false) AND operator, which allows the establishments of logical relations among concepts;
c) research modalities: advanced search;
d) limits: time limits: papers published in the last 20 years; humans; languages: English;
e) manual search performed by senior researchers who are experienced in clinical nutrition through the revision of reviews and individual articles on the management of respiratory gas exchanges, inflammation and oxidative stress by dietary approach in COPD published in journals qualified in the Index Medicus.
Results
This review included 77 eligible studies and the dedicated flowchart is shown in .
Carbohydrates
This research was conducted based on the keywords: “chronic obstructive pulmonary disease “AND “inflammation” AND “carbohydrates” AND “diet” AND “gas exchanges”; four articles were sourced: all clinical studies.
Lipids (OMEGA-3)
This research was conducted based on the keywords: “chronic obstructive pulmonary disease “AND “inflammation” AND “lipids” AND “PUFA” AND “omega-3”; six articles were sourced: two cross-sectional investigation; one clinical study; one placebo controlled, randomized, double blind study; one systematic review and a meta-analysis; one observational study.
Protein
This research was conducted based on the keywords: “aging” AND “nutrition” AND “dietary protein” AND “ exercise” AND “physical function”; eleven articles were sourced: seven case-control study; one recommendations from the ESPEN Expert Group’, Clinical Nutrition; one position paper; one review; one single-blind randomized crossover design.
Fiber and Antioxidants
This research was conducted based on the keywords: “chronic obstructive pulmonary disease” AND “fruit” AND “inflammation” AND “oxidative stress” AND “vegetables”; eleven articles were sourced: three were randomized controlled trials; two prospective cohort studies; three cross‐sectional investigations; one multiple linear regression analysis; one clinical study; one case-control study.
Alcohol
This research was conducted based on the keywords: “alcohol consumption ”AND “chronic obstructive pulmonary disease”; three articles were sourced: one cross‐sectional investigation; two prospective cohort study.
Salt/Sodium
This research was carried out based on the keywords: “chronic bronchitis”, AND “chronic obstructive pulmonary disease”, AND “diet” AND “respiratory”. Eleven articles were sourced: three prospective cohort studies; three review; one clinical study; one retrospective study; one “in vitro” study”; ine report; one EFSA reference.
Vitamin D
This research was conducted based on the keywords: “”pulmonary function” AND “respiratory tract infection” AND “inflammation” AND “oxidative stress” AND “vitamin D” OR “25 hydroxyvitamin D” OR “25(OH)D” AND “vitamin D deficiency”; nine articles were sourced, as follows: three cross‐sectional investigations; four randomized clinical trial studies; one prospective cohort study; one clinical study.
Dietary Supplement or Foods for Specified Medical Purpose
This research was conducted based on the keywords: “dietary supplement” AND “chronic obstructive pulmonary disease ”AND “pulmonary rehabilitation”; four articles were sourced: two systematic reviews and meta-analysis; one prospective randomized and controlled study; one clinical study.
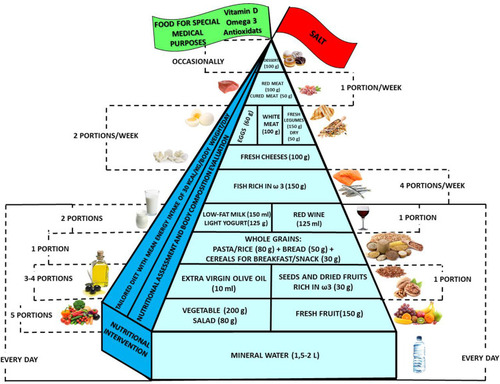
It was therefore decided to graphically represent in a simple and intuitive way what should be proper nutrition for the COPD patient, specifying the quality and amount of food, in order to counter the states of chronic inflammation and increased oxidative stress, along with the management of carbon dioxide produced and oxygen consumed.
This pyramid, presented in , is divided into three parts as follows:
(a) foods that should be consumed daily;
(b) foods that must be consumed 1, 2 or 4 times per week;
(c) foods to be eaten occasionally;
The food amounts are estimates based on nutritional and practical considerations.
The pennant at the top of the pyramid means that COPD subjects need a specific customized dietary supplementation with Vitamin D, n-3 fatty acids and antioxidants, such as Vitamin C.
Discussion
Carbohydrate
It is necessary to take into account the known respiratory difficulties in patients with COPD, and consequently evaluate the impact of nutrition on the production of carbon dioxide (V’CO2). High-calorie intakes, especially those with high carbohydrate content, increase V’CO2 and can precipitate respiratory failure in patients with severe lung disease.Citation47 The respiratory quotient (RQ) is the ratio of CO2 production to O2 consumption. An RQ of 1 indicates 100% oxidation of carbohydrates, while the general value of 0.7 indicates the oxidation of 100% of lipids; this means that the production of CO2 is greater by carbohydrates than lipids. Increased CO2 production leads to an increase in alveolar ventilation with a greater workload from the respiratory system.Citation47 Respiratory impairment, rather than guaranteeing oxygen intake, does not succeed in reducing, with an adequate exchange, the excessive concentration of carbon dioxide. Correct nutrition has a decisive role in these patients, both to rebalance nutritional status and to achieve that which is the priority objective in the presence of hypercapnia, ie the reduction of CO2 levels. This objective, which cannot be achieved by increasing the elimination of CO2, can be achieved by reducing the production of CO2 and optimizing the metabolic conditions of the patient. Respiratory impairment, rather than guaranteeing oxygen intake, does not succeed in reducing, with an adequate exchange, the excessive concentration of carbon dioxide. Several studies have proposed a lower percentage of carbohydrates in the diet in favor of a higher percentage of lipids; in fact, it seems that a diet rich in lipids is more advantageous for the patient with COPD than a diet with a high content of carbohydrates for ventilatory exchange,Citation48 as a reduced calorie intake of carbohydrates reduces all unfavorable physiological anomalies in chronic respiratory failure.Citation49 This data is also confirmed by other research in which a 22% improvement in forced vital capacity (CVF or FVC) and maximum expiratory volume in 1 second (FEV1) was obtained with a low carbohydrate diet, equal to 28%, with 55% of lipids.Citation50 Another study found that with a carbohydrate intake not exceeding 200 g per day (with further improvement when the total of carbohydrates does not exceeded 50 g per day) resulted in a better general well-being of patients with chronic respiratory insufficiency.Citation51
In conclusion, in COPD patients, it is useful to reduce the amount of carbohydrates to less than 200 grams of daily integral carbohydrates (equal to 1 portion of pasta or rice or other whole grains [80 g portion], 1 portion of whole meal bread [50 g portion] and 1 portion of whole grains for breakfast [30 g portion]Citation52 in favor of lipids with percentages that can reach up to 55% depending on the severity of the disease. In the initial phase of the disease, it will be useful to increase the lipid part (30–35% of total calories) with a controlled amount of carbohydrates (45–50 %).
Lipids (OMEGA-3)
Increasing the daily lipid quota, it will be necessary to choose the quality. Although the topic is still being studied, some authors have positively evaluated the intake of polyunsaturated fatty acids (PUFA), given their anti-inflammatory action, and the establishment of a condition of chronic inflammation typical of the disease itself.Citation53
When it comes to inflammation, some authors investigated for the first time in 2012 the existence of an association between consumption of omega-3 and omega-6 fatty acids using a food frequency questionnaire and some inflammatory blood markers in patients with COPD who are clinically stable. The results showed that a higher intake of ALA (α-linolenic acid, an essential fatty acid, founder of the omega-3, anti-inflammatory) was associated with lower levels of TNF-α, while higher assumptions of AA (arachidonic acid, pro-inflammatory omega-6) was correlated with higher concentrations of IL-6 and CRP.Citation54 High levels of blood DHA (decosaesanoic acid, a semi-essential fatty acid) have been shown to be useful in reducing the risk of developing COPD, and therefore having a preventive role for chronic inflammatory conditions of the lung.Citation55 More general intake of omega 3 fatty acids was associated with a higher FEV1,Citation56 and thus also assuming a role in the treatment of the disease.
Furthermore, PUFAs (polyunsaturated fatty acids) have shown beneficial effects on the ability to respond to exercise in patients with COPD, during a targeted rehabilitation programCitation57 and also specific improvement in the 6-minute walking test.Citation58
In summary, lipids should be well represented and omega-3 fatty acids (α-linolenic acid, docosahexaenoic acid, eicosapentaenoic acid), with their anti-inflammatory action, are useful both in the prevention of the risk and treatment of the chronic inflammatory state that occurs in patients. Omega 3 has also proved useful in improving the response to exercise. So, in COPD patients, the diet should contain a higher percentage of lipids (50%, preferably omega-3: fish, 4 servings/week; seeds, nuts: 30 grams/day). The risks regarding dioxin and polychlorinated biphenyls (PCB) intake are small and the health benefits are greater than, or at least similar to, the health risks, as recently demonstrated in a study that evaluated quantitative benefit-risk assessment.Citation59
Protein
Disturbances in intermediary protein metabolism have been demonstrated in patients with COPD. Yoneda T found in underweight COPD patients a decrease in plasma levels of branched-chain amino acids (BCAA) in relation to hypermetabolism, possibly resulting from the severity of COPD and respiratory muscle weakness, and various disturbances in plasma amino-acid levels.Citation60
The results of the study by Yoneda T confirm previous studies.Citation61,Citation62
This low plasma BCAA levels in COPD are associated with disturbances in muscle energy metabolism during exercise,Citation63 suggesting that preservation of BCAA levels is of importance in COPD. In contrast, COPD patients with preserved body weight have increased leucine turnoverCitation64 and preserved plasma leucine levels.Citation62
Given this background, various studies assessed the effectiveness of multi-supplementation (milk protein, hydrolyzed casein, whey protein, soy protein, amino acids) on whole body protein anabolism in COPD patients, but conflicting results were found.Citation65–Citation67
However, although this topic has been studied extensively, there are no specific indications regarding amino acids/protein intake or supplementation in patients with COPD. So, considering that it is estimated that the percentage of sarcopenic COPD patients ranges from 12 to 39%, for the prevention of sarcopenia, a protein quota of 1–1.2 g/kg/body weight is recommended, while in the patient with overt sarcopenia the protein intake should be 1.5 g/kg of body weight, as reported in the guidelines of the European artificial nutrition societyCitation68 and in the position paper of the PROT-AGE study group.Citation69
These indications are also reiterated in a recent review of 2019.Citation28
So, in COPD patients, the diet should contain foods of animal origin (white meat: 3 portions/week, red meat: 1 portion/week, milk and yogurt: 1 portion/day, twice a week cheese, eggs: 2 portions/week, legumes: 2–3 portions/week, fish, 4 servings/week) to prevent or treat muscle loss.
Fiber and Antioxidants
In addition to polyunsaturated fatty acids, another important nutritional factor is the consumption of fruit and vegetables, as they are rich in fiber and antioxidants. While the increased intake of fruit and vegetables for a short time (12 weeks) did not lead to significant improvements in markers of systemic inflammation, airways and oxidative stress,Citation70 long-term consumption of fruit, investigated with a food frequency questionnaire, was inversely associated with the incidence of COPD;Citation71 in addition to fruit, fiber intake, through the consumption of whole grains, was associated with a lower incidence of new COPD diagnoses.Citation5 The same conclusion emerged from the Morgen study, lasting 3 years, during which a questionnaire was administered to 13,651 subjects, with results showing an indirect beneficial association between the consumption of whole grains (> 45 g/day) and fruit (> 180 g/day) with the incidence of COPD.Citation2 Lifestyle changes, with increased consumption of fruit and vegetables compared to the usual diet, improved lung function.Citation72 Vice versa, a reduction in fruit consumption has proven to lead to a deterioration of FEV1.Citation73 A strong inverse association between total fiber consumption and the incidence of COPD in smokers and ex-smokers was also observed.Citation71 Vitamin C and Vitamin E have been studied as antioxidant factors. Blood levels of vitamin C and E were lower in patients with COPD.Citation74 Vitamin C intake was positively correlated with FEV1,Citation75 while vitamin E supplementation (400 IU per day) reduced blood markers of oxidative stress.Citation76 Furthermore, a nutritional supplement characterized by the presence of both, with specific contributions equal to 180 mg/day of vitamin C and 30 mg day α-tocopherol, associated with zinc (15 mg/day) and selenium (50 μg/day) in patients with COPD undergoing pulmonary rehabilitation treatment showed significant improvements in muscle strength, suggesting a potential “add-on” effect.Citation77 Other anti-oxidants, such as beta-cryptoxanthin, lutein zeaxanthin, retinol, beta-carotene and lycopene, both blood and food, were positively associated with FEV1%, supporting the hypothesis that an imbalance in the state antioxidant/oxidant is associated with chronic airflow limitation.Citation78
In conclusion, it is favorably useful to modify the lifestyle by adding (a) large quantities of fruit, vegetables (5 portions per day: 3 of fruit and 2 of vegetables, 1 raw and cooked),Citation52 (b) whole foods, to increase the fiber share,Citation79 and (c) antioxidants in order to improve FEV1 and oxidative stress. Particular attention should be paid to the consumption of Vitamin C. The foods that contain the greatest quantities are citrus fruits, red fruits, kiwi, peppers, tomatoes, spinach, broccoli, lettuce.Citation79 Another vitamin that is particularly required is Vitamin E, which is highly available when contained in extra virgin olive oil, wheat germ, dried fruit.Citation79 Two minerals that are highly recommended by the examined studies are zinc, which is available in meat, fish, eggs, dairy products, seeds and legumesCitation79 and selenium, which is present in foods of marine origin.Citation79
Finally, it is important to note that these recommendations on vitamins were mainly derived from isolated studies, not from randomized-controlled studies, so such recommendations could be true for a minority of the patients, and should not be generalized.
Alcohol
Alcohol intake was also taken into consideration in the examined studied. Within the MORGEN Study, in addition to the intake of fruit and whole grains, the intake of alcohol in small quantities was positively correlated with COPD; in subjects who declared consumption between 1–30 g/day, a higher FEV1 is described and a lower prevalence of symptoms compared to those who did not consume alcohol or those who consumed more.Citation2
Some authors demonstrated in 2012 a positive association between white wine intake and higher levels of FEV1 in the general population with a consequent lower risk of moderate airway obstruction.Citation80
In another recent prospective cohort study carried out on more than 44,000 Swedish men, the lowest risk of COPD incidence was highlighted in subjects who declared, through a food frequency questionnaire, alcohol consumption between 18 and 23.9 g of ethanol per day.Citation81
In conclusion, the protective role of moderate alcohol intake is confirmed, in the proportion of 125 mL of wine per day.Citation52
Salt/Sodium
The quantity of sodium intake, as often happens in pathological conditions, must be kept under control.
Sodium is unfortunately well represented in the diet called “western diet” (mainly based on the consumption of fast foods, packaged foods, red meats and processed meats, sweets and carbonated and/or sweetened drinks) which, as already explained, are related to risk of COPD.Citation3,Citation5-7
Moreover, there is some evidence, even as the topic is under debate,Citation82 that suggests a high sodium intake may accentuate airway reactivity and reduce flows,Citation83 through potentiation of the electrogenic sodium pump in the membrane of the airway smooth muscle.Citation84,Citation85 Furthermore, the contractile response of airway smooth muscle cells to specific antigen has been demonstrated to be dependent on the level of hyperpolarization resulting from sodium influx.Citation86 Therefore, a diet with a high salt content could predispose people toward the development of airway disease, particularly airway hyperreactivity.
Finally, a study was carried out on the Chinese population of Singapore, in which there was a 1.4-fold increase in the risk of cough with phlegm in subjects who had a meat-rich diet for a short period of time, or preserved or fried foods and noodles.Citation87
An intake of about 2 g of sodium is the amount recommended by EFSA.Citation88
Dietary Supplements
On top of the food pyramid for the dietary management of COPD, there is a pennant to draw attention to the fact that subjects with COPD require special dietary supplements: Vitamin D, n-3 fatty acids, antioxidants (Vitamin C and E, selenium, zinc).
Vitamin D
Several studies suggest that patients with COPD whose vitamin D (25-OH) values are lower than 20 ng/mL (deficient) may be at greater risk of exacerbations of the disease, worsening pulmonary function and decline in lung function over time.Citation89–Citation94 In subjects with severe deficiency (blood Vitamin D values <10 ng/mL) who received supplementation with Vitamin D at high doses (100,000 IU per4 weeks), a reduction in exacerbations was demonstratedCitation93 and an improvement in FEV1 in patients with severe and very severe COPD was observed (100,000 IU once a month for 6 months).Citation95 Daily Vitamin D supplementation has a significant effect in reducing the number of acute exacerbations when it is given for a prolonged period (2000 IU per day for 6 months).Citation96
Foods that contain a good quantity of Vitamin D, such as fish, in particular fish oil and liver, are consumed less, while foods that are consumed daily, such as milk and eggs, are represented in smaller quantities.Citation79 The impact of nutrition on the level of Vitamin D is therefore low and almost all of Vitamin D is synthesized in the skin through adequate sun exposure.
The calcium requirement in adults is 1000 mg per day while in menopausal women and in the elderly it is 1200 mg per day.
The vitamin D requirement in adults is 15 µg per day while in the elderly it is 20µg per day.Citation97
To conclude, it is necessary for all patients with COPD to monitor blood levels of Vitamin D and provide adequate supplementation, and patients should be reminded that since Vitamin D is a fat-soluble vitamin, supplementation should be carried out during a meal in which there are lipids consumed.
Foods for Special Medical Purposes
To promote a significant increase in weight and muscle strength in malnourished patients, it is not enough to have a correct personalized dietary approach that brings adequate amounts of macro- and micronutrients; it is also important to get nutritional supplementation with foods for special medical purposes.Citation43,Citation44 In subjects who received a combination of nutritional supplementation with respiratory rehabilitation, improvements were noted, particularly in lean mass, compared to respiratory rehabilitation alone.Citation98 Given the greater production of CO2 following carbohydrate intake compared to lipid intake, the ideal nutritional supplementation should mainly contain lipids. A drink rich in carbohydrates compared to a lipid-rich one leads to a significant increase in V’CO2 values with a worsening of performance in the 6-minute walk test with a greater risk of falling.Citation47 It should be emphasized that nutritional supplements are effective when they are needed; normal-fed patients may not respond in the same way to nutritional supplementation.Citation44
Conclusion
In conclusion, for the patient with COPD, it is necessary first of all to provide a nutritional and body composition assessment in order to estimate their needs and then build a personalized normocaloric dietary scheme if the patient is of normal-weight or overweight (BMI between 18 and 30 kg/m2), a high-calorie diet if the patient is malnourished (BMI <18 kg/m2), and a low-calorie diet if the patient is obese (BMI> 30 kg/m2). The diet should contain a higher percentage of lipids (up to about 50%), and a reduced amount of carbohydrates (about 30%) compared to that for healthy patient. The choice of fats should be directed towards polyunsaturated fats, preferably omega-3 (fish: 4 servings per week, seeds and nuts; 30 grams per day). The supply of antioxidants must be constant, especially with the use of extra virgin olive oil (2–3 servings per day of 10 mL) and nuts (rich in Vitamin E) in the portion of 30 grams per day, 5 portions in between fruits and vegetables every day, in particular citrus fruits, kiwis, red fruits, peppers, tomatoes, spinach, broccoli, lettuce due to the high Vitamin C content, and foods of animal origin such as meat (3 portions a week of white meat, 1 portion per week of red meat), milk (daily 1 portion of milk and 1 of yogurt, twice a week cheese), eggs (2 portions per week), legumes (2–3 portions per week) and fish (4 portions per week) for the high zinc and selenium content.
Also, the fiber must be well represented, with whole grains, in the quantity of at least 25 g per day.Citation97
The protein content must be significantly present to prevent or treat muscle loss. In the former case, the protein intake must be 1–1.2 g/kg/lost body, while in the second case the proteins must be equal 1.5 g/kg/lost body with a specific supply of leucine equal to 2.5–2.8 g (meat, cheese, fish, eggs).
The calcium content will have to cover the estimated needs (1000 mg for the adult, 1200 mg for menopausal women), to prevent osteoporosis, through the daily consumption of water rich in calcium (2 liters), milk, yogurt and bi-weekly consumption of cheeses. It will also be necessary to evaluate the possibility of initiating specific integration with Vitamin D in the event of proven deficiency or insufficient levels.
The need to insert a food for special medical purposes specific for the disease with a quantity of lipids higher than that of carbohydrates will have to be assessed individually, through a personalized nutritional evaluation.
Two examples of diet (the first with 50% of lipids and 30% of carbohydrates, while in the second diet the percentages of lipids and carbohydrates are inverted.), with the related bromatological analysis, are presented in and .
Table 1 Bromatological Analysis and Example of Diet with 50% of Lipids and 30% of Carbohydrates
Table 2 Bromatological Analysis and Example of Diet with 30% of Lipids and 50% of Carbohydrates
In conclusion, the nutrition pyramid described in our narrative review is hypothetical, even in light of several limitations of the present review; the main limitation is the fact that to date there are no randomized controlled trials in the literature clearly showing that improved nutrition, via the regulation of carbon dioxide produced/oxygen consumed, inflammation and oxidative stress, improves symptoms and/or the progression of COPD. Further, to build the pyramid, we could only make a narrative review of the literature, not a meta-analysis.
Even if this nutritional pyramid is hypothetical, we hope that it can serve the valuable purpose of helping researchers focus on the often-ignored possible connections between nutrition and COPD. Further investigation is needed in the future and, specifically, more randomized clinical trials should be conducted that directly study nutrition and symptoms and/or progression of COPD in order to understand the specific mechanisms that interconnect the regulation of carbon dioxide produced/oxygen consumed, inflammation, oxidative stress and nutrition.
Abbreviation
AA, arachidonic acid; ALA, α-linolenic acid; ATS, American Thoracic Society; BCAA, branched-chain amino acids; BEE, Basal Energy Expenditure; BIA, bioempedentiometry; BMI, body mass index; BODE, Body-mass index, airflow Obstruction, Dyspnea, and Exercise; COPD, Chronic obstructive pulmonary disease; CRP, C-reactive protein; DHA, decosaesanoic acid; DXA, double-beam X densitometer; EFSA, European Food Safety Authority; ERS, European Respiratory Society; ESPEN, European Society for Clinical Nutrition and Metabolism; FEV1, maximum expiratory volume in 1 second; FFM, Fat Free Mass; FM, Fat Mass; FVC (or CVF), forced vital capacity; g, grams; kcal, kilocalories; Kg, kilograms; mg, milligrams; m, meter; NCBI, National Center of Biotechnology Information; NF-kB, Nuclear Factor kappa-light-chain-enhancer of activated B cells; PAL, Physical Activity Levels; PCB, polychlorinated biphenyls; PUFA, polyunsaturated fatty acids; RQ, respiratory quotient; SPPB, Short Physical Performance Battery; TEE, total energy expenditure; TNF-α, tumor necrosis factor α; V’CO2 - CO2, carbon dioxide; μg, micrograms.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors report no conflicts of interest in this work.
Acknowledgments
The authors want to thank Dr. Antonella Riva and Dr. Giovanna Petrangolini, from INDENA SpA, as they have dealt with the revision for English language.
References
- WillettWC, SacksF, TrichopoulouA, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6):1402S–1406S. doi:10.1093/ajcn/61.6.1402S7754995
- TabakC, SmitHA, HeederikD, OckéMC, KromhoutD. Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study). Clin Exp Allergy. 2001;31(5):747–755. doi:10.1046/j.1365-2222.2001.01064.x.11422134
- VarrasoR, FungTT, BarrRG, HuFB, WillettW, CamargoCA. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am J Clin Nutr. 2007;86(2):488–495. doi:10.1093/ajcn/86.2.48817684223
- VarrasoR, KauffmannF, LeynaertB, et al. Dietary patterns and asthma in the E3N study. Eur Respir J. 2009;33(1):33–41. doi:10.1183/09031936.0013080718829673
- VarrasoR, ChiuveSE, FungTT, et al. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: prospective study. BMJ. 2015;350(feb03 7):h286–h286. doi:10.1136/bmj.h28625649042
- WoodLG, GargML, GibsonPG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol. 2011;127(5):1133–1140. doi:10.1016/j.jaci.2011.01.03621377715
- BerthonBS, WoodLG. Nutrition and respiratory health—feature review. Nutrients. 2015;7(3):1618–1643. doi:10.3390/nu703161825751820
- McKeeverTM, LewisSA, CassanoPA, et al. Patterns of dietary intake and relation to respiratory disease, forced expiratory volume in 1 s, and decline in 5-y forced expiratory volume. Am J Clin Nutr. 2010;92(2):408–415. doi:10.3945/ajcn.2009.2902120554789
- LandboC, PrescottE, LangeP, VestboJ, AlmdalTP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. doi:10.1164/ajrccm.160.6.990211510588597
- HallinR, GudmundssonG, Suppli UlrikC, et al. Nutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD). Respir Med. 2007;101(9):1954–1960. doi:10.1016/j.rmed.2007.04.00917532198
- CaoC, WangR, WangJ, BunjhooH, XuY, XiongW. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. simpson C, ed. PLoS One. 2012;7(8):e43892. doi:10.1371/journal.pone.004389222937118
- CelliBR, CoteCG, MarinJM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa02132214999112
- EisnerMD, BlancPD, SidneyS, et al. Body composition and functional limitation in COPD. Respir Res. 2007;8(1):7. doi:10.1186/1465-9921-8-717261190
- Montes de OcaM, TálamoC, Perez-PadillaR, et al. Chronic obstructive pulmonary disease and body mass index in five Latin America cities: the PLATINO study. Respir Med. 2008;102(5):642–650. doi:10.1016/j.rmed.2007.12.02518314321
- LainscakM, von HaehlingS, DoehnerW, et al. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J Cachexia Sarcopenia Muscle. 2011;2(2):81–86. doi:10.1007/s13539-011-0023-921766053
- ScholsAM, BroekhuizenR, Weling-ScheepersCA, WoutersEF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82(1):53–59. doi:10.1093/ajcn.82.1.5316002800
- ScholsAM, FerreiraIM, FranssenFM, et al. Nutritional assessment and therapy in COPD: A European respiratory society statement. Eur Respir J. 2014;44(6):1504–1520. doi:10.1183/09031936.0007091425234804
- FilleyGF, BeckwittHJ, ReevesJT, MitchellRS. Chronic obstructive bronchopulmonary disease. II. Oxygen transport in two clinical types. Am J Med. 1968;44(1):26–38. doi:10.1016/0002-9343(68)90234-95635286
- NguyenHT, CollinsPF, PaveyTG, NguyenNV, PhamTD, GallegosDL. Nutritional status, dietary intake, and health-related quality of life in outpatients with COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:215–226. doi:10.2147/COPD.S18132230666102
- RemelsAHV, GoskerHR, LangenRCJ, ScholsAMWJ. The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol. 2013;114(9):1253–1262. doi:10.1152/japplphysiol.00790.201223019314
- SteutenLMG, CreutzbergEC, VrijhoefHJM, WoutersEF. COPD as a multicomponent disease: inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006;15(2):84–91. doi:10.1016/j.pcrj.2005.09.00116701766
- VozorisNT, O’DonnellDE. Prevalence, risk factors, activity limitation and health care utilization of an obese population-based sample with chronic obstructive pulmonary disease. Can Respir J. 2012;19(3):e18–e24. doi:10.1155/2012/73261822679617
- ZapateroA, BarbaR, RuizJ, et al. Malnutrition and obesity: influence in mortality and readmissions in chronic obstructive pulmonary disease patients. J Hum Nutr Diet. 2013;26(SUPPL.1):16–22. doi:10.1111/jhn.1208823656492
- JeeSH, SullJW, ParkJ, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355(8):779–787. doi:10.1056/NEJMoa05401716926276
- SpeltaF, Fratta PasiniAM, CazzolettiL, FerrariM. Body weight and mortality in COPD: focus on the obesity paradox. Eat Weight Disord Stud Anorexia Bulim Obes. 2018;23(1):15–22. doi:10.1007/s40519-017-0456-z
- Van BorstB, Den, GoskerHR, KosterA, et al. The influence of abdominal visceral fat on inflammatory pathways and mortality risk in obstructive lung disease. Am J Clin Nutr. 2012;96(3):516–526. doi:10.3945/ajcn.112.04077422811442
- GanzoniA, HeiligP, SchönenbergerK, HügliO, FittingJW, BrändliO. High-caloric nutrition in chronic obstructive lung disease. Schweiz Rundsch Med Prax. 1994;83(1):13–16.8029587
- CollinsPF, YangIA, ChangY-C, VaughanA. Nutritional support in chronic obstructive pulmonary disease (COPD): an evidence update. J Thorac Dis. 2019;11(Suppl 17):S2230–S2237. doi:10.21037/jtd.2019.10.4131737350
- BaarendsEM, ScholsAMWJ, PannemansDLE, WesterterpKR, WoutersEFM. Total free living energy expenditure in patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155(2):549–554. doi:10.1164/ajrccm.155.2.90321939032193
- WilsonDO, DonahoeM, RogersRM, PennockBE. Metabolic rate and weight loss in chronic obstructive lung disease. J Parenter Enter Nutr. 1990;14(1):7–11. doi:10.1177/014860719001400107
- ScholsAMWJ, FredrixEWHM, SoetersPB, WesterterpKR, WoutersEFM. Resting energy expenditure in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 1991;54(6):983–987. doi:10.1093/ajcn/54.6.9831957831
- NordensonA, GrönbergAM, HulthénL, LarssonS, SlindeF. A validated disease specific prediction equation for resting metabolic rate in underweight patients with COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:271–276. doi:10.2147/copd.s1254420856826
- SlindeF, GrönbergAM, SvantessonU, HulthénL, LarssonS. Energy expenditure in chronic obstructive pulmonary disease-evaluation of simple measures. Eur J Clin Nutr. 2011;65(12):1309–1313. doi:10.1038/ejcn.2011.11721697822
- BarreiroE, RabinovichR, Marin-CorralJ, BarberàJA, GeaJ, RocaJ. Chronic endurance exercise induces quadriceps nitrosative stress in patients with severe COPD. Thorax. 2009;64(1):13–19. doi:10.1136/thx.2008.10516318835959
- SharmaR, AnkerSD. Cytokines, apoptosis and cachexia: the potential for TNF antagonism. Int J Cardiol. 2002;85(1):161–171. doi:10.1016/S0167-5273(02)00244-9.12163221
- LangenRCJ, HaegensA, VernooyJHJ, et al. NF-κB activation is required for the transition of pulmonary inflammation to muscle atrophy. Am J Respir Cell Mol Biol. 2012;47(3):288–297. doi:10.1165/rcmb.2011-0119OC22538866
- CostaTM, DaRL, CostaFM, et al. Sarcopenia in COPD: relationship with COPD severity and prognosis. J Bras Pneumol. 2015;41(5):415–421. doi:10.1590/S1806-3713201500000004026578132
- JonesP, Dalziel, SR, LamdinR, et al. Oral non-steroidal anti-inflammatory drugs versus other oral analgesic agents for acute soft tissue injury. Cochrane Database Syst Rev. 2015;2015(7). doi:10.1002/14651858.CD007789.pub2
- ByunMK, ChoEN, ChangJ, AhnCM, KimHJ. Sarcopenia correlates with systemic inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:669–675. doi:10.2147/COPD.S13079028255238
- de BlasioF, Di GregorioA, de BlasioF, BiancoA, BellofioreB, ScalfiL. Malnutrition and sarcopenia assessment in patients with chronic obstructive pulmonary disease according to international diagnostic criteria, and evaluation of raw BIA variables. Respir Med. 2018;134:1–5. doi:10.1016/j.rmed.2017.11.00629413494
- EL-GazzarAG, AbdallaME, AlmahdyMA. Study of Osteoporosis in chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2013;62(1):91–95. doi:10.1016/J.EJCDT.2013.01.009
- Graat-VerboomL, SmeenkFWJM, Van Den BorneBEEM, et al. Progression of osteoporosis in patients with COPD: a 3-year follow up study. Respir Med. 2012;106(6):861–870. doi:10.1016/j.rmed.2011.12.02022369986
- CollinsPF, StrattonRJ, EliaM. Nutritional support in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1385–1395. doi:10.3945/ajcn.111.02349922513295
- FerreiraIM, BrooksD, WhiteJ, GoldsteinR. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD000998. doi:10.1002/14651858.CD000998.pub323235577
- CelliBR, DecramerM, WedzichaJA, et al. An official american thoracic society/european respiratory society statement: research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(7):e4–e27. doi:10.1164/rccm.201501-0044ST25830527
- EggerM, SmithGD, AltmanDG. Systematic Reviews in Health Care: Meta-Analysis in Context. BMJ Books; 2001.
- EfthimiouJ, MounseyPJ, BensonDN, MadgwickR, ColesSJ, BensonMK. Effect of carbohydrate rich versus fat rich loads on gas exchange and walking performance in patients with chronic obstructive lung disease. Thorax. 1992;47(6):451–456. doi:10.1136/thx.47.6.4511496505
- KuoCD, ShiaoGM, LeeJD. The effects of high-fat and high-carbohydrate diet loads on gas exchange and ventilation in COPD patients and normal subjects. Chest. 1993;104(1):189–196. doi:10.1378/chest.104.1.1898325067
- TirlapurVG, MirMA. Effect of low calorie intake on abnormal pulmonary physiology in patients with chronic hypercapneic respiratory failure. Am J Med. 1984;77(6):987–994. doi:10.1016/0002-9343(84)90177-36507470
- AngelilloVA, BediS, DurfeeD, DahlJ, PattersonAJ, O’DonohueWJ. Effects of low and high carbohydrate feedings in ambulatory patients with chronic obstructive pulmonary disease and chronic hypercapnia. Ann Intern Med. 1985;103(6 (Pt 1)):883–885. doi:10.7326/0003-4819-103-6-8833933397
- KwanR, MirMA. Beneficial effects of dietary carbohydrate restriction in chronic cor pulmonale. Am J Med. 1987;82(4):751–758. doi:10.1016/0002-9343(87)90011-83105310
- SINU. Standard quantitativi delle porzioni. Available from: http://www.sinu.it/public/20141111_LARN_Porzioni.pdf.
- GanWQ, ManSFP, SenthilselvanA, SinDD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574–580. doi:10.1136/thx.2003.01958815223864
- de BatlleJ, SauledaJ, BalcellsE, et al. Association between Ω3 and Ω6 fatty acid intakes and serum inflammatory markers in COPD. J Nutr Biochem. 2012;23(7):817–821. doi:10.1016/j.jnutbio.2011.04.00521889886
- ShaharE, BolandLL, FolsomAR, TockmanMS, McGovernPG, EckfeldtJH. Docosahexaenoic acid and smoking-related chronic obstructive pulmonary disease. The atherosclerosis risk in communities study investigators. Am J Respir Crit Care Med. 1999;159(6):1780–1785. doi:10.1164/ajrccm.159.6.981006810351918
- Garcia-LarsenV, AmigoH, BustosP, BakolisI, RonaRJ. Ventilatory function in young adults and dietary antioxidant intake. Nutrients. 2015;7(4):2879–2896. doi:10.3390/nu704287925884660
- BroekhuizenR, WoutersEFM, CreutzbergEC, Weling-ScheepersCAPM, ScholsAMWJ. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax. 2005;60(5):376–382. doi:10.1136/thx.2004.03085815860712
- MatsuyamaW, MitsuyamaH, WatanabeM, et al. Effects of omega-3 polyunsaturated fatty acids on inflammatory markers in COPD. Chest. 2005;128(6):3817–3827. doi:10.1378/chest.128.6.381716354850
- TuomistoJT, AsikainenA, MeriläinenP, HaapasaariP. Health effects of nutrients and environmental pollutants in Baltic herring and salmon: a quantitative benefit-risk assessment. BMC Public Health. 2020;20(1):64. doi:10.1186/s12889-019-8094-131941472
- YonedaT, YoshikawaM, FuA, TsukaguchiK, OkamotoY, TakenakaH. Plasma levels of amino acids and hypermetabolism in patients with chronic obstructive pulmonary disease. Nutrition. 2001;17(2):95–99. doi:10.1016/S0899-9007(00)00509-811240335
- PouwEM, ScholsAM, DeutzNE, WoutersEF. Plasma and muscle amino acid levels in relation to resting energy expenditure and inflammation in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(3):797–801. doi:10.1164/ajrccm.158.3.97080979731007
- EngelenMP, WoutersEF, DeutzNE, MenheerePP, ScholsAM. Factors contributing to alterations in skeletal muscle and plasma amino acid profiles in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2000;72(6):1480–1487. doi:10.1093/ajcn/72.6.148011101475
- KutsuzawaT, ShioyaS, KuritaD, HaidaM. Plasma branched-chain amino acid levels and muscle energy metabolism in patients with chronic obstructive pulmonary disease. Clin Nutr. 2009;28(2):203–208. doi:10.1016/j.clnu.2009.01.01919250720
- KaoCC, HsuJWC, BandiV, HananiaNA, KheradmandF, JahoorF. Resting energy expenditure and protein turnover are increased in patients with severe chronic obstructive pulmonary disease. Metabolism. 2011;60(10):1449–1455. doi:10.1016/j.metabol.2011.02.01321550084
- JonkerR, DeutzNEP, ScholsAMWJ, et al. Whole body protein anabolism in COPD patients and healthy older adults is not enhanced by adding either carbohydrates or leucine to a serving of protein. Clin Nutr. 2019;38(4):1684–1691. doi:10.1016/j.clnu.2018.08.00630150004
- EngelenMPKJ, De CastroCLN, RuttenEPA, WoutersEFM, ScholsAMWJ, DeutzNEP. Enhanced anabolic response to milk protein sip feeding in elderly subjects with COPD is associated with a reduced splanchnic extraction of multiple amino acids. Clin Nutr. 2012;31(5):616–624. doi:10.1016/j.clnu.2012.04.00622682082
- EngelenMPKJ, RuttenEPA, De CastroCLN, WoutersEFM, ScholsAMWJ, DeutzNEP. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2007;85(2):431–439. doi:10.1093/ajcn/85.2.43117284740
- DeutzNEP, BauerJM, BarazzoniR, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–936. doi:10.1016/j.clnu.2014.04.00724814383
- BauerJ, BioloG, CederholmT, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the prot-age study group. J Am Med Dir Assoc. 2013;14(8):542–559. doi:10.1016/j.jamda.2013.05.02123867520
- BaldrickFR, ElbornJS, WoodsideJV, et al. Effect of fruit and vegetable intake on oxidative stress and inflammation in COPD: a randomised controlled trial. Eur Respir J. 2012;39(6):1377–1384. doi:10.1183/09031936.0008601122088966
- KaluzaJ, HarrisH, WallinA, LindenA, WolkA. Dietary fiber intake and risk of chronic obstructive pulmonary disease: a prospective cohort study of men. Epidemiology. 2018;29(2):254–260. doi:10.1097/EDE.000000000000075028901975
- KeranisE, MakrisD, RodopoulouP, et al. Impact of dietary shift to higher-antioxidant foods in COPD: a randomised trial. Eur Respir J. 2010;36(4):774–780. doi:10.1183/09031936.0011380920150206
- CareyIM, StrachanDP, CookDG. Effects of changes in fresh fruit consumption on ventilatory function in healthy British adults. Am J Respir Crit Care Med. 1998;158(3):728–733. doi:10.1164/ajrccm.158.3.97120659730997
- LinY-C, WuT-C, ChenP-Y, HsiehL-Y, YehS-L. Comparison of plasma and intake levels of antioxidant nutrients in patients with chronic obstructive pulmonary disease and healthy people in Taiwan: a case-control study. Asia Pac J Clin Nutr. 2010;19(3):393–401.20805084
- SchwartzJ, WeissST. Relationship between dietary vitamin C intake and pulmonary function in the first national health and nutrition examination survey (NHANES I). Am J Clin Nutr. 1994;59(1):110–114. doi:10.1093/ajcn/59.1.1108279390
- DagaMK, ChhabraR, SharmaB, MishraTK. Effects of exogenous vitamin E supplementation on the levels of oxidants and antioxidants in chronic obstructive pulmonary disease. J Biosci. 2003;28(1):7–11. doi:10.1007/BF2970125.12682418
- GouziF, MauryJ, HéraudN, et al. Additional effects of nutritional antioxidant supplementation on peripheral muscle during pulmonary rehabilitation in COPD patients: a randomized controlled trial. Oxid Med Cell Longev. 2019;2019:5496346. doi:10.1155/2019/549634631178967
- Ochs-BalcomHM, GrantBJB, MutiP, et al. Antioxidants, oxidative stress, and pulmonary function in individuals diagnosed with asthma or COPD. Eur J Clin Nutr. 2006;60(8):991–999. doi:10.1038/sj.ejcn.160241016482071
- BDAIEO. Banca Dati di Composizione degli Alimenti per studi epidemiologici in Italia; 2015 Available from: http://www.bda-ieo.it.
- SiedlinskiM, BoerJMA, SmitHA, PostmaDS, BoezenHM. Dietary factors and lung function in the general population: wine and resveratrol intake. Eur Respir J. 2012;39(2):385–391. doi:10.1183/09031936.0018411021852339
- KaluzaJ, HarrisHR, LindenA, Alcohol ConsumptionWA. Risk of chronic obstructive pulmonary disease: a prospective cohort study of men. Am J Epidemiol. 2019;188(5):907–916. doi:10.1093/aje/kwz02030877760
- BrittonJ, PavordI, RichardsK, et al. Dietary sodium intake and the risk of airway hyperreactivity in a random adult population. Thorax. 1994;49(9):875–880. doi:10.1136/thx.49.9.8757940426
- RomieuI, TrengaC. Diet and obstructive lung diseases. Epidemiol Rev. 2001;23(2):268–287. doi:10.1093/oxfordjournals.epirev.a00080612192737
- BurneyP. A Diet rich in sodium may potentiate asthma. Chest. 1987;91(6):143S–148S. doi:10.1378/chest.91.6_supplement.143s3581956
- KnoxAJ, AjaoP, BrittonJR, TattersfieldAE. Effect of sodium-transport inhibitors on airway smooth muscle contractility in vitro. Clin Sci. 1990;79(4):315–323. doi:10.1042/cs07903152171851
- MonteleoneCA, ShermanAR. Nutrition and asthma. Arch Intern Med. 1997;157(1):23–34. doi:10.1001/archinte.1997.00440220027005.8996038
- ButlerLM, KohWP, LeeHP, TsengM, YuMC, LondonSJ. Prospective study of dietary patterns and persistent cough with phlegm among Chinese Singaporeans. Am J Respir Crit Care Med. 2006;173(3):264–270. doi:10.1164/rccm.200506-901OC16239624
- EFSA. Tenori di sodio e cloruro di riferimento per l’alimentazione umanaitle. Available from: https://www.efsa.europa.eu/it/press/news/190403.
- BlackPN, ScraggR. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128(6):3792–3798. doi:10.1378/chest.128.6.379216354847
- GindeAA, MansbachJM, CamargoCA. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third national health and nutrition examination survey. Arch Intern Med. 2009;169(4):384–390. doi:10.1001/archinternmed.2008.56019237723
- ShaheenSO, JamesonKA, RobinsonSM, et al. Relationship of vitamin D status to adult lung function and COPD. Thorax. 2011;66(8):692–698. doi:10.1136/thx.2010.15523421653927
- LangeNE, SparrowD, VokonasP, LitonjuaAA. Vitamin D deficiency, smoking, and lung function in the normative aging study. Am J Respir Crit Care Med. 2012;186(7):616–621. doi:10.1164/rccm.201110-1868OC22822023
- LehouckA, MathieuC, CarremansC, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105–114. doi:10.7326/0003-4819-156-2-201201170-0000422250141
- PuhanMA, SiebelingL, FreiA, ZollerM, Bischoff-FerrariH, Ter RietG. No association of 25-hydroxyvitamin D with exacerbations in primary care patients with COPD. Chest. 2014;145(1):37–43. doi:10.1378/chest.13-129624008868
- ZendedelA, GholamiM, AnbariK, GhanadiK, BachariEC, AzargonA. Effects of vitamin D intake on FEV1 and COPD exacerbation: a randomized clinical trial study. Glob J Health Sci. 2015;7(4):243–248. doi:10.5539/gjhs.v7n4p24325946929
- KhanDM, UllahA, RandhawaFA, IqtadarS, ButtNF, WaheedK. Role of Vitamin D in reducing number of acute exacerbations in chronic obstructive pulmonary disease (COPD) patients. Pakistan J Med Sci. 2017;33(3):610–614. doi:10.12669/pjms.333.12397
- SINU. Tabelle Larn. Available from: http://www.sinu.it/html/pag/tabelle_larn_2014_rev.asp.
- GurgunA, DenizS, ArgınM, KarapolatH. Effects of nutritional supplementation combined with conventional pulmonary rehabilitation in muscle-wasted chronic obstructive pulmonary disease: a prospective, randomized and controlled study. Respirology. 2013;18(3):495–500. doi:10.1111/resp.1201923167516