Abstract
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable lung disease characterized by airflow limitation that is not fully reversible. In a significant proportion of patients with COPD, reduced lung elastic recoil combined with expiratory flow limitation leads to lung hyperinflation during the course of the disease. Development of hyperinflation during the course of COPD is insidious. Dynamic hyperinflation is highly prevalent in the advanced stages of COPD, and new evidence suggests that it also occurs in many patients with mild disease, independently of the presence of resting hyperinflation. Hyperinflation is clinically relevant for patients with COPD mainly because it contributes to dyspnea, exercise intolerance, skeletal muscle limitations, morbidity, and reduced physical activity levels associated with the disease. Various pharmacological and nonpharmacological interventions have been shown to reduce hyperinflation and delay the onset of ventilatory limitation in patients with COPD. The aim of this review is to address the more recent literature regarding the pathogenesis, assessment, and management of both static and dynamic lung hyperinflation in patients with COPD. We also address the influence of biological sex and obesity and new developments in our understanding of hyperinflation in patients with mild COPD and its evolution during progression of the disease.
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable lung disease characterized by airflow limitation that is not fully reversible.Citation1 COPD is a leading cause of mortality and morbidity worldwide, even if it remains largely underdiagnosed.Citation2,Citation3 Currently, the prevalence of the disease is estimated to be around 10% in the population aged >40 yearsCitation4 and could reach around 20%–30%Citation5,Citation6 when including milder patients (Global initiative for chronic Obstructive Lung Disease [GOLD] stage 1).Citation1
In a significant proportion of patients with COPD, reduced lung elastic recoil combined with expiratory flow limitation eventually leads to lung hyperinflation during the course of the disease.Citation7 In patients with COPD, the lung can be hyperinflated at rest (static hyperinflation) and/or during exercise (dynamic hyperinflation) when ventilatory requirements are increased and expiratory time is shortened. Hyperinflation is clinically relevant for patients with COPD mainly because it contributes to the dyspneaCitation8 and morbidity associated with the disease.Citation9 In fact, although measurement of expiratory flows is a prerequisite for the diagnosis and staging of COPD, the effects of the disease on static and dynamic lung volumes correlate better with patient symptoms and impairment in functional capacity than spirometric indices of the disease.Citation10 Moreover, dynamic lung hyperinflation is related to reduced daily physical activity in COPD,Citation11 which is an important component of quality of life.Citation12
Despite the difficulties in establishing a cause-effect relationship, exercise intolerance and lung hyperinflation are closely interrelated in COPD.Citation13,Citation14 While exercise intolerance in patients with COPD is complex and multifactorial,Citation15–Citation17 dynamic hyperinflation remains a major contributor to exercise limitation that is consistently observed in this disease.Citation18 During exercise, hyperinflation may impede cardiacCitation19,Citation20 and respiratory muscle function and increase the work of breathing.Citation21 Finally, this phenomenon can also occur in patients with mild disease,Citation22–Citation24 a category of individuals likely representing a great portion of patients diagnosed with COPD.Citation5
This review addresses the more recent literature regarding the pathogenesis of both static and dynamic lung hyperinflation. The pathophysiology and physiological consequences of lung hyperinflation are summarized, as well as management, pharmacological treatment, and the impact of pulmonary rehabilitation on hyperinflation. We also address the influence of biological sex and obesity and new developments in our understanding of hyperinflation in mild COPD patients and its evolution during progression of the disease. The review is based on literature available on the PubMed database, irrespective of the year of publication.
Pathophysiology of hyperinflation
Lung volumes can be divided into several compartments defined by the normal cycle of tidal breathing and the maximum capacity to inhale and exhale (). In health, during relaxed tidal breathing, the lungs tend to return to a basal level of inflation, which is termed functional residual capacity (FRC) or end-expiratory lung volume (EELV). During the hyperpnea of exercise, both tidal volume (VT) and respiratory rate increase to meet the increased ventilatory requirements. Therefore, maintenance of stable lung volumes requires that expiratory muscles must be recruited to elevate pleural and alveolar pressure, increase expiratory flow, and force the increased VT to be completely exhaled before the next inhalation.Citation25
Figure 1 Lung volumes and capacities at rest and during exercise.
Abbreviations: COPD, chronic obstructive pulmonary disease; EELV, end-expiratory lung volume; EILV, end-inspiratory lung volume; ERV, expiratory reserve volume; FRC, functional residual capacity; FVC, forced vital capacity; IC, inspiratory capacity; IRV, inspiratory reserve volume; RV, residual volume; VT, tidal volume.
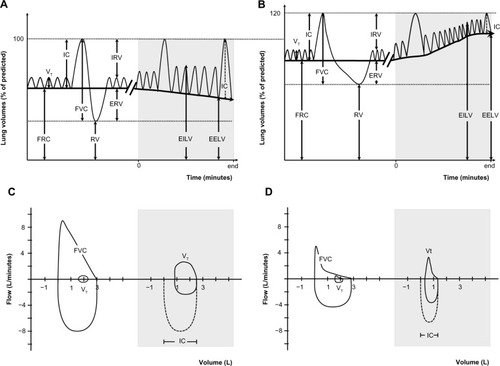
Hyperinflation, defined as an increased volume of air remaining in the lung at the end of spontaneous expirations, is present when resting FRC or EELV is increased above normal.Citation26 Two types of hyperinflation can be distinguished, ie, static and dynamic hyperinflation. A significant proportion of patients with COPD have some degree of lung hyperinflation, which often remains undetected in the absence of detailed physiological analysis (see section on assessment). Both static and dynamic effects of breathing contribute differently to lung hyperinflation in COPD.
Static and dynamic hyperinflation
Static hyperinflation
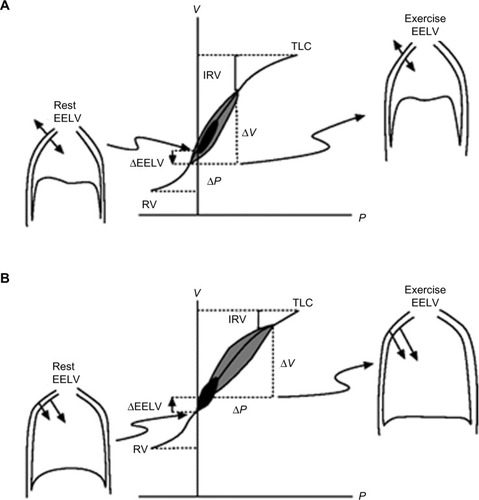
Under normal physiological conditions, for a given change in pleural pressure generated by the respiratory muscles, the attainable end-inspiratory lung volume (EILV) and EELV are determined by the passive pressure–volume relationship of the respiratory system ().Citation26,Citation27 In healthy subjects, elastic recoil pressure of the respiratory system decreases progressively during exhalation, reaching zero at FRC or EELV and the elastic work of breathing is minimized by maintaining VT within 20%–80% of the vital capacity range. With advancing age, damage to the connective tissue of the lung occurs, resulting in a reduction of the lung elastic recoil pressure.Citation28 The equilibrium point (FRC or EELV) therefore occurs at a higher lung volume than in younger subjects, with a consequence of an increased volume of air remaining in the lung at the end of spontaneous expirations. This is referred to as static hyperinflation, which exists at rest.Citation28 In COPD with emphysema, the lung recoil pressure is further reduced by a reduced elastic load related to smoking or α1-antitrypsin deficiency.Citation29 Therefore, the elastic recoil pressure of the respiratory system falls to zero at a larger FRC or EELV, resulting in more static hyperinflation.
Figure 2 Pressure–volume relationships of the total respiratory system.
Abbreviations: COPD, chronic obstructive pulmonary disease; EELV, end-expiratory lung volume; IRV, inspiratory reserve volume; P, pressure; RV, residual volume; TLC, total lung capacity; V, volume.
Dynamic hyperinflation
Dynamic lung hyperinflation refers to the temporary increase in EELV above the resting value during periods of increased ventilatory needs (eg, exercise). It is dependent on operational lung volumes and expiratory time, and is thus a key mechanistic consequence of expiratory flow limitation.Citation14
During exercise, respiratory rate increases and VT expands to accommodate increased respiratory demands. The hyperpnea induces phasic activity of expiratory muscles in both healthy individuals and in those with COPD.Citation30,Citation31 In healthy individuals, the increased expiratory effort progressively decreases EELV and expiratory airflows are sufficient to allow complete exhalation of the inhaled VT before the next inhalation, even when breathing approaches maximal ventilation. In contrast, the combined effects of decreased lung elastic recoil pressure and increased airways resistance in patients with COPD results in an increased mechanical time constant for lung emptying in many alveolar units. Thus, as the respiratory rate and expiratory flow increases, the expiratory time available for exhalation can become insufficient and complete exhalation of VT to the relaxation volume becomes increasingly compromised, and EELV usually increases with hyperpnea.Citation32 In addition, similar to healthy subjects, patients with COPD recruit expiratory muscles to increase their pleural and alveolar pressures, in an effort to increase expiratory flow. However, in these patients, the airways typically collapse when the pleural pressure becomes positive, thereby preventing increased expiratory flow.Citation33 As a result, exhalation may not be completed prior to the onset of the next breath, causing an increase in operational lung volumes and progressive air retention called “air trapping”.Citation13,Citation26,Citation34 This is referred to as dynamic hyperinflation, which can occur independently of static hyperinflation. Usually observed during exercise, the onset of dynamic hyperinflation will also occur at lower minute ventilations as disease severity limiting exhalation worsens, and may even occur during quiet breathing in severe patients or during an acute exacerbation.Citation14,Citation35
Natural history of hyperinflation
Development of hyperinflation during the course of COPD is insidious. In early COPD, the forced expiratory volume in one second (FEV1) may not be the optimal indicator of small airways obstruction.Citation36 In fact, considering the extent of small airway inflammation reported in patients with mild COPD,Citation37 it is conceivable that substantial structural damages could have taken place before marked expiratory flow limitation is objectively measured via FEV1.Citation38 Early changes observed in pulmonary function of heavy smokers without COPD likely imply increased total lung capacity (TLC) and residual volume because of the loss of elastic recoil.Citation39 These early changes reflecting lung hyperinflation are observed without any apparent reduction in FEV1.Citation39 In mild COPD, measures of TLC, FRC, and residual volume were found to be significantly above predicted values while vital capacity and inspiratory capacity (IC) were preserved.Citation40 Throughout the continuum of hyperinflation from mild to more severe COPD, vital capacity and IC decrease linearly with the progression of airflow obstruction (FEV1 decline). On the other hand, the progressive increase in TLC, FRC, and residual volume appears to be exponential with the worsening airflow limitation during the course of COPD.Citation40
During exercise, some studies report that dynamic hyperinflation is already present in patients with mild disease (GOLD stage 1), even when resting hyperinflation is slightly presentCitation22,Citation23 or absent.Citation24,Citation41–Citation43 Even if patients with mild COPD usually have preserved resting IC, they still exhibit dynamic hyperinflation and abnormal ventilatory mechanics during exercise when compared with healthy controls.Citation22,Citation44 At peak exercise, notwithstanding the severity of the disease, patients seem to show a consistent fall of approximately 20% of their resting IC at peak exercise.Citation22–Citation24,Citation45–Citation48
In patients with moderate-to-severe COPD, the level of dynamic hyperinflation is poorly related to FEV1.Citation49 However, when comparing two patients with similar FEV1, the one presenting with a reduced diffusion capacity, more severe small airway obstruction, and a higher ventilatory response to exercise will tend to develop more dynamic hyperinflation early during exercise.Citation13 Moderate levels of dynamic hyperinflation can even be observed in healthy elderly individuals aged >70 years without any pulmonary disease following normal aging of the lung parenchyma.Citation50–Citation52 Likewise, the ventilatory response during exercise of a healthy elderly subject could be similar to that of a patient with GOLD stage 1 COPD.Citation53
Physiological and sensory consequence of lung hyperinflation
Dyspnea
The interrelation between hyperinflation and dyspnea has been evaluated indirectly using regression analysis. O’Donnell and WebbCitation54 evaluated 23 patients with severe COPD and found that the change in EILV from baseline was the strongest predictor of the change in Borg dyspnea ratings (r=0.63, P<0.001). In this study, EELV and VT (both components of EILV) combined with breathing frequency accounted for 61% of the variance in dyspnea intensity. A subsequent study in a larger cohort of COPD patients (n=105) demonstrated that the VT/IC ratio, an index of EILV and VT constraint, was the strongest predictor of exertional dyspnea based on multiple linear regression analysis.Citation13 Moreover, interventions that deflate the lungs (ie, reduce EILV and EELV) and delay the onset of critical VT constraints consistently reduce dyspnea intensity in patients with COPD during exercise.Citation45,Citation46,Citation55,Citation56
Although dynamic hyperinflation is a cardinal feature of COPD with important physiological consequences, a small proportion of patients (~15%–20%) do not dynamically hyperinflate during exercise even though they still experience intolerable dyspnea.Citation13,Citation42,Citation57 Guenette et alCitation42 recently evaluated the effects of dynamic hyperinflation on dyspnea by comparing a group of well characterized COPD patients who did not acutely increase their EELV during exercise (nonhyperinflators, n=65) with those that did increase their EELV (hyperinflators, n=65). Despite being well matched for age, sex, body mass index, and baseline airflow obstruction, the authors were not able to show that the hyperinflators experienced more dyspnea than the nonhyperinflators. The authors concluded that perhaps the regulation of EILV provides a better index of critical constraints to ventilation (and therefore dyspnea) during exercise than the behavior of dynamic EELV per se. This finding does not necessarily diminish the physiological and sensory significance of dynamic hyperinflation, but rather shows that some individuals with airflow obstruction can still experience similar critical VT constraints (and thus similar dyspnea ratings), regardless of how they regulate EELV.
Respiratory and limb muscle function
Respiratory muscles
Static lung hyperinflation alters the geometry of the thorax and shortens the diaphragm,Citation58 thereby placing the diaphragm in a suboptimal contractile position to generate pressure. This mechanical disadvantage reduces the force-generating capacity of the inspiratory muscles and is likely to become further exaggerated in patients who dynamically hyperinflate.Citation59 Indeed, the ability of the respiratory muscles to generate pressure decreases at high lung volumes in humans.Citation58,Citation60 These functionally weakened respiratory muscles coupled with the increased elastic and threshold loading of the inspiratory musclesCitation61 results in a substantial increase in the work and oxygen cost of breathing.Citation21,Citation62
Despite the known deleterious effects of static and dynamic hyperinflation on respiratory muscle function, some have postulated that respiratory muscle strength and function may actually be preserved in some patients with COPD.Citation58,Citation63,Citation64 Chronic exposure to lung hyperinflation may result in physiological adaptations to preserve inspiratory muscle strength and perhaps obviate the development of diaphragmatic fatigue.Citation65 Some of the documented adaptations include: an increase in the relative fraction of fatigue-resistant slow-twitch (type I) muscle fibersCitation66 that can occur even in mild-to-moderate COPD;Citation67 a reduction in sarcomere length which permits an increase in pressure production at higher lung volumes;Citation68 increased mitochondrial density;Citation68 and/or an improvement in mitochondrial respiratory chain capacity.Citation69
Limb muscles
A direct link between dynamic hyperinflation and peripheral muscle function has not been fully established. Studies in healthy subjects suggest that high levels of respiratory muscle work may result in a sympathetically mediated metaboreflex which causes redistribution of blood flow from the locomotor muscles to the respiratory muscles.Citation70–Citation72 A reduction in locomotor muscle blood flow could result in an accelerated rate of development of limb muscle fatigue during exercise. This contention is supported by studies that show reduced limb muscle fatigue and corresponding improvements in perceived leg discomfort when the work of breathing is mechanically unloaded during exercise in healthy humansCitation73 and in patients with COPD.Citation74 In theory, dynamic hyperinflation and the associated increase in work and oxygen cost of breathing may compromise blood flow to the periphery, leading to compromised oxygen delivery and therefore causing increased leg fatigue. Indeed, studies that have unloaded the respiratory muscles of hyperinflated patients with bronchodilators or heliox resulted in an improvement in indices of limb muscle fractional oxygen extraction.Citation75,Citation76 For example, Louvaris et alCitation77 recently demonstrated that improving operating lung volumes in hyperinflated COPD patients with heliox enhanced oxygen delivery to the quadriceps muscles during exercise by increasing arterial oxygen content and blood flow to the quadriceps muscles. The authors speculated that this was likely due to blood flow redistribution from the respiratory muscles since cardiac output was similar between heliox and room air.
Cardiac function
Lung hyperinflation has been shown to adversely affect cardiovascular function in patients with COPD. Lung hyperinflation reduces right ventricular preload and venous return at rest and during exercise.Citation78–Citation81 Left ventricular afterload may also increase due to the high intrathoracic pressure swings needed to overcome the high elastic and resistive loads encountered by patients with COPD during exercise.Citation80 In addition, right ventricular afterload increases during exercise because there is an increase in pulmonary vascular resistance resulting from patients breathing at a high EILV.Citation82,Citation83 There is also indirect evidence to suggest that lung hyperinflation is associated with pulmonary hypertension. A number of mechanisms have been proposed to explain this association as recently described,Citation84 including increased intrathoracic pressures, cardiovascular effects, increased lung volume, altered gas exchange, pulmonary vascular remodeling, and endothelial dysfunction. Collectively, these cardiovascular consequences of lung hyperinflation likely contribute, in highly variable combinations, to the reduced cardiac performance observed in some COPD patients during exercise.Citation20,Citation85 However, it should be acknowledged that not all studies have been able to demonstrate a direct link between dynamic hyperinflation and cardiac performance during exercise. For example, Stark-Leyva et alCitation86 found that voluntary hyperinflation in healthy subjects did not adversely affect cardiac output during exercise. It remains to be determined if these findings in a healthy model can be extrapolated to patients with both static and dynamic hyperinflation, such as those with COPD.
Exercise tolerance
The mechanisms of exercise intolerance in COPD are complex and multifactorial and have been the subject of rigorous scientific debate.Citation16–Citation18 Potential mechanisms include abnormal ventilatory mechanics, limb muscle dysfunction, and impaired cardiac function, among other factors.Citation87 All of these mechanisms are related, at least in part, to lung hyperinflation as previously described. Thus, it is difficult to directly demonstrate a cause–effect relationship between hyperinflation and exercise performance because interventions that reduce hyperinflation may also improve any one or a combination of these contributory factors to varying degrees. Nevertheless, correlative evidence indicates that there is a link between exercise performance and indices of lung hyperinflation. For example, peak VT relative to predicted vital capacity was found to be the best predictor of peak aerobic capacity (r=0.68, P<0.0005) in 105 patients with COPD.Citation13 Work from other groups supports these results by showing a significant correlation between resting IC and peak work rate and peak oxygen uptake, particularly in patients with demonstrable expiratory flow limitation at rest.Citation88 The notion that lung hyperinflation is inversely related to exercise tolerance is also supported, albeit indirectly, by studies showing statistically significant correlations between improvements in resting and exercise IC and improvements in peak oxygen uptake and cycle endurance time following different interventions.Citation46,Citation56,Citation89,Citation90
Influence of comorbidities and sex on hyperinflation
Obesity
Obesity is an abnormal or excessive fat accumulation that may impair health.Citation91 Added weight on the thorax and abdomen (and also the neck), can significantly affect static and dynamic lung volumes along with respiratory mechanics,Citation92–Citation112 usually in a dose-response fashion. While several studies have addressed this issue, significant variability has been observed when evaluating the effects of obesity on lung volumes. These discrepancies may arise from heterogeneity in the severity of obesity and/or fat distribution, the precision of its measurement, or other confounding factors, such as underlying lung disease or sex differences. These uncertainties may well be exaggerated when the respiratory effects of obesity are studied alongside another heterogeneous disease such as COPD. As such, caution is recommended in drawing conclusions.
Total respiratory system compliance is usually reduced in obese patients. Obesity alone appears to have a “deflationary” effect. Obese patients consistently have a reduced expiratory reserve volume (or FRC) proportional to the magnitude of obesity.Citation99,Citation108,Citation110,Citation113–Citation120 Total lung capacity is usually not affected (ie, it remains within the lower limits of normal values), although some studies report decreases in cases of very severe obesity (body mass index >45 kg/m2).Citation110,Citation112,Citation121 Obesity is associated with a small decrease in FEV1 and forced vital capacity (although they remain within normal values)Citation108,Citation122,Citation123 and the FEV1/forced vital capacity ratio is preserved.Citation124 The physiological consequences of a combination of obesity and COPD are not well known and could theoretically provide advantages and disadvantages. On the one hand, both of these pathologies may have opposing effects in terms of lung volumes, COPD being primarily hyperinflating and obesity being deflating. This could provide an advantage to patients with COPD who are obese by reducing the deleterious effects of dynamic hyperinflation. On the other hand, this combination could increase mechanical loading and airway closure,Citation125 and thus worsen trapping of air in the lung.
While very few studies have addressed the impact of the combination of obesity and COPD on lung volumes, available results suggest that compared with normal weight patients, obese patients with COPD have reduced TLC and FRCCitation126–Citation129 and that lower lung volumes are maintained throughout exercise.Citation126,Citation128,Citation129 Obese patients with COPD still hyperinflate to a similar degree (Δ IC from rest to peak exercise capacity) than their normal weight counterparts.Citation126,Citation128,Citation129 Obesity in COPD appears to have a deflating effect at rest, and as a consequence, even if patients hyperinflate at a similar rate, they remain at lower volumes during exercise. Therefore, these studies all report that obese patients with COPD have either preserved or increased exercise capacity,Citation126–Citation129 except when walking is the testing modality.Citation127 Mechanistic dataCitation126 showed that the elastic properties of the lung were better preserved and that diaphragmatic function appeared not to be better in obese patients with COPD. Also, the increased metabolic load induced by obesity appeared to be compensated by an increased ventilatory efficiency (ie, lower ventilatory equivalent for CO2) in these patients. The precise mechanisms by which obesity and COPD interact to affect lung volumes are presently not well known. They are likely influenced by several factors, such as COPD phenotypeCitation42 and fat distribution.Citation96
Sex
Respiratory volumes and flows are significantly different between the sexes, as shown by the reference equations for lung function.Citation130,Citation131 These differences (mainly smaller lungs and maximal flow rates in women) may also affect dynamic volumes because fit women may suffer from expiratory flow limitation that induces an increase in EELV.Citation132–Citation134 It is therefore possible that COPD affects women differently than men. Women with COPD appear to be more susceptible to resting hyperinflation, despite lower tobacco use and younger age.Citation135 When restricted to emphysema, women also present a different pattern of disease compared with men, ie, smaller airway lumen and thicker airway walls.Citation136,Citation137 During constant work rate cycle exercise testing at the same relative intensity, women with COPD hyperinflated at a rate similar to that in men (Δ IC).Citation138 However, considering their smaller lung volumes, they reached a critical inspiratory reserve volume sooner than men and thus stopped exercise earlier than men. Similar results were obtained in another sample of COPD patients.Citation139 It would appear that women may be more susceptible to the deleterious effects of COPD because of their smaller respiratory systems compared with men.
Assessment of hyperinflation
Static assessments
In order to calculate lung hyperinflation at baseline, two subdivisions of the vital capacity must be measured. These are the IC and the expiratory reserve volume ().Citation140 Methods used for assessment of these parameters in COPD are body plethysmography, nitrogen washout, and helium dilution techniques.Citation141 Body plethysmography is considered the gold standard. This test is performed in a body plethysmograph allowing measurement of intrathoracic gas while airflow is occluded. Based on Boyle’s law,Citation142 changes in thoracic volumes caused by a compression or decompression of the gas in the lungs during respiratory maneuvers can be computed. FRC is thus obtained and constitutes the key measurement of static hyperinflation. A minimum of three values must be obtained, and the difference between the lowest and the highest FRC must be within 5% to be considered reliable. The mean value is then reported. In elderly healthy subjects, residual volume and FRC represent 30% and 55% of TLC, respectively.Citation130 In COPD, these values can be increased to 70% and 85% of the TLC for residual volume and FRC, respectively.Citation143 Usually, lung volumes/capacities exceeding 120%–130% of the predicted value are considered to be clinically relevant in COPD, but this remains arbitrary given that no consensus about the definition or severity of lung hyperinflation is available.Citation131,Citation141 It seems that the FRC calculated by body plethysmography is overestimated because it includes both ventilated and nonventilated lung compartments.Citation130,Citation142 In contrast, nitrogen washout and helium dilution techniques underestimate FRC in the presence of severe airflow obstruction or emphysema.Citation32,Citation140 Complete details about these three techniques are available in the latest American Thoracic Society/European Respiratory Society task force document.Citation141 Finally, because of a lack of standardization, radiographic techniquesCitation144–Citation147 are not commonly used clinically to measure static hyperinflation in COPD.Citation32 In fact, lung volumes calculated from radiographic techniques are based on the volume of gas within the outline of the thoracic cage and thus include the volume of tissue as well as the lung gas volume.Citation140 This method is usually reserved for patients with a limited ability to correctly perform the other techniques. Nevertheless, high-resolution computed tomography might constitute a useful upcoming technique to assess hyperinflation in COPD.Citation32
Dynamic assessments
Dynamic hyperinflation is determined from assessment of EELV (). This volume can be used interchangeably with FRC, although it is usually more appropriate to use it during exercise because this value is temporarily increased. EELV is commonly measured during exercise or any condition increasing minute ventilation by assessment of serial IC measurements as recently described by Guenette et al.Citation41 As for the resting EELV, a minimum of three IC maneuvers must be performed at rest. Values within 10% or 150 mL of the largest acceptable IC are usually considered reproducible. During exercise, patients are asked to take a deep inspiration after a normal expiration at specific intervals ranging from 1 to 3 minutes as well as at symptom limitation and during recovery. Because TLC remains stable during exercise,Citation148,Citation149 a temporary decrease in IC reflects a temporary increase in EELV (). More than 80% of patients with moderate-to-severe COPD showed significant increases in EELV during exercise.Citation11,Citation13,Citation46,Citation150 This volume has been shown to be reliably measurable and is responsive to treatment in COPD.Citation57,Citation89 Moreover, inspiratory-to-total lung capacity ratio <25% has also been used as a prognostic tool in COPD.Citation151 A recent study showed that reduction of the inspiratory reserve volume (IC – VT, ) reflecting “room to breathe” was even more related to exercise dyspnea than EELVCitation42 (). Finally, other methods such as optoelectronic plethysmographyCitation152 and respiratory inductance plethysmographyCitation153 are available for the assessment of dynamic hyperinflation, but they are still mainly used for research purposes in COPD.
Management and treatment of hyperinflation
Bronchodilator therapy
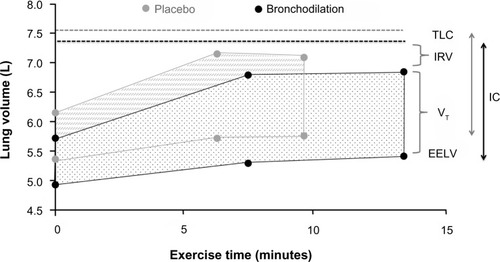
Pharmacological interventions that reduce operating lung volumes and delay the onset of ventilatory limitation consistently reduce the intensity of dyspnea during exercise in patients with COPD.Citation46,Citation55,Citation56 It should be noted, however, that the rates of increase in EELV (dynamic hyperinflation) and dyspnea symptoms during exercise are not modified after administration of bronchodilators. Rather, pharmacotherapy delays the development of restrictive ventilatory mechanics during exercise by deflating the lungs and decreasing EELV at rest. The resulting increase in resting IC causes a parallel downward shift in operating lung volumes during exercise in comparison with exercise performed without bronchodilation ().Citation41,Citation150 Thus, for any given exercise intensity or ventilation, patients breathe on the more linear portion of the respiratory system pressure–volume curve, with attendant improvements in neuromechanical coupling and, by extension, dyspnea. However, the absolute magnitude of dynamic hyperinflation does not change, and may even increase during peak exercise, reflecting the higher levels of ventilation that can be achieved following pharmacotherapy.Citation10,Citation45,Citation90
Figure 3 Acute effects of bronchodilation therapy on operational volume during constant work rate cycle ergometry in patients with COPD.
Abbreviations: COPD, chronic obstructive pulmonary disease; EELV, end-expiratory lung volume; IC, inspiratory capacity; IRV, inspiratory reserve volume; TLC, total lung capacity; VT, tidal volume.
Nonpharmacological interventions
Ventilatory support
The use of noninvasive ventilatory support consistently increases endurance time and reduces perception of dyspnea during constant load cycling tasks in patients with COPD.Citation154,Citation155 However, assisting ventilation by continuous positive airway pressure or pressure support will not affect EELV at rest or the increase in EELV during exercise.Citation156 The use of ventilatory support techniques will therefore not directly impact either static or dynamic lung hyperinflation. The effects of these interventions on dyspnea are probably mostly related to unloading of the inspiratory muscles during exercise.Citation155,Citation157–Citation160 Respiratory muscle function is often impaired in patients with COPD.Citation161 As previously described, these muscles have to overcome higher elastic and threshold loads during exercise which increases the work and oxygen cost of breathing in comparison with healthy subjects.Citation26,Citation162 Optimal continuous positive airway pressure reduces the elastic work of breathing throughout inspiration, counterbalances intrinsic positive end expiratory pressure, and takes away the threshold load on the inspiratory muscles while pressure support provides variable resistive and elastic unloading of the ventilatory muscles.Citation156,Citation163
Unloading respiratory muscles by proportional assisted ventilation improved leg blood flow and exercise performance during sustained high intensity exercise in healthy trained cyclists, indicating a competition for blood flow between respiratory and limb muscles.Citation164,Citation165 One study has so far investigated these mechanisms in patients with moderate-to-severe COPD.Citation166 These authors found positive effects of respiratory muscle unloading by proportional assisted ventilation during a relatively short (average of 4–5 minutes) constant load cycling task on endurance time, leg muscle oxygenation, and dyspnea and leg fatigue symptoms.Citation166
Oxygen/heliox administration
Supplemental oxygen during exercise consistently improves endurance and maximal exercise capacity and reduces ventilation and dyspnea at isotime during endurance exercise testing in COPD patients with and without resting hypoxemia.Citation167 Oxygen supplementation during exercise delays the attainment of ventilatory limitation and accompanying intolerable symptoms of dyspnea during exercise by reducing ventilatory demand.Citation168,Citation169 Oxygen supplementation will however not affect EELV and IC at rest and will also not change EELV for a given level of ventilation during exercise.Citation168,Citation170 The improvements observed at a given level of exertion are therefore not caused by a direct effect on static or dynamic hyperinflation. Both improved oxygen delivery to the peripheral muscles (resulting in less reliance on anaerobic metabolism), and attenuated peripheral chemoreceptor stimulation have been proposed as possible explanations for the reduction in ventilatory demand for a given level of exertion.Citation168,Citation169
Heliox is a low density gas mixture (79% helium, 21% oxygen) that has been used in patients with COPD to reduce airflow resistance with increasing ventilatory requirements during exercise.Citation171 Heliox supplementation has been shown to improve exercise intensity and endurance in patients with COPD in comparison with room air breathing.Citation172 Effects on dyspnea are likely but less clearly documented in the current literature.Citation172 Two papers evaluating dyspnea at isotime during an endurance cycling task however consistently showed significant reductions in perception of dyspnea.Citation171,Citation173 Heliox breathing increases the size of the maximal resting flow–volume envelope and seems to actually slow down the increase in EELV during exercise by decreasing airflow resistance, thereby directly altering dynamic hyperinflation.Citation170,Citation171 The response with regard to exercise capacity seems to be correlated with the magnitude of change in EELV during exercise.Citation171 In three studies, the responses to hyperoxic helium (60%–70% helium, 30%–40% oxygen) and oxygen supplementation alone were compared during a constant load cycling task in patients with moderate (nonhypoxemic),Citation173 severe,Citation174 and very severe (on long-term oxygen therapy) symptoms.Citation175 These studies all found significant differences in endurance time in favor of the hyperoxic helium group.Citation173–Citation175 They further demonstrated reductions in the resistive work of breathing,Citation173 and reductions in exercise-induced dynamic hyperinflation (increases in EELV)Citation174,Citation175 in comparison with hyperoxia alone.
Lung volume reduction surgery
In selected patients, lung volume reduction surgery decreases static and dynamic hyperinflation, and improves neuromechanical coupling, respiratory muscle function, exertional dyspnea, and exercise performance.Citation176–Citation179 Lung volume reduction surgery increases maximal ventilatory capacity as evidenced by increases in both maximal voluntary ventilation and maximal minute ventilation at peak exercise.Citation176–Citation178,Citation180–Citation182 The positive effects of this intervention on airflow obstruction have been ascribed to increases in lung elastic recoil or to reductions in TLC and residual volume leading to an increased vital capacity and improvements in respiratory muscle function.Citation183,Citation184 However, the understanding of the exact mechanisms of improvement in lung function remains incomplete and needs to be improved to select the optimal patients for this procedure.Citation183,Citation184 Besides the effects on static hyperinflation, it seems that the intervention also exerts a direct effect on dynamic hyperinflation during exercise.Citation176–Citation178 While minute ventilation has been reported to be stable at comparable work rates after lung volume reduction surgery, decreases in EELV have been observed, with reductions in breathing frequency and increases in VT.Citation183 Thus, lung volume reduction surgery improves airway conductance and lung emptying both at rest (comparable with bronchodilators) and during exercise (comparable with heliox breathing).
Exercise
The improvements in dyspnea and exercise capacity during constant load cycling tasks after properly conducted exercise training programs are larger than those observed with any of the previously described interventions.Citation185,Citation186 Several physiological and psychological factors, including a reduction in dynamic hyperinflation, have been proposed to explain these improvements.Citation187–Citation189 It is generally accepted that exercise training, unlike bronchodilators, does not have an impact on resting pulmonary mechanics.Citation190 From the available data, it also appears that, unlike heliox breathing or lung volume reduction surgery, exercise training does not have a direct effect on the rate of increase in EELV (dynamic hyperinflation) during exercise.Citation170 Similar to the acute effects of oxygen supplementation, exercise training reduces ventilatory needs for a given level of exertion.Citation170,Citation190,Citation191 This decrease in ventilatory needs is probably related to improvements in limb muscle function after training with an accompanying reduced reliance on anaerobic metabolism during exercise.Citation187,Citation189 Less ventilation will allow patients to reduce their respiratory rate, increase VT, and reduce EELV for a given workload and will eventually result in reduced symptoms of dyspnea and improved exercise endurance.Citation187,Citation189 For a given level of ventilation, EELV seems, however, not to be altered after exercise training.Citation187–Citation189
Breathing techniques
Pursed lip breathing is used spontaneously by some patients with severe dyspnea, airflow obstruction, and lung hyperinflation.Citation192 Therapeutically, it has been applied to reduce breathing frequency and increasing VT during exercise in several small studies, with mixed results in terms of dyspnea reduction and improvements in exercise capacity.Citation192–Citation194 Spahija et alCitation192 observed that during constant work bicycle exercise, a reduction in dyspnea during application of pursed lip breathing was related to changes in EELV and pressure generation of the inspiratory muscles. Even though the evidence base is limited, pursed lip breathing might be used on a trial-and-error basis in individual patients. A recent study by Collins et alCitation195 used a computerized ventilation feedback intervention aimed at slowing respiratory rate in combination with an exercise training program and showed reductions in respiratory rate, ventilation, and dynamic hyperinflation at isotime during a constant load cycling task. Feasibility of this approach on a larger scale needs to be addressed.
Inspiratory muscle training
Strengthening inspiratory muscles by specific training programs has been applied frequently in patients with COPD with the aim to alleviate dyspnea and improve exercise capacity. Reduced contractile muscle effort has been proposed as an important dyspnea relieving mechanism in studies that used ventilatory support to unload these muscles during exercise.Citation157–Citation160 Inspiratory muscle training aims to increase the capacity of these muscles to allow them to function at a lower fraction of their maximal capacity during exercise. Strong evidence supports effects of inspiratory muscle training to improve inspiratory muscle function (strength and endurance) and to reduce dyspnea and improve exercise capacity when applied as a standalone intervention.Citation196 Positive effects of inspiratory muscle training on operational lung volumes and breathing patterns during exercise have so far only been demonstrated in a single study.Citation197 More research into the mechanisms linking inspiratory muscle training to reduction of dyspnea during daily activities is warranted.
Summary
Although measurement of FEV1 is mandatory to establish a diagnosis of COPD, research in recent years has clearly demonstrated that hyperinflation, at rest and/or during exercise, is more closely associated with important clinical outcomes such as dyspnea and exercise intolerance than with expiratory flow indices. Hyperinflation has become an important endpoint in several clinical trials evaluating the efficacy of pharmacological and nonpharmacological therapeutic approaches to COPD. These trials have shown that measuring hyperinflation at rest and/or during exercise in the context of a multicenter randomized trial is feasible and valid. These trials have also confirmed that reducing hyperinflation in patients with COPD is a realistic therapeutic objective and is associated with relevant clinical benefits.
Disclosure
The authors report no conflicts of interest in this work.
References
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- ManninoDMGagnonRCPettyTLLydickEObstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994Arch Intern Med2000160111683168910847262
- HillKGoldsteinRSGuyattGHPrevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary careCMAJ2010182767367820371646
- HalbertRJNatoliJLGanoABadamgaravEBuistASManninoDMGlobal burden of COPD: systematic review and meta-analysisEur Respir J200628352353216611654
- BuistASMcBurnieMAVollmerWMInternational variation in the prevalence of COPD (the BOLD Study): a population-based prevalence studyLancet2007370958974175017765523
- KohansalRMartinez-CamblorPAgustiABuistASManninoDMSorianoJBThe natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohortAm J Respir Crit Care Med2009180131019342411
- [No authors listed]Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic SocietyAm J Respir Crit Care Med199915913213409872857
- O’DonnellDELavenezianaPDyspnea and activity limitation in COPD: mechanical factorsCOPD20074322523617729066
- SorianoJBVisickGTMuellerovaHPayvandiNHansellALPatterns of comorbidities in newly diagnosed COPD and asthma in primary careChest200512842099210716236861
- O’DonnellDELamMWebbKASpirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1999160254254910430726
- Garcia-RioFLoresVMedianoODaily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflationAm J Respir Crit Care Med2009180650651219542481
- JonesPWActivity limitation and quality of life in COPDCOPD20074327327817729072
- O’DonnellDERevillSMWebbKADynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2001164577077711549531
- O’DonnellDEHyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary diseaseProc Am Thorac Soc20063218018416565429
- PepinVSaeyDLavioletteLMaltaisFExercise capacity in chronic obstructive pulmonary disease: mechanisms of limitationCOPD20074319520417729063
- AlivertiAMacklemPTThe major limitation to exercise performance in COPD is inadequate energy supply to the respiratory and locomotor musclesJ Appl Physiol2008105274975118678622
- DebigareRMaltaisFThe major limitation to exercise performance in COPD is lower limb muscle dysfunctionJ Appl Physiol2008105275175318678623
- O’DonnellDEWebbKAThe major limitation to exercise performance in COPD is dynamic hyperinflationJ Appl Physiol2008105275375518678624
- WatzHWaschkiBMeyerTDecreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflationChest20101381323820190002
- TzaniPAielloMEliaDDynamic hyperinflation is associated with a poor cardiovascular response to exercise in COPD patientsRespir Res20111215022074289
- LoringSHGarcia-JacquesMMalhotraAPulmonary characteristics in COPD and mechanisms of increased work of breathingJ Appl Physiol2009107130931419359620
- OfirDLavenezianaPWebbKALamYMO’DonnellDEMechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2008177662262918006885
- O’DonnellDELavenezianaPOraJWebbKALamYMOfirDEvaluation of acute bronchodilator reversibility in patients with symptoms of GOLD stage I COPDThorax200964321622319052054
- GagnonPSaeyDProvencherSWalking exercise response to bronchodilation in mild COPD: a randomized trialRespir Med2012106121695170522999808
- HenkeKGSharrattMPegelowDDempseyJARegulation of end-expiratory lung volume during exerciseJ Appl Physiol19886411351463356631
- O’DonnellDELavenezianaPPhysiology and consequences of lung hyperinflation in COPDEur Respir Rev2006151006167
- MeadJTurnerJMMacklemPTLittleJBSignificance of the relationship between lung recoil and maximum expiratory flowJ Appl Physiol1967221951086017658
- PrideNBAgeing and changes in lung mechanicsEur Respir J200526456356516204583
- ThurlbeckWMOverview of the pathology of pulmonary emphysema in the humanClin Chest Med1983433373506357598
- GrimbyGGoldmanMMeadJRespiratory muscle action inferred from rib cage and abdominal V-P partitioningJ Appl Physiol1976415 Pt 1739751993162
- DoddDSBrancatisanoTEngelLAChest wall mechanics during exercise in patients with severe chronic air-flow obstructionAm Rev Respir Dis1984129133386230971
- O’DonnellDELavenezianaPThe clinical importance of dynamic lung hyperinflation in COPDCOPD20063421923217361503
- BarnesPJChronic obstructive pulmonary diseaseN Engl J Med2000343426928010911010
- Milic-EmiliJDynamic pulmonary hyperinflation and intrinsic PEEP: consequences and management in patients with chronic obstructive pulmonary diseaseRecenti Prog Med19908111733737 Italian2126881
- HaluszkaJChartrandDAGrassinoAEMilic-EmiliJIntrinsic PEEP and arterial PCO2 in stable patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis19901415 Pt 1119411972111105
- BarnesNBushAHowling for the moonThorax201166864564621415246
- HoggJCChuFUtokaparchSThe nature of small-airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med2004350262645265315215480
- McDonoughJEYuanRSuzukiMSmall-airway obstruction and emphysema in chronic obstructive pulmonary diseaseN Engl J Med2011365171567157522029978
- CorbinRPLovelandMMartinRRMacklemPTA four-year follow-up study of lung mechanics in smokersAm Rev Respir Dis19791202293304475151
- DeesomchokAWebbKAForkertLLung hyperinflation and its reversibility in patients with airway obstruction of varying severityCOPD20107642843721166631
- GuenetteJAChinRCCoryJMWebbKAO’DonnellDEInspiratory capacity during exercise: measurement, analysis, and interpretationPulm Med2013201395608123476765
- GuenetteJAWebbKAO’DonnellDEDoes dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD?Eur Respir J201240232232922183485
- ChinRCGuenetteJAChengSDoes the respiratory system limit exercise in mild chronic obstructive pulmonary disease?Am J Respir Crit Care Med2013187121315132323590271
- BabbTGRodarteJRLung volumes during low-intensity steady-state cyclingJ Appl Physiol19917029349372022587
- O’DonnellDEVoducNFitzpatrickMWebbKAEffect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary diseaseEur Respir J2004241869415293609
- O’DonnellDEFlugeTGerkenFEffects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPDEur Respir J200423683284015218994
- NederJAFuldJPOverendTEffects of formoterol on exercise tolerance in severely disabled patients with COPDRespir Med2007101102056206417658249
- MaltaisFCelliBCasaburiRAclidinium bromide improves exercise endurance and lung hyperinflation in patients with moderate to severe COPDRespir Med2011105458058721183326
- CalverleyPMKoulourisNGFlow limitation and dynamic hyperinflation: key concepts in modern respiratory physiologyEur Respir J200525118619915640341
- JohnsonBDDempseyJADemand vs capacity in the aging pulmonary systemExerc Sport Sci Rev1991191712101936085
- JohnsonBDReddanWGPegelowDFSeowKCDempseyJAFlow limitation and regulation of functional residual capacity during exercise in a physically active aging populationAm Rev Respir Dis19911435 Pt 19609672024851
- JohnsonBDReddanWGSeowKCDempseyJAMechanical constraints on exercise hyperpnea in a fit aging populationAm Rev Respir Dis19911435 Pt 19689772024852
- LavenezianaPParkerCMO’DonnellDEVentilatory constraints and dyspnea during exercise in chronic obstructive pulmonary diseaseAppl Physiol Nutr Metab20073261225123818059601
- O’DonnellDEWebbKAExertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflationAm Rev Respir Dis19931485135113578239175
- BelmanMJBotnickWCShinJWInhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med199615339679758630581
- O’DonnellDEHamiltonALWebbKASensory-mechanical relationships during high-intensity, constant-work-rate exercise in COPDJ Appl Physiol200610141025103516675610
- O’DonnellDETraversJWebbKAReliability of ventilatory parameters during cycle ergometry in multicentre trials in COPDEur Respir J200934486687419282342
- SimilowskiTYanSGauthierAPMacklemPTBellemareFContractile properties of the human diaphragm during chronic hyperinflationN Engl J Med1991325139179231881417
- SinderbyCSpahijaJBeckJDiaphragm activation during exercise in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200116371637164111401887
- PolkeyMIHamnegardCHHughesPDRaffertyGFGreenMMoxhamJInfluence of acute lung volume change on contractile properties of human diaphragmJ Appl Physiol1998854132213289760323
- GeaJCasadevallCPascualSOrozco-LeviMBarreiroERespiratory diseases and muscle dysfunctionExpert Rev Respir Med201261759022283581
- ShindohCHidaWKikuchiYOxygen consumption of respiratory muscles in patients with COPDChest199410537907978131542
- ByrdRBHyattREMaximal respiratory pressures in chronic obstructive lung diseaseAm Rev Respir Dis19689858488565683479
- SinghBEastwoodPRFinucaneKEVolume displaced by diaphragm motion in emphysemaJ Appl Physiol20019151913192311641325
- MadorMJKufelTJPinedaLASharmaGKDiaphragmatic fatigue and high-intensity exercise in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2000161111812310619807
- LevineSKaiserLLeferovichJTikunovBCellular adaptations in the diaphragm in chronic obstructive pulmonary diseaseN Engl J Med199733725179918069400036
- DoucetMDebigareRJoanisseDRAdaptation of the diaphragm and the vastus lateralis in mild-to-moderate COPDEur Respir J200424697197915572541
- Orozco-LeviMGeaJLloretaJLSubcellular adaptation of the human diaphragm in chronic obstructive pulmonary diseaseEur Respir J199913237137810065684
- RiberaFN’GuessanBZollJMitochondrial electron transport chain function is enhanced in inspiratory muscles of patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2003167687387912493645
- St CroixCMMorganBJWetterTJDempseyJAFatiguing inspiratory muscle work causes reflex sympathetic activation in humansJ Physiol2000529Pt 249350411101657
- SheelAWDerchakPAMorganBJPegelowDFJacquesAJDempseyJAFatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humansJ Physiol2001537Pt 127728911711580
- DempseyJASheelAWSt CroixCMMorganBJRespiratory influences on sympathetic vasomotor outflow in humansRespir Physiol Neurobiol2002130132012380012
- RomerLMLoveringATHaverkampHCPegelowDFDempseyJAEffect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humansJ Physiol2006571Pt 242543916373384
- AmannMReganMSKobitaryMImpact of pulmonary system limitations on locomotor muscle fatigue in patients with COPDAm J Physiol Regul Integr Comp Physiol20102991R314R32420445160
- ChiappaGRQueirogaFJrMedaEHeliox improves oxygen delivery and utilization during dynamic exercise in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2009179111004101019299497
- LavenezianaPPalangePOraJMartoliniDO’DonnellDEBronchodilator effect on ventilatory, pulmonary gas exchange, and heart rate kinetics during high-intensity exercise in COPDEur J Appl Physiol2009107663364319711095
- LouvarisZZakynthinosSAlivertiAHeliox increases quadriceps muscle oxygen delivery during exercise in COPD patients with and without dynamic hyperinflationJ Appl Physiol201211371012102322879534
- MahlerDABrentBNLokeJZaretBLMatthayRARight ventricular performance and central circulatory hemodynamics during upright exercise in patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis198413057227296388444
- LightRWMintzHMLindenGSBrownSEHemodynamics of patients with severe chronic obstructive pulmonary disease during progressive upright exerciseAm Rev Respir Dis198413033913956476589
- Montes de OcaMRassuloJCelliBRRespiratory muscle and cardiopulmonary function during exercise in very severe COPDAm J Respir Crit Care Med19961545128412898912737
- VizzaCDLynchJPOchoaLLRichardsonGTrulockEPRight and left ventricular dysfunction in patients with severe pulmonary diseaseChest199811335765839515827
- RanieriVMDambrosioMBrienzaNIntrinsic PEEP and cardiopulmonary interaction in patients with COPD and acute ventilatory failureEur Respir J199696128312928804950
- Oswald-MammosserMApprillMBachezPEhrhartMWeitzenblumEPulmonary hemodynamics in chronic obstructive pulmonary disease of the emphysematous typeRespiration1991585–63043101792422
- WrobelJPThompsonBRWilliamsTJMechanisms of pulmonary hypertension in chronic obstructive pulmonary disease: a pathophysiologic reviewJ Heart Lung Transplant201231655756422502811
- VassauxCTorre-BouscouletLZeineldineSEffects of hyperinflation on the oxygen pulse as a marker of cardiac performance in COPDEur Respir J20083251275128218550609
- Stark-LeyvaKNBeckKCJohnsonBDInfluence of expiratory loading and hyperinflation on cardiac output during exerciseJ Appl Physiol20049651920192714729724
- VogiatzisIZakynthinosSFactors limiting exercise tolerance in chronic lung diseasesCompr Physiol2012231779181723723024
- DiazOVillafrancaCGhezzoHRole of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at restEur Respir J200016226927510968502
- O’DonnellDELamMWebbKAMeasurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19981585 Pt 1155715659817708
- PetersMMWebbKAO’DonnellDECombined physiological effects of bronchodilators and hyperoxia on exertional dyspnoea in normoxic COPDThorax200661755956716467067
- [No authors listed]Obesity: preventing and managing the global epidemic. Report of a WHO consultationWorld Health Organ Tech Rep Ser2000894ixii125311234459
- LittletonSWImpact of obesity on respiratory functionRespirology2012171434922040049
- SalomeCMKingGGBerendNPhysiology of obesity and effects on lung functionJ Appl Physiol2010108120621119875713
- LeoneNCourbonDThomasFLung function impairment and metabolic syndrome: the critical role of abdominal obesityAm J Respir Crit Care Med2009179650951619136371
- SutherlandTJGouldingAGrantAMThe effect of adiposity measured by dual-energy X-ray absorptiometry on lung functionEur Respir J2008321859118353855
- BabbTGWyrickBLDeLoreyDSChasePJFengMYFat distribution and end-expiratory lung volume in lean and obese men and womenChest2008134470471118641101
- OfirDLavenezianaPWebbKAO’DonnellDEVentilatory and perceptual responses to cycle exercise in obese womenJ Appl Physiol200710262217222617234804
- HamouiNAnthoneGCrookesPFThe value of pulmonary function testing prior to bariatric surgeryObes Surg200616121570157317217631
- JonesRLNzekwuMMThe effects of body mass index on lung volumesChest2006130382783316963682
- ParameswaranKToddDCSothMAltered respiratory physiology in obesityCan Respir J200613420321016779465
- JubberASRespiratory complications of obesityInt J Clin Pract200458657358015311557
- CanoyDLubenRWelchAAbdominal obesity and respiratory function in men and women in the EPIC-Norfolk Study, United KingdomAm J Epidemiol2004159121140114915191931
- KoenigSMPulmonary complications of obesityAm J Med Sci2001321424927911307867
- RochesterDFObesity and pulmonary functionAlpertMAlexanderJThe Heart and Lung in ObesityArmonk, NY, USAFutura Publishing Co1998
- PankowWPodszusTGutheilTPenzelTPeterJVon WichertPExpiratory flow limitation and intrinsic positive end-expiratory pressure in obesityJ Appl Physiol1998854123612439760311
- LazarusRGoreCJBoothMOwenNEffects of body composition and fat distribution on ventilatory function in adultsAm J Clin Nutr199868135419665094
- CollinsLCHobertyPDWalkerJFFletcherECPeirisANThe effect of body fat distribution on pulmonary function testsChest19951075129813027750322
- ZerahFHarfAPerlemuterLLorinoHLorinoAMAtlanGEffects of obesity on respiratory resistanceChest19931035147014768486029
- JenkinsSCMoxhamJThe effects of mild obesity on lung functionRespir Med19918543093111947368
- RubinsteinIZamelNDuBarryLHoffsteinVAirflow limitation in morbidly obese, nonsmoking menAnn Intern Med1990112118288322378649
- SurattPMWilhoitSCHsiaoHSAtkinsonRLRochesterDFCompliance of chest wall in obese subjectsJ Appl Physiol Respir Environ Exerc Physiol19845724034076469810
- RayCSSueDYBrayGHansenJEWassermanKEffects of obesity on respiratory functionAm Rev Respir Dis198312835015066614644
- LadoskyWBotelhoMAAlbuquerqueJPJrChest mechanics in morbidly obese non-hypoventilated patientsRespir Med200195428128611316110
- KellyTMJensenRLElliottCGCrapoROMaximum respiratory pressures in morbidly obese subjectsRespiration198854273773231898
- ThomasPSCowenERHulandsGMilledgeJSRespiratory function in the morbidly obese before and after weight lossThorax19894453823862503905
- EmirgilCSobolBJThe effects of weight reduction on pulmonary function and the sensitivity of the respiratory center in obesityAm Rev Respir Dis197310848318424741877
- BiringMSLewisMILiuJTMohsenifarZPulmonary physiologic changes of morbid obesityAm J Med Sci1999318529329710555090
- PelosiPCrociMRavagnanIThe effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesiaAnesth Analg19988736546609728848
- ColletFMallartABervarJFPhysiologic correlates of dyspnea in patients with morbid obesityInt J Obes (Lond)200731470070617006440
- WatsonRAPrideNBPostural changes in lung volumes and respiratory resistance in subjects with obesityJ Appl Physiol200598251251715475605
- BurkiNKBakerRWVentilatory regulation in eucapnic morbid obesityAm Rev Respir Dis198412945385436424520
- SchachterLMSalomeCMPeatJKWoolcockAJObesity is a risk for asthma and wheeze but not airway hyperresponsivenessThorax20015614811120896
- SinDDJonesRLManSFObesity is a risk factor for dyspnea but not for airflow obstructionArch Intern Med2002162131477148112090884
- BabbTGWyrickBLChasePJWeight loss via diet and exercise improves exercise breathing mechanics in obese menChest2011140245446021273293
- BoisellePMLitmanovichDEMichaudGDynamic expiratory tracheal collapse in morbidly obese COPD patientsCOPD201310560461023837455
- OraJLavenezianaPWadellKPrestonMWebbKAO’DonnellDEEffect of obesity on respiratory mechanics during rest and exercise in COPDJ Appl Physiol20111111101921350021
- SavaFLavioletteLBernardSBretonMJBourbeauJMaltaisFThe impact of obesity on walking and cycling performance and response to pulmonary rehabilitation in COPDBMC Pulm Med2010105521054892
- LavioletteLSavaFO’DonnellDEEffect of obesity on constant workrate exercise in hyperinflated men with COPDBMC Pulm Med2010103320509967
- OraJLavenezianaPOfirDDeesomchokAWebbKAO’DonnellDECombined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise toleranceAm J Respir Crit Care Med20091801096497119897773
- StocksJQuanjerPHReference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory SocietyEur Respir J1995834925067789503
- QuanjerPHTammelingGJCotesJEPedersenOFPeslinRYernaultJCLung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory SocietyEur Respir J Suppl1993165408499054
- McClaranSRHarmsCAPegelowDFDempseyJASmaller lungs in women affect exercise hyperpneaJ Appl Physiol (1985)1998846187218819609779
- GuenetteJAWittJDMcKenzieDCRoadJDSheelAWRespiratory mechanics during exercise in endurance-trained men and womenJ Physiol2007581Pt 31309132217412775
- DeruelleFNourryCMucciPDifference in breathing strategies during exercise between trained elderly men and womenScand J Med Sci Sports200818221322017490460
- LavioletteLLacasseYDoucetMChronic obstructive pulmonary disease in womenCan Respir J2007142939817372636
- MartinezFJCurtisJLSciurbaFSex differences in severe pulmonary emphysemaAm J Respir Crit Care Med2007176324325217431226
- CampPGCoxsonHOLevyRDSex differences in emphysema and airway disease in smokersChest200913661480148819617404
- LavioletteLO’DonnellDEWebbKAHamiltonALKestenSMaltaisFPerformance during constant workrate cycling exercise in women with COPD and hyperinflationCOPD20096534035119863363
- GuenetteJAJensenDWebbKAOfirDRaghavanNO’DonnellDESex differences in exertional dyspnea in patients with mild COPD: physiological mechanismsRespir Physiol Neurobiol2011177321822721524719
- WangerJClausenJLCoatesAStandardisation of the measurement of lung volumesEur Respir J200526351152216135736
- PellegrinoRViegiGBrusascoVInterpretative strategies for lung function testsEur Respir J200526594896816264058
- CoatesALPeslinRRodensteinDStocksJMeasurement of lung volumes by plethysmographyEur Respir J1997106141514279192953
- FishmanAMartinezFNaunheimKA randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysemaN Engl J Med2003348212059207312759479
- BurkiNKKrumpelmanJLCorrelation of pulmonary function with the chest roentgenogram in chronic airway obstructionAm Rev Respir Dis198012122172237362131
- SimonGPrideNBJonesNLRaimondiACRelation between abnormalities in the chest radiograph and changes in pulmonary function in chronic bronchitis and emphysemaThorax197328115234685207
- NakanoYMuroSSakaiHComputed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung functionAm J Respir Crit Care Med20001623 Pt 11102110810988137
- de JongPAMullerNLParePDCoxsonHOComputed tomographic imaging of the airways: relationship to structure and functionEur Respir J200526114015215994401
- StubbingDGPengellyLDMorseJLJonesNLPulmonary mechanics during exercise in subjects with chronic airflow obstructionJ Appl Physiol Respir Environ Exerc Physiol19804935115157204175
- VogiatzisIGeorgiadouOGolematiSPatterns of dynamic hyperinflation during exercise and recovery in patients with severe chronic obstructive pulmonary diseaseThorax200560972372915964912
- MaltaisFHamiltonAMarciniukDImprovements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPDChest200512831168117816162703
- CasanovaCCoteCde TorresJPInspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2005171659159715591470
- AlivertiAStevensonNDellacaRLLo MauroAPedottiACalverleyPMRegional chest wall volumes during exercise in chronic obstructive pulmonary diseaseThorax200459321021614985554
- ClarenbachCFSennOBrackTKohlerMBlochKEMonitoring of ventilation during exercise by a portable respiratory inductive plethysmographChest200512831282129016162719
- van’t HulAKwakkelGGosselinkRThe acute effects of noninvasive ventilatory support during exercise on exercise endurance and dyspnea in patients with chronic obstructive pulmonary disease: a systematic reviewJ Cardiopulm Rehabil200222429029712202851
- van’t HulAGosselinkRHollanderPPostmusPKwakkelGAcute effects of inspiratory pressure support during exercise in patients with COPDEur Respir J2004231344014738228
- O’DonnellDEVentilatory limitations in chronic obstructive pulmonary diseaseMed Sci Sports Exerc200133Suppl 7S647S65511462073
- O’DonnellDESaniiRYounesMImprovement in exercise endurance in patients with chronic airflow limitation using continuous positive airway pressureAm Rev Respir Dis19881386151015143059897
- PetrofBJCalderiniEGottfriedSBEffect of CPAP on respiratory effort and dyspnea during exercise in severe COPDJ Appl Physiol19906911791882203722
- MaltaisFReissmannHGottfriedSBPressure support reduces inspiratory effort and dyspnea during exercise in chronic airflow obstructionAm J Respir Crit Care Med19951514102710337697226
- PolkeyMIKyroussisDMillsGHInspiratory pressure support reduces slowing of inspiratory muscle relaxation rate during exhaustive treadmill walking in severe COPDAm J Respir Crit Care Med19961544 Pt 1114611508887619
- Orozco-LeviMStructure and function of the respiratory muscles in patients with COPD: impairment or adaptation?Eur Respir J Suppl20034641s51s14621106
- RochesterDFThe respiratory muscles in COPD. State of the artChest198485Suppl 647S50S6373180
- AmbrosinoNStrambiSNew strategies to improve exercise tolerance in chronic obstructive pulmonary diseaseEur Respir J200424231332215332404
- HarmsCABabcockMAMcClaranSRRespiratory muscle work compromises leg blood flow during maximal exerciseJ Appl Physiol1997825157315839134907
- HarmsCAWetterTJSt CroixCMPegelowDFDempseyJAEffects of respiratory muscle work on exercise performanceJ Appl Physiol200089113113810904044
- Borghi-SilvaAOliveiraCCCarrascosaCRespiratory muscle unloading improves leg muscle oxygenation during exercise in patients with COPDThorax2008631091091518492743
- BradleyJMLassersonTElbornSMacmahonJO’NeillBA systematic review of randomized controlled trials examining the short-term benefit of ambulatory oxygen in COPDChest2007131127828517218587
- O’DonnellDED’ArsignyCWebbKAEffects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2001163489289811282762
- SomfayAPorszaszJLeeSMCasaburiRDose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patientsEur Respir J2001181778411510809
- DolmageTEEvansRAGoldsteinRSDefining hyperinflation as ‘dynamic’: moving toward the slopeRespir Med2013107795395823478191
- PalangePValliGOnoratiPEffect of heliox on lung dynamic hyperinflation, dyspnea, and exercise endurance capacity in COPD patientsJ Appl Physiol20049751637164215234959
- HuntTWilliamsMTFrithPSchembriDHeliox, dyspnoea and exercise in COPDEur Respir Rev201019115303820956163
- EvesNDPetersenSRHaykowskyMJWongEYJonesRLHelium-hyperoxia, exercise, and respiratory mechanics in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2006174776377116840742
- HussainOCollinsEGAdiguzelNLangbeinWETobinMJLaghiFContrasting pressure-support ventilation and helium-oxygen during exercise in severe COPDRespir Med2011105349450520851591
- QueirogaFJrNunesMMedaEExercise tolerance with helium-hyperoxia versus hyperoxia in hypoxaemic patients with COPDEur Respir J201342236237023180584
- O’DonnellDEWebbKABertleyJCChauLKConlanAAMechanisms of relief of exertional breathlessness following unilateral bullectomy and lung volume reduction surgery in emphysemaChest1996110118278681624
- BendittJOWoodDEMcCoolFDLewisSAlbertRKChanges in breathing and ventilatory muscle recruitment patterns induced by lung volume reduction surgeryAm J Respir Crit Care Med199715512792849001325
- MartinezFJde OcaMMWhyteRIStetzJGaySECelliBRLung-volume reduction improves dyspnea, dynamic hyperinflation, and respiratory muscle functionAm J Respir Crit Care Med19971556198419909196106
- LaghiFJubranATopeliAEffect of lung volume reduction surgery on neuromechanical coupling of the diaphragmAm J Respir Crit Care Med199815724754839476861
- BendittJOLewisSWoodDEKlimaLAlbertRKLung volume reduction surgery improves maximal O2 consumption, maximal minute ventilation, O2 pulse, and dead space-to-tidal volume ratio during leg cycle ergometryAm J Respir Crit Care Med19971562 Pt 15615669279240
- CordovaFO’BrienGFurukawaSKuzmaAMTravalineJCrinerGJStability of improvements in exercise performance and quality of life following bilateral lung volume reduction surgery in severe COPDChest199711249079159377952
- KellerCARuppelGHibbettAOsterlohJNaunheimKSThoracoscopic lung volume reduction surgery reduces dyspnea and improves exercise capacity in patients with emphysemaAm J Respir Crit Care Med1997156160679230727
- MarchandEGayan-RamirezGDe LeynPDecramerMPhysiological basis of improvement after lung volume reduction surgery for severe emphysema: where are we?Eur Respir J199913368669610232448
- FesslerHEScharfSMIngenitoEPMcKennaRJJrSharafkhanehAPhysiologic basis for improved pulmonary function after lung volume reductionProc Am Thorac Soc20085441642018453348
- LacasseYGuyattGHGoldsteinRSThe components of a respiratory rehabilitation program: a systematic overviewChest19971114107710889106590
- CasaburiRPorszaszJReduction of hyperinflation by pharmacologic and other interventionsProc Am Thorac Soc20063218518916565430
- CasaburiRZuWallackRPulmonary rehabilitation for management of chronic obstructive pulmonary diseaseN Engl J Med2009360131329133519321869
- O’DonnellDEOraJWebbKALavenezianaPJensenDMechanisms of activity-related dyspnea in pulmonary diseasesRespir Physiol Neurobiol2009167111613219450767
- TroostersTGosselinkRJanssensWDecramerMExercise training and pulmonary rehabilitation: new insights and remaining challengesEur Respir Rev201019115242920956162
- PorszaszJEmtnerMGotoSSomfayAWhippBJCasaburiRExercise training decreases ventilatory requirements and exercise-induced hyperinflation at submaximal intensities in patients with COPDChest200512842025203416236851
- Puente-MaestuLAbadYMPedrazaFSanchezGStringerWWA controlled trial of the effects of leg training on breathing pattern and dynamic hyperinflation in severe COPDLung2006184315916716902841
- SpahijaJMarchieMGhezzoHGrassinoAFactors discriminating spontaneous pursed-lips breathing use in patients with COPDCOPD20107425426120673034
- CasciariRJFairshterRDHarrisonAMorrisonJTBlackburnCWilsonAFEffects of breathing retraining in patients with chronic obstructive pulmonary diseaseChest19817943933987226902
- GarrodRDallimoreKCookJDaviesVQuadeKAn evaluation of the acute impact of pursed lips breathing on walking distance in nonspontaneous pursed lips breathing chronic obstructive pulmonary disease patientsChron Respir Dis200522677216279153
- CollinsEGLangbeinWEFehrLCan ventilation-feedback training augment exercise tolerance in patients with chronic obstructive pulmonary disease?Am J Respir Crit Care Med2008177884485218202351
- GosselinkRDe VosJvan den HeuvelSPSegersJDecramerMKwakkelGImpact of inspiratory muscle training in patients with COPD: what is the evidence?Eur Respir J201137241642521282809
- PetrovicMReiterMZipkoHPohlWWankeTEffects of inspiratory muscle training on dynamic hyperinflation in patients with COPDInt J Chron Obstruct Pulmon Dis2012779780523233798
