Abstract
Background
This study sought to explore the underlying mechanism of miR-21 mediated apoptosis and inflammation in chronic obstructive pulmonary disease (COPD) induced by cigarette smoke (CS).
Methods
We detected levels and PTEN/Akt/NF-κB axis protein levels in peripheral lung tissues of COPD patients and CS-exposed mice and HBE cells. Western blotting assay was used to determine the expression of cleaved caspase-3. IL-6 and IL-8 protein was detected in cell supernatant from cells by ELISA. HBE cells were transfected with a miR-21 inhibitor, and co-culture with A549.
Results
Increased miR-21 expression, reduced PTEN expression and following activation of Akt in in peripheral lung tissues of COPD patients and CS-exposed mice and HBE cells. Inhibition of miR-21 showed enhanced PTEN levels and reduced the expression of phosphorylated form of Akt and NF-κB. Decreased expression of cleaved caspase-3, IL-6 and IL-8 in A549 cells co cultured with HBE cells transfected with miR-21 inhibitor compared with transfected with miR-21 control inhibitor.
Conclusion
MiR-21 contributes to COPD pathogenesis by modulating apoptosis and inflammation through the PTEN/Akt/NF-κB pathway. Targeting miR-21 may increase PTEN expression and inhibit Akt/NF-κB pathway, offering potential diagnostic and therapeutic value in COPD management.
Introduction
Chronic obstructive pulmonary disease (COPD) represents a significant global health challenge, contributing extensively to morbidity, mortality, and healthcare utilization. Primarily induced by cigarette smoking,Citation1,Citation2 COPD is marked by persistent oxidative stress and inflammation, culminating in irreversible airflow restriction due to the narrowing of small airways and the development of lung emphysema.Citation3,Citation4 Currently, there are no definitive treatments to halt COPD progression, largely attributable to an incomplete understanding of its pathogenic mechanisms.
Continuous exposure to deleterious particles or gases, particularly those found in tobacco smoke, is recognized as a primary factor in intensifying inflammatory responses.Citation5 For nearly all smokers, this starts with inflammation on the periphery of the airways and eventually spreads throughout the entire airway, causing a rapid loss of lung function.Citation6 The main cause of chronic inflammation is immune cell infiltration into the small airways, which may lead to airway remodeling and blockage. Chronic inflammation produces inflammatory mediators and damaging enzymes.Citation7,Citation8 When a cell responds to environmental stimuli, which includes DNA damage and oxidative stress, it is said to be undergoing apoptosis.Citation9 According to a study conducted by Demedts et alCitation10 lung structural cell death may be a significant upstream event in the pathogenesis of COPD. CS triggers an excessive production of ROS in lung tissues, disrupting the balance between oxidative and antioxidant systems. This imbalance leads to cellular dysfunction and triggers apoptosis.Citation2,Citation11,Citation12 Individuals with COPD have more apoptotic alveolar epithelial cells in their lungs. The growth of structural cells is unable to counteract these pathological alterations, which results in the degeneration of lung tissue and the development of emphysema.Citation12
The mechanisms underlying COPD have not been fully elucidated. Several studies suggest that microRNAs (miRs), including miR-21, may significantly influence the pathogenesis of COPD.Citation13 Research indicates a correlation between miR-21 and the activation as well as the severity of the inflammatory response.Citation14,Citation15 Prior studies have demonstrated an elevated expression of miR-21 in peripheral serum, mononuclear cells of COPD-afflicted rats and COPD patients.Citation16,Citation17 Furthermore, research by He et al identified that the knockdown of miR-21 (miR-21−/−) exerts a protective effect against COPD.Citation18 According to these results, miR-21 levels within the lungs may be a good measure of the severity of COPD, and miR-21 targeting may be a useful treatment strategy for the disease in its earliest phases.
A negative modulator of the PI3K and AKT signaling pathways, phospholipase and tensin homologue (PTEN), significantly influencing cell proliferation, migration, invasion, and apoptosis.Citation19 In COPD patients, oxidative stress has been shown to reduce PTEN protein levels, consequently leading to continuous activation of the PI3K/Akt pathway. Furthermore, Akt is the primary upstream component that both directly and indirectly phosphorylates p65 by IκB kinase (IKK), activating nuclear factor-κB (NF-κB).Citation20 The activated NF-κB blocks apoptosis through triggering the expression of anti-apoptosis genes including caspase inhibitors, and regulate cell proliferation and the inflammation.Citation21 This activation triggers the release of proinflammatory mediators and modulates cell apoptosis.Citation22,Citation23 MiR-21 specifically targets PTEN to mitigate its inhibition of the PI3K/Akt pathway.Citation24 The relevance of this pathway to COPD, however, remains uncertain.
In this study, we substantiated the hypothesis that miR-21 contributes to the development of COPD. This is achieved by miR-21’s modulation of cellular apoptosis and inflammatory responses, mediated through the regulation of the PTEN/Akt/NF-κB pathway.
Materials and Methods
Study Participants and Specimens
Participants from Wuxi People’s Hospital were separated into three groups: non-smokers without COPD, asymptomatic smokers, and individuals diagnosed with COPD. COPD is diagnosed per GOLD guidelines, focusing on respiratory symptoms and risk factor exposure, with confirmation via a post-bronchodilator FEV₁/FVC ratio below 70%.
Peripheral lung tissue samples were collected from patients undergoing lobectomy for small nodules or lung transplantation at Wuxi People’s Hospital. These samples were immediately preserved in liquid nitrogen post-collection for subsequent analysis. At the recruitment stage, peripheral blood specimens were obtained and treated with anticoagulants. Initially, these specimens underwent a centrifugation process at 3000 × g for 10 minutes at 4°C. After that, the resultant supernatant had been aliquoted along with went through a second centrifugation phase during 16,000 × g over 10 minutes, also at 4°C. For future analyses, these serum samples were preserved at a temperature of −80°C.
All human experimental was conformed to the Declaration of Helsinki and the Ethics Committee of Wuxi People’s Hospital. All subjects provided written informed consent.
Establishment About Murine COPD Model
Male C57BL6 mice, 6–8 weeks old, had been sourced through Shanghai SLRC and housed in Wuxi People’s Hospital. These mice underwent CS exposure using Daqianmen brand cigarettes (China) within a full-body exposure setup, receiving 4 hours of exposure daily over a period of 12 weeks. For comparative purposes, age-similar mice were housed under comparable conditions but without CS exposure, serving as the control group. All animal experiments complied with the ethical guidelines of the International Council for Laboratory Animal Science and the Ethics Committee of Nanjing Medical University.
Cell Culture and Treatment
HBE cells, sourced from CHI Scientific Inc., and A549 cells, purchased from the American Type Culture Collection (Manassas, VA, USA), were cultured in a controlled environment. Cell lines were cultured within DMEM (10% FBS, penicillin, streptomycin) in a 37 °C, 5% CO2 environment. Bi-daily subcultured HBE cells, at 70–80% confluence, were PBS-washed and incubated in 5% CSE-DMEM for 48 hours.
Preparation of Cigarette Smoke Extract (CSE)
Cigarette smoke was infused into 10 mL of serum-free DMEM to prepare the CSE. The standardization of CSE was conducted through assessing the absorbance at 540 nm (A540), with an acceptable quality range defined between 0.9 and 1.2 for A540. This preparation was considered as the 100% CSE concentration. To ensure sterility, CSE was cleaned employing a 0.22-um pore size filter as well as subsequently diluted with the culture medium for experimental use within 30 minutes.
Hematoxylin-Eosin Staining
The bronchial alveolar tissue samples from mice were subjected to a series of histological preparations. Initially, the tissue was dehydrated and cleared before being embedded in paraffin. Then, the embedded tissue was sectioned. Glass slides carrying tissue sections were dewaxed, rehydrated and stained with hematoxylin for 5 minutes. Following a rinse with PBS, the tissue underwent a brief differentiation in hydrochloric acid-ethanol solution for 3 seconds and was subsequently stained with eosin for 2 minutes. Finally, neutral resin was used to mount the stained slices. The prepared slides were examined under an optical microscope to assess the pathological alterations in the mouse bronchial alveolar tissue.
RNA Extraction and Quantitative Real-Time PCR
RNA was extracted from cells and serum using Takara’s kits. MiRNA quantification involved reverse transcription of 1 μg cellular RNA or 200 ng serum RNA with PrimeScript RT. PCR, using TB Green premix on ABI 9600, consisted of initial 95 °C for 30 seconds, then 40 cycles at 95 °C for 15 seconds and 60 °C for 34 minutes. U6 snRNA and GAPDH were the controls.
Western Bolt Analysis
Proteins isolated from HBE cells, A549 cells, as well as mouse and human lung tissues, were subjected to SDS-PAGE separation and subsequently transferred onto PVDF membranes. s. These membranes, post-blocking, were exposed to a series of antibodies sourced from Cell Signaling Technology, United States. These antibodies targeted PTEN, phosphorylated Akt (p-Akt) (Ser473), Akt, phosphorylated NF-κB (p-NF-κB) (Ser536), NF-κB, cleaved caspase-3, and β-actin. Using Image J software, a quantitative analysis for the protein band density has been performed.
Luciferase Reporter Assay
Luciferase activity was measured according to earlier reports. 293T cells were co-transfected with wild-type or mutant PTEN 3’UTR luciferase mimics and miR-21-5p mimics or NC mimics employing Lipofectamine 2000 (Thermo Fisher Scientific, USA). The cells were extracted for detection 48 hours after transfection utilizing the Dual‐Luciferase Reporter assay system (Promega, USA) with an Infinite 200 PRO multimode microplate reader (Tecan Group, Ltd., Switzerland). Activities of Renilla luciferase were employed to standardize the transfection efficiency.
Cell Transfection
The HBE cell line was transfected with the miR-21 inhibitor and control inhibitor using Lipofectamine 2000 (Invitrogen, USA), following the manufacturer’s protocol. Post 48 hours of transfection, HBE cells were subjected to 5% CSE for an additional 48 hours.
Transwell Assays
In a co-culture model, HBE cells were seeded onto hanging cell culture inserts with 0.4-μm pores (Millipore, Merck, Germany) and transfected with miR-21 inhibitors or control inhibitor as described earlier. Concurrently, A549 cells were cultured in 6-well plates. Following a 48-hour transfection period, 5% CSE was applied to the HBE cells, and inserts had been subsequently co-cultured with A549 cells for a further 48 hours.
Cell Proliferation Assay
After HBE cell co-culture, A549 proliferation was assessed. Cells at 2×103 cells/well were cultured in 96-well plates. After being exposed to 10 μL of CCK-8 solution for 24, 48, and 72 hours, cells were remained under dark-incubated for 2 hours. Proliferation was determined by measuring 450 nm absorbance.
Statistical Analysis
Every single data point was presented as mean ± SD. For comparisons between two groups, the Student’s t-test was employed. In contrast, for analyzing data involving three or more groups, one-way ANOVA was utilized, supplemented by post hoc tests such as Bonferroni or Dunnett’s T3. P <0.05 values have been accepted as statistically noteworthy.
Results
Demographic Characteristics for Study Population
In this study, lung tissues from seven COPD patients, seven smokers, and five non-smokers without COPD were collected for qRT-PCR analysis. The participants in the COPD, smoker, and control groups were carefully matched in terms of age, gender, and current smoking status. provides a summary of each participant’s specific baseline features.
Table 1 Clinical Characteristics of Enrolled Subjects from Whom Lung Tissues Were Collected
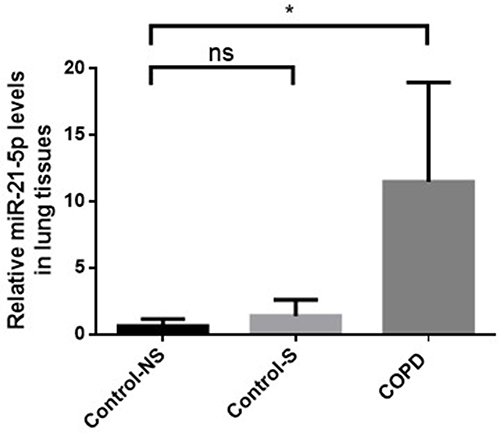
Enhanced miR-21 Expression in Lung Tissue of COPD Patients
Our analysis confirmed that miR-21 expression was significantly elevated in the tissue of COPD patients compared with non-smokers without COPD. However, a marginal increase in miR-21 expression was observed in smokers without COPD, although this increase had no statistical difference when compared with the control group ().
Figure 1 Enhanced miR-21 expression in lung tissue of COPD patients. Assessment of MiR-21 via qRT-PCR across groups: Con-NS (non-smokers, no COPD) (n =5), Con-S (smokers, no COPD) (n =7), and COPD (patients with COPD) (n =7). Data represent mean ± standard deviation. Statistical thresholds: *P < 0.05, (via one-way ANOVA).
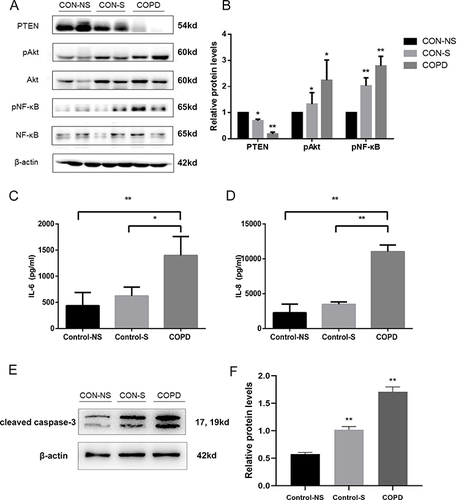
Decreased PTEN Expression, Activating Akt/NF-κB Pathway, and Increasing Inflammation and Apoptosis in COPD Patients
In pursuit of identifying miR-21’s target gene, the PTEN expression in our specimens was quantified using Western blot analysis (). During these evaluations, a notable diminution in PTEN protein concentrations was evident in individuals with COPD when contrasted with both non-smokers and smokers without COPD, who exhibited normal pulmonary function (). Furthermore, an inverse relationship was discerned between the levels of PTEN protein and the phosphorylated versions of Akt and NF-κB. Inflammatory factor IL‐6 and IL-8 in the Peripheral blood serum were increased compared with that in control-NS and Control group ( and ). An increased expression of cleaved caspased-3 in the peripheral lung tissue of COPD patients ( and ). This suggests a link between elevated miR-21 levels and increased inflammatory and apoptosis activity.
Figure 2 Decreased PTEN expression, activating Akt/NF-κB pathway, and increasing inflammation and apoptosis in COPD patients. (A) Protein levels of PTEN, phosphorylated Akt (p-Akt), Akt, phosphorylated NF-κB (p-NF-κB), NF-κB, and β-actin in Western blot analysis. (B) Comparison of PTEN, p-Akt, p-NF-κB protein levels. (C) IL-6 and (D) IL-8 levels were measured by ELISA. (E)Western blot were determined and (F) relative protein levels of cleaved caspase-3 in the peripheral lung tissue. Density of bands measured using ImageJ. β-actin for normalization. Group classification: Con-NS (non-smokers, no COPD) (n =5), Con-S (smokers, no COPD) (n =7), COPD (COPD patients) (n =7). Data: average values ± standard deviation. Statistical thresholds: *P < 0.05, **P < 0.01, via one-way ANOVA.
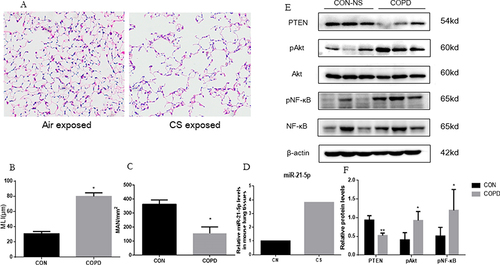
Increased miR-21 Expression, Reduced PTEN Expression and Following Activation of Akt in Lung from COPD Mice
In the animal model of COPD, mice exposed to cigarette smoke for 12 weeks exhibited notable pathological changes, including airway space enlargement, alveolar wall destruction, and significant infiltration of inflammatory cells in the small airways (). Concurrent with these changes, MLI increased significantly and MAN significantly decreased ( and ), substantiating the COPD mouse model’s successful creation. Moreover, an upsurge in miR-21 expression within the lung tissues of these mice was noted, detailed (). Complementarily, a reduction in PTEN and an elevation in phosphorylated Akt and NF-κB were detected, mirroring the miR-21 level variations ( and ).
Figure 3 Increased miR-21 expression, reduced PTEN expression and following activation of Akt in lung from COPD mice. (A) Lung tissue of mice exposed to air and cigarette smoke (CS) analyzed via Hematoxylin-eosin staining, revealing tissue destruction and airway dilation (original magnification ×100). Bar: 200 μm. (B and C) Evaluation of lung architecture through Mean Linear Intercept (MLI) quantification and Emphysema assessment via Mean Alveolar Numbers (MAN). (D) Determination of miR-21 levels in lung tissue by quantitative Real-Time PCR (qRT-PCR). (E) Protein expression of PTEN, phosphorylated Akt (p-Akt), Akt, phosphorylated NF-κB (p-NF-κB), NF-κB assessed by Western Blot. (F) Band density quantified using ImageJ. Data presented as mean ± standard deviation, n = 3. Statistical analysis: *P < 0.05, **P < 0.01, via one-way ANOVA.
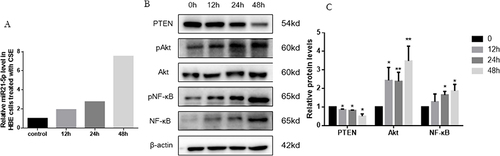
CSE Inducing Increase of miR-21 and Decrease of PTEN in HBE Cells
In the experiment involving CSE-treated HBE cells, these cells were exposed to 5% CSE for durations of 12, 24, and 48 hours. It was observed that miR-21 levels in the HBE cells increased progressively over these time intervals (). This elevation in miR-21 levels showed a negative correlation with the protein levels of PTEN ( and ). Furthermore, there was a time-dependent increase in phosphorylated Akt and NF-κB, which was in line with the observed reduction in PTEN levels.
Figure 4 CSE inducing increase of miR-21 and decrease of PTEN in HBE cells. (A) Exposure of HBE cells to 5% CSE at intervals of 12h, 24h, and 48h. (B) Assessment of miR-21 levels via quantitative Real-Time PCR (qRT-PCR). (C) Protein expression evaluated using Western blot, with relative levels determined. Band densities quantified by ImageJ. Data expressed as mean ± standard deviation, n = 3. Statistical significance established at *P < 0.05, **P < 0.01, through one-way ANOVA.
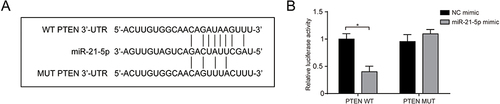
miR-21 Targeting PTEN
We verified that miR-21 binds to PTEN directly through luciferase reporter assay. The connection points of miR-21 and PTEN were predicted from bioinformatics analysis (). The miR-21-5p mimic group exhibited inhibited luciferase activity of WT plasmids but unchanged between the other groups (), which revealed an inversely correlated regulation between miR-21 and PTEN.
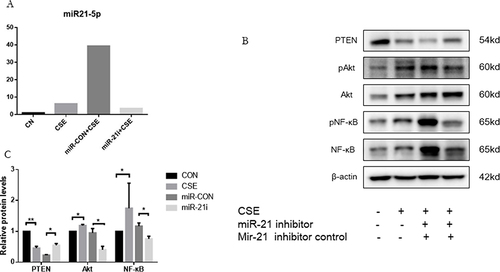
MiR-21 Inhibiting PTEN to Activate the Akt/NF-κB Pathway in HBE Cells
Investigating the influence of miR-21 on PTEN regulation in HBE cells, HBE cells were transfected with a miR-21 inhibitor and a control counterpart separately for 48 hours (). This was followed by subjecting the HBE cells exposed to 5% CSE for the same duration. It was observed that the suppression of miR-21 led to an increase in PTEN levels and a concurrent decrease in phosphorylated Akt and NF-κB, thus suggesting miR-21’s role in modulating the PTEN/Akt/NF-κB signaling in HBE cells exposed to CSE ( and ).
Figure 6 MiR-21 activating Akt/NF-κB pathway via inhibition of PTEN in HBE cells. (A) Treatment of HBE cells involved miR-21 inhibitor and a control inhibitor over a 48-hour period, followed by CSE exposure for another 48 hours. (B) MiR-21 expression quantified using qRT-PCR. Analysis of PTEN, p-Akt, and p-NF-κB protein levels via Western blot. (C) ImageJ utilized for measuring band densities. Labels: MiR-21i (miR-21 inhibitor), Con-inhibitor (control for miR-21 inhibitor). Statistical data: averages ± standard deviation, n = 3, significance denoted as *P < 0.05, **P < 0.01, determined through one-way ANOVA.
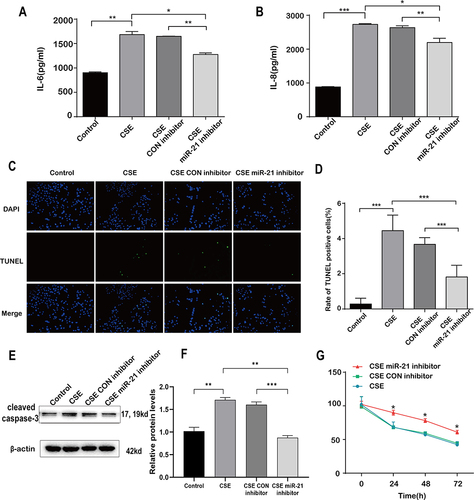
Decreased Inflammation and Apoptosis of A549 in Co Culture with HBE Cells Transfected with miR-21 Inhibitor
Within the co-culture framework, where the concentrations of IL-6 and IL-8 in cell supernatant from A549 cells were comparatively lower when co-cultured with HBE cells transfected with the miR-21 inhibitor ( and ). It was observed that A549 cells exhibited a notable decline in TUNEL-positive staining ( and ) and cleaved caspase-3 expression ( and ), when co-cultured with HBE cells that had been transfected with the miR-21 inhibitor, in contrast to those transfected with a standard control inhibitor ( and ). Cell viability was Increased compared with the control group measured by CCK-8 assay (). This phenomenon indicates a suppression of apoptosis in A549 cells under co-culture conditions with HBE cells treated with the miR-21 inhibitor. These findings highlight miR-21’s role in the cellular response to CSE treatment.
Figure 7 Decreased inflammation and apoptosis of A549 in co culture with HBE cells transfected with miR-21 inhibitor. HBE cells were co-cultured with A549 cells which were transfected with control inhibitor or miR-21 inhibitor and then treated with CSE for 48h. A549 cells are divided into four groups according to co-cultured HBE cells: control, CSE, CSE plus control inhibitor and CSE plus miR-21 inhibitor. (A) IL-6 and (B) IL-8 levels were measured by ELISA. (C) TUNEL-positive staining (in green) and DAPI (in blue) of A549 cells co-cultured with HBE cells (200x). (D) Quantitative analysis of TUNEL positive cells. (E)Western blot were determined and (F) relative protein levels of caspase-3 in A549 cells co-cultured with HBE cells. (G) Cell viability was measured by CCK-8 assay after co-cultured with HBE cells. Dates were mean ± SD, n=3, *P<0.05; **P < 0.01; ***P < 0.001.
Discussion
COPD predominantly caused by smoking, is the third most common cause of mortality worldwide. In individuals with COPD, the disease is marked by a persistent inflammatory process, which manifests as chronic bronchitis, obstruction of small airways, and emphysematous destruction. This condition is further characterized by the degradation of alveolar structures, abnormal activation of bronchial epithelial cells, and ongoing lung inflammation.Citation8,Citation25 Despite this, the precise pathogenic mechanisms underlying COPD remain elusive and poorly understood.
PTEN known for its antagonistic role against the PI3K/Akt pathway, has garnered interest for its functions in maintaining genomic stability, regulating cell survival, proliferation, and metabolism in various conditions including tumors, asthma, idiopathic pulmonary fibrosis, and arthritis.Citation26–28 Earlier investigations have indicated a marked decrease in PTEN concentrations within the peripheral lung tissues of individuals diagnosed with COPD. Importantly, this decrease in PTEN was found to have a direct positive correlation with the intensity of airflow obstruction.Citation22 Moreover, Bozinovski and Anderson came to the conclusion that the primary causes of COPD are acquired somatic mutations of PTEN in smokers’ epithelium. PTEN mutation exacerbated inflammation via triggering the NF-κB pathways in COPD patients.Citation29,Citation30 Moreover, the PI3K/Akt/NF-κB pathway has been found to be persistently activated, contributing to inflammation, release of inflammatory mediators, skeletal muscle wasting, metabolic disturbances,Citation31 epithelial-mesenchymal transition,Citation32 and apoptosis in pulmonary structural cells.Citation33 In our investigation, we confirmed the suppression of PTEN and the activation of the PI3K/Akt/NF-κB pathway in patients with COPD, reflecting similar outcomes observed in mouse studies conducted previously. Smoking, identified as a pivotal risk factor, is linked to the decrease in PTEN levels, predominantly through ROS-induced oxidation leading to the inactivation of PTEN, thus initiating the PI3K/Akt pathway.Citation34 These insights were corroborated by our results, which indicated diminished PTEN expression and an increase in phosphorylated Akt and NF-κB in non-COPD smokers and HBE cells exposed to CSE.
MiR-21, widely present in various cell types within mammals, is known for its role in numerous tumors, influencing cell proliferation, migration and invasion through the regulation of the PTEN/PI3K/Akt pathway.Citation35,Citation36 Prior research has shown that smoke or CSE exposure increases miR-21 expression, both in a COPD mouse modelCitation18 and in vitro studies involving HBE and A549 tumor cells.Citation37 In asymptomatic heavy smokers, who had a smoking index exceeding 20 pack-years, and in COPD patients, serum levels of miR-21 were found to be higher than in non-smokers.Citation16 We discovered that patients with COPD had substantially higher levels for miR-21 in their peripheral lungs. However, due to the limited availability of COPD patient samples, we could not extensively explore the relationship between miR-21 levels, PI3K/Akt pathway activity, and the severity of air-flow obstruction. Additionally, our research identified a temporal increase in miR-21 expression in both a COPD mouse model and HBE cells, highlighting its potential as a diagnostic biomarker for COPD. Suppressing miR-21 could counter the decline in PTEN expression and boost the levels of phosphorylated Akt and NF-κB, thus alleviating ROS-induced apoptosis in alveolar epithelial cells. As a result, targeting miR-21 may offer a defensive mechanism against alveolar damage induced by apoptosis and inflammation.
Our study firstly elucidated the role of miR-21 in the pathogenesis of COPD via the PTEN/Akt/NF-κB pathway. We also have some limitations. The decisive role of miR-21 and PTEN/PI3K/Akt pathway were not confirmed in vivo experiments. We will continue to explore its mechanism in vivo in subsequent studies.
Conclusions
In conclusion, our results revealed that miR-21 exerts a suppressive effect on PTEN expression and plays a role in triggering the Akt/NF-κB pathway, which is related to the occurrence and development of COPD. These findings highlight the therapeutic and diagnostic potential of targeting miR-21 in COPD.
Ethics Approval and Consent to Participate
The study was approved by the ethical review board of Wuxi People’s Hospital Affiliated to Nanjing Medical University. The ethics approval number was KS2023119 and KS2019008.
Disclosure
Xiaoyan Sai, Chu Qin, and Zixiao Zhang are co-first authors for this study. The authors declare that there is no conflict of interest in this work.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Additional information
Funding
References
- Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. doi:10.1016/s0140-6736(22)00470-6
- Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD--implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis. 2014;9:1207–1224. doi:10.2147/copd.S51226
- Guo P, Li R, Piao TH, Wang CL, Wu XL, Cai HY. Pathological mechanism and targeted drugs of COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1565–1575. doi:10.2147/copd.S366126
- Barnes PJ. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020;33:101544. doi:10.1016/j.redox.2020.101544
- Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–743. doi:10.1016/s0140-6736(09)61303-9
- Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(23):2445–2454. doi:10.1056/NEJMra0804752
- Vij N, Chandramani-Shivalingappa P, Van Westphal C, Hole R, Bodas M. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am J Physiol Cell Physiol. 2018;314(1):C73–C87. doi:10.1152/ajpcell.00110.2016
- Ortiz-Quintero B, Martínez-Espinosa I, Pérez-Padilla R. Mechanisms of lung damage and development of COPD due to household biomass-smoke exposure: inflammation, oxidative stress, MicroRNAs, and gene polymorphisms. Cells. 2022;12(1):3.
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi:10.1080/01926230701320337
- Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006;7(1):53. doi:10.1186/1465-9921-7-53
- Zinellu E, Zinellu A, Fois AG, Carru C, Pirina P. Circulating biomarkers of oxidative stress in chronic obstructive pulmonary disease: a systematic review. Respir Res. 2016;17(1):150. doi:10.1186/s12931-016-0471-z
- Song Q, Chen P, Liu XM. The role of cigarette smoke-induced pulmonary vascular endothelial cell apoptosis in COPD. Respir Res. 2021;22(1):39. doi:10.1186/s12931-021-01630-1
- Stolzenburg LR, Harris A. The role of microRNAs in chronic respiratory disease: recent insights. Biol Chem. 2018;399(3):219–234. doi:10.1515/hsz-2017-0249
- Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi:10.1038/nature03702
- Wu H, Neilson JR, Kumar P, et al. miRNA profiling of naïve, effector and memory CD8 T cells. PLoS One. 2007;2(10):e1020. doi:10.1371/journal.pone.0001020
- Xie L, Wu M, Lin H, et al. An increased ratio of serum miR-21 to miR-181a levels is associated with the early pathogenic process of chronic obstructive pulmonary disease in asymptomatic heavy smokers. Mol Biosyst. 2014;10(5):1072–1081. doi:10.1039/c3mb70564a
- He S, Chen D, Hu M, et al. Bronchial epithelial cell extracellular vesicles ameliorate epithelial-mesenchymal transition in COPD pathogenesis by alleviating M2 macrophage polarization. Nanomedicine. 2019;18:259–271. doi:10.1016/j.nano.2019.03.010
- He S, Li L, Sun S, Zeng Z, Lu J, Xie L. A novel murine chronic obstructive pulmonary disease model and the pathogenic role of MicroRNA-21. Front Physiol. 2018;9:503. doi:10.3389/fphys.2018.00503
- Abeyrathna P, Su Y. The critical role of Akt in cardiovascular function. Vascul Pharmacol. 2015;74:38–48. doi:10.1016/j.vph.2015.05.008
- Sun L, Li W, Li W, Xiong L, Li G, Ma R. Astragaloside IV prevents damage to human mesangial cells through the inhibition of the NADPH oxidase/ROS/Akt/NF‑κB pathway under high glucose conditions. Int J Mol Med. 2014;34(1):167–176. doi:10.3892/ijmm.2014.1741
- Han SS, Yun H, Son DJ, et al. NF-kappaB/STAT3/PI3K signaling crosstalk in iMyc E mu B lymphoma. Mol Cancer. 2010;9:97. doi:10.1186/1476-4598-9-97
- Yanagisawa S, Baker JR, Vuppusetty C, et al. Decreased phosphatase PTEN amplifies PI3K signaling and enhances proinflammatory cytokine release in COPD. Am J Physiol Lung Cell Mol Physiol. 2017;313(2):L230–L239. doi:10.1152/ajplung.00382.2016
- Sun X, Chen L, He Z. PI3K/Akt-Nrf2 and anti-inflammation effect of macrolides in chronic obstructive pulmonary disease. Curr Drug Metab. 2019;20(4):301–304. doi:10.2174/1389200220666190227224748
- Xie X, Qu P, Wu H, et al. Circulating exosomal miR-21 mediates HUVEC proliferation and migration through PTEN/PI3K/AKT in Crohn’s disease. Ann Transl Med. 2022;10(5):258. doi:10.21037/atm-22-475
- Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. doi:10.1146/annurev.pathol.4.110807.092145
- Cai B, Yang L, Do Jung Y, et al. PTEN: an emerging potential target for therapeutic intervention in respiratory diseases. Oxid Med Cell Longev. 2022;2022:4512503. doi:10.1155/2022/4512503
- Qiu T, Tian Y, Gao Y, et al. PTEN loss regulates alveolar epithelial cell senescence in pulmonary fibrosis depending on Akt activation. Aging. 2019;11(18):7492–7509. doi:10.18632/aging.102262
- Boosani CS, Gunasekar P, Agrawal DK. An update on PTEN modulators - a patent review. Expert Opin Ther Pat. 2019;29(11):881–889. doi:10.1080/13543776.2019.1669562
- Anderson GP, Bozinovski S. Acquired somatic mutations in the molecular pathogenesis of COPD. Trends Pharmacol Sci. 2003;24(2):71–76. doi:10.1016/s0165-6147(02)00052-4
- Hosgood HD, Menashe I, He X, Chanock S, Lan Q. PTEN identified as important risk factor of chronic obstructive pulmonary disease. Respir Med. 2009;103(12):1866–1870. doi:10.1016/j.rmed.2009.06.016
- Li M, Zhong X, Xu WT. Substance P promotes the progression of bronchial asthma through activating the PI3K/AKT/NF-κB pathway mediated cellular inflammation and pyroptotic cell death in bronchial epithelial cells. Cell Cycle. 2022;21(20):2179–2191. doi:10.1080/15384101.2022.2092166
- Jiang B, Guan Y, Shen HJ, et al. Akt/PKB signaling regulates cigarette smoke-induced pulmonary epithelial-mesenchymal transition. Lung Cancer. 2018;122:44–53. doi:10.1016/j.lungcan.2018.05.019
- Tsai MJ, Chang WA, Jian SF, Chang KF, Sheu CC, Kuo PL. Possible mechanisms mediating apoptosis of bronchial epithelial cells in chronic obstructive pulmonary disease - A next-generation sequencing approach. Pathol Res Pract. 2018;214(9):1489–1496. doi:10.1016/j.prp.2018.08.002
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–990. doi:10.1016/j.cellsig.2012.01.008
- Sheedy FJ. Turning 21: induction of miR-21 as a key switch in the inflammatory response. Front Immunol. 2015;6:19. doi:10.3389/fimmu.2015.00019
- Zhang S, Sun P, Xiao X, Hu Y, Qian Y, Zhang Q. MicroRNA-21 promotes epithelial-mesenchymal transition and migration of human bronchial epithelial cells by targeting poly (ADP-ribose) polymerase-1 and activating PI3K/AKT signaling. Korean J Physiol Pharmacol. 2022;26(4):239–253. doi:10.4196/kjpp.2022.26.4.239
- Pace E, Di Vincenzo S, Di Salvo E, et al. MiR-21 upregulation increases IL-8 expression and tumorigenesis program in airway epithelial cells exposed to cigarette smoke. J Cell Physiol. 2019;234(12):22183–22194. doi:10.1002/jcp.28786